Abstract
Objective
Iron is essential for triggering oligodendrocytes to myelinate, however, in gray matter (GM) iron increases with age and is associated with age-related degenerative brain diseases. Women have lower iron levels than men, both in the periphery and in the brain, particularly in white matter (WM), possibly due to iron loss through menstruation. We tested the hypothesis that hysterectomy could increase WM iron levels.
Methods
We assessed three WM and five GM regions in 39 post-menopausal women, of whom 15 had premenopausal hysterectomy, utilizing a validated magnetic resonance imaging technique called FDRI that quantifies ferritin iron. A group of 54 matched male subjects was included for comparison.
Results
Amongst women, hysterectomy was associated with significantly higher frontal lobe WM iron. Men had higher iron levels than women without hysterectomy in three brain regions but did not differ from women with hysterectomy in any region.
Conclusions
The results suggest that menstruation-associated blood loss is a source of gender differences in brain iron. It is possible that brain iron can be influenced by peripheral iron levels and may thus be a modifiable risk factor for age-related degenerative diseases.
Keywords: hysterectomy, iron, metal, dementia, risk, gender, myelin, oligodendrocytes, white matter, gray matter, treatment, prevention
1. Introduction
Iron is essential for cell function and is a key requirement for myelination (Sow et al., 2006), however, increased tissue iron can promote tissue oxidative damage to which the brain is especially vulnerable (Zecca et al., 2004). In the human brain, age-related increases in iron contribute to the development of proteinopathies (abnormal deposits of proteins) associated with several prevalent degenerative brain diseases such as Alzheimer's disease (AD), Parkinson’s disease (PD), and Dementia with Lewy Bodies (DLB) (Bush, 2003; Ke and Ming Qian, 2003; Turnbull et al., 2003) (reviewed in Bartzokis, 2004, 2011; Kell, 2009; Zecca et al., 2004). Iron levels are abnormally elevated in gray matter (GM) of these neurodegenerative diseases, suggesting that increased iron levels may be a risk-factor (Bartzokis et al., 1993; Bartzokis, 2011) as well as impact their age at onset, especially in males (Barker et al., 2002; Bartzokis et al., 1999; Bartzokis et al., 2004b; Miech et al., 2002; Raber et al., 2004).
A previous study by our research group identified brain iron differences between men and women, reporting that as is the case in peripheral measures of ferritin and iron (Fleming et al., 2001; Whitfield et al., 2003), women have lower brain ferritin iron levels than men (Bartzokis et al., 2007b). The gender differences in brain iron were most prominent in all three white matter (WM) regions assessed, which had different levels of iron: frontal lobe WM (FWM) > genu of corpus callosum WM (GWM) > splenium of corpus callosum WM (SWM). In normal WM, oligodendrocytes are the predominant cell type and contain the highest levels of iron of any brain cell (reviewed in Todorich et al., 2009). Iron levels in WM regions are very likely reflective of oligodendrocytes and their myelin since, unlike GM, the number of other cells (astrocytes and microglia) is minimal in WM (Peters and Sethares, 2002) and axonal iron levels are very low (Quintana et al., 2006; Zhang et al., 2005). High iron levels are essential for oligodendrocytes to proceed with myelination and later-myelinating white matter regions such as FWM contain higher levels of myelin and iron than earlier-myelinating regions (Bartzokis et al., 2007b).
In the context of evidence that brain iron is responsive to peripheral iron status (Borten et al., 2004; Pinero et al., 2000) (for review see Beard and Connor, 2003), the gender-related differences in peripheral (Fleming et al., 2001; Whitfield et al., 2003) and brain (Bartzokis et al., 2007b) iron suggest that lower peripheral iron levels could in turn reduce brain iron levels. This possibility, first noted by Hallgren and Sourander (Hallgren and Sourander, 1958), was based on their anecdotal observations made during their landmark post mortem study of human nonheme brain iron. They observed that subjects with known hemorrhages or severe anemia ante mortem had lower brain iron levels as measured post mortem, and suggested that brain iron may be mobilized for metabolic needs outside the brain (Hallgren and Sourander, 1958).
Menstruation reduces peripheral iron levels in premenopausal women and may contribute to the previously observed gender differences in brain iron (Bartzokis et al., 2007b; Fleming et al., 2001; Whitfield et al., 2003). We investigated a possible source of those gender differences in brain iron indirectly by examining the effect of premature cessation of menstrual blood loss through hysterectomy. We compared postmenopausal women who underwent hysterectomy with those who did not. We hypothesized that WM regions and especially FWM, which myelinates later and achieves the highest density of oligodendrocytes and iron, may be most impacted by increased iron levels expected from hysterectomy (Brett, 2005; Howard et al., 2005).
Hysterectomy is the most common nonobstetrical surgery among women in the United States (Kozak et al., 2004), with one in three women in the United States having had a hysterectomy by age 60. According to estimates, 50% also involve “bilateral oophorectomy,” the surgical removal of both ovaries (Lepine et al., 1997). Hysterectomy will result in the cessation of menses if the uterus is removed, whether or not one or both ovaries are removed (Brett, 2005). However, if one or both ovaries are not removed during hysterectomy, a potential endogenous source of sex hormones may remain.
Brain iron levels can be measured in vivo using magnetic resonance imaging (MRI) through the effect of iron on transverse relaxation rates (R2) (Bartzokis et al., 1993; Vymazal et al., 1996a). The bulk of brain iron is stored in ferritin molecules (Floyd and Carney, 1993; Morris et al., 1992) and an in vivo MRI method called field-dependent R2 increase (FDRI) can obtain specific measures of the iron content of ferritin molecules (ferritin iron) (Bartzokis et al., 1993). The method takes advantage of the fact that ferritin iron increases R2 linearly with the field strength of the MRI instrument to produce a highly specific and reproducible measure of this tissue iron store (Bartzokis et al., 1993). Briefly, FDRI is the difference in measures of brain R2 obtained with two different field-strength MRI instruments. In the presence of ferritin, R2 increases with increasing magnetic field-strength (Bartzokis et al., 1993; Vymazal et al., 1996a; Vymazal et al., 1996b). This field-dependent R2 increase is specifically associated with the total iron contained in ferritin molecules (Bartzokis et al., 1993; Vymazal et al., 1996a) and has been shown to be independent of the amount of iron loading (number of iron atoms per molecule of ferritin) (Vymazal et al., 1996b) and to increase linearly with field-strength (Bartzokis et al., 1993; Vymazal et al., 1996a; Vymazal et al., 1996b). Thus, FDRI is a specific measure of the total iron contained in ferric oxyhydroxide particles that form the mineral core of ferritin molecules. In human tissue, ferritin and its breakdown product (hemosiderin) are the only known physiologic sources of such particles (Bartzokis et al., 1993; Bulte et al., 1997; Vymazal et al., 1996a). The FDRI measure will therefore be referred to herein as ferritin iron (Bartzokis et al., 2007b).
The current study tested the hypothesis that women who had hysterectomies before menopause will have higher brain iron levels in WM regions than women who did not have hysterectomies. The postulated underlying mechanism is that hysterectomy will lead to increased peripheral iron levels (Brett, 2005; Fleming et al., 2001; Howard et al., 2005; Whitfield et al., 2003), which in turn may lead to increased brain iron levels.
2. Methods
2.1. Subjects
The data for regional brain iron for the subjects in this study were included in prior publications by our research group (Bartzokis et al., 2007b; Bartzokis et al., 2011). Because the focus of the current study was on the effects of hysterectomy, the sample consisted of only post-menopausal female subjects; however, a group of matched male subjects (Table 1b) was included for comparison. Healthy adult volunteers that participated in the study were recruited from the community and hospital staff. Prior to study participation all subjects received written and oral information about the study and signed written informed consents approved by the local institutional review board. Potential subjects were excluded if they had a history of neurological disorder or a family history of AD or other neurodegenerative disorder. The subjects were excluded if they were obese, or if they had a current or prior serious illness, or a medical history of diabetes, cardiovascular disease, or difficult to control hypertension.
Table 1.
| a. Demographics of Hysterectomy Groups | |||||
|---|---|---|---|---|---|
| Female |
Student t-test (two-tailed) |
||||
| HYST− (n=24) | HYST+ (n=15) | df | t | p | |
| Age | 66.0 (7.7) | 66.5 (5.9) | 37 | −0.22 | 0.83 |
| Education, yrs | 14.7 (2.2) | 16.2 (2.7) | 37 | −1.9 | 0.066 |
| Age at Hyst/Meno | 49.6 (4.8) | 45.5 (6.4) | 37 | 2.27 | 0.029 |
| Years from Hyst/Meno to MRI date | 16.4 (9.9) | 20.9 (8.6) | 37 | −1.45 | 0.16 |
|
Chi-square test |
|||||
| df | Χ2 | p | |||
| Race (C/As/AA)* | 18 / 5 / 1 | 9 / 3 / 3 | 2 | 2.56 | 0.28 |
| HRT (Yes/No) | 10 / 14 | 11 / 4 | 1 | 3.72 | 0.054 |
| b. Demographics of Women and Men | |||||
|---|---|---|---|---|---|
| Female vs. Male |
Student t-test (two-tailed) |
||||
| Female (n=39) | Male (n=54) | df | t | p | |
| Age | 66.2 (7.0) | 66.2 (6.9) | 91 | −0.02 | 0.98 |
| Education, yrs | 15.3 (2.5) | 16.3 (2.8) | 91 | −1.85 | 0.068 |
|
Chi-square test |
|||||
| df | Χ2 | p | |||
| Race (C/As/AA)* | 27 / 8 / 4 | 40 / 9 / 5 | 2 | 0.28 | 0.87 |
HYST+: female subjects who had a premenopausal hysterectomy.
HYST−: female subjects who did not undergo hysterectomy.
The above table contains unadjusted means and standard deviations, except for Race and hormone replacement treatment (HRT) which present the frequency (number of subjects in each category).
For Race: C = Caucasian; As = Asian; AA = African American.
The above table contains unadjusted means and standard deviations, except for Race which presents the frequency (number of subjects in each category).
Race: C = Caucasian; As = Asian; AA = African American.
The women were divided into two subgroups based on whether or not they had a hysterectomy. This information was not available for a subset (n=14) of the women, which were not included in any of the analyzed subgroups. Of women with hysterectomies, only one reported being post-menopausal before the date of hysterectomy (seven years) and this subject was excluded from analyses. The final normal female population consisted of 39 subjects, ranging in age from 47 to 80 years (mean=66.2, SD=7.0) and their racial distribution was 27 (69%) Caucasian, 8 (21%) Asian, and 4 (10%) African-American. The normal male comparison population consisted of 54 subjects, ranging in age from 49 to 80 years (mean=66.2, SD=6.9) and their racial distribution was 40 (74%) Caucasian, 9 (17%) Asian, and 5 (9%) African-American.
All subjects were functioning independently and had no evidence of neurocognitive impairment on clinical interview. All of the 37 female subjects who were over the age of 55 were administered the Mini-Mental State Examination (MMSE) and their scores all fell in the normal range (between 27 and 30; mean=28.8, SD=1.0). Two female subjects were under the age of 55 and were not administered the MMSE. Of the males, 50 out of 51 subjects who were over the age of 55 were administered the MMSE and their scores all fell in the normal range (between 27 and 30; mean=28.4, SD=0.9). Four male subjects were not administered the MMSE: one was 74 years old, and three were not over age of 55.
Two subgroups were created from the 39 female subjects described above: subjects who had a hysterectomy (HYST+; n=15) and those who did not (HYST−; n=24). Table 1a presents demographic data for these two HYST groups. Education, race, MMSE scores, and exposure to estrogen replacement (HRT) did not differ between the two HYST groups (Table 1a). The mean age at time of MRI scan did not differ between the groups. The HYST+ women had a hysterectomy on average 20.9 years prior to their MRI scan, at a mean age of 45.5 years. The HYST− women reported menopause an average of 16.4 years prior to their MRI scan, at a mean age of 49.6 years. The age at hysterectomy for the HYST+ group was 4.1 years less than the age at menopause for the HYST− group (p=.029). Thus, on average the women in the HYST− group continued to menstruate for 4.1 years longer than those in the HYST+ group.
2.2. MRI protocol
The methods have been described in detail elsewhere (Bartzokis et al., 1993; Bartzokis et al., 2004a) and will only be summarized here. The subjects were all scanned using two MR instruments operating at different field strengths (1.5 T and 0.5 T instruments), and the two scans were done within one hour of each other using the same imaging protocol. Coronal and sagittal pilot scans were first obtained to specify the location and spatial orientation of the head and the position of the axial image acquisition grid. The axial image acquisition sequence acquired interleaved contiguous slices using a Carr Purcell Meiboom Gill dual spin-echo sequence (2500/20,90/2, 3 mm slice thickness, 192 gradient steps, and 25 cm field of view). The coronal and sagittal pilot scans were used to determine the alignment and accuracy of head repositioning in the second MRI instrument. To consistently position the actual image slices identically within the brain and thus sample the same volume of tissue, the axial slice-select grid was adjusted so that the anterior commissure was contained within the same slice in both high and low field-strength instruments (Bartzokis et al., 1993).
2.3. Image analysis
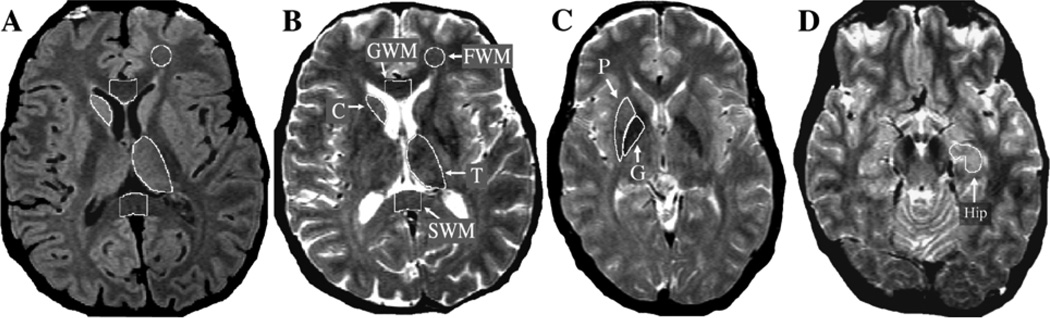
The same eight brain regions (three white matter and five gray matter regions) were analyzed as in our prior study (Bartzokis et al., 2007b). Data for each region of interest (ROI) were obtained from contiguous pairs of slices (see Figure 1).
Figure 1. Regions of Interest (ROI) Delineation.
Regions of Interest. Region of interest (ROI) definition is depicted on axial MRI TE20 (image A) and TE90 (images B, C, D). The TE20 has optimal contrast between GM (appears light gray) and WM (appears dark gray). The TE90 has optimal contrast between brain (appears gray) and CSF (appears white). Both TE20 and TE90 images are used to draw each ROI as this combination of slices maximizes contrast needed for accurate ROI definition. As an example, the use of both contrasts is depicted in the thalamus ROI that borders WM laterally and CSF medially and posteriorly (images A and B). Data for each region of interest (ROI) are obtained from contiguous pairs of slices. Only one hemisphere ROI is depicted on the figures although ROIs are measured bilaterally for all regions except for midline corpus callosum regions (GWM and SWM).
A&B: Frontal lobe white matter (FWM): the second and third slices superior to the orbitofrontal gray matter are chosen. A standard circular ROI template is first positioned within the desired area. The ROI is then manually edited to exclude any unwanted gray matter (which appears hyperintense) or other hyperintensities.
Genu (GWM): the two slices are chosen on which the angle formed by the left and right sides of the genu appears the most horizontal (linear). This results in a sample consistently in the middle of the structure, which contains primarily fibers connecting the prefrontal cortices.
Splenium (SWM): the second and third lowest slices on which the fibers of the splenium connected in the midline are chosen. This results in a sample primarily in the lower half of the splenium, which contains predominantly primary sensory (visual) fibers. For the two corpus callosum regions (GWM and SWM), a standard rectangular ROI template is first positioned on the midline, and then the anterior and posterior borders are manually edited to exclude non-corpus callosum tissue. Lateral borders are defined by the dimensions of the rectangular template.
Caudate nucleus (C): the third and fourth slices above the anterior commissure (not shown) are chosen.
Thalamus (T): the second and third highest slices on which thalamus appears are chosen. The lateral border of the ROI is drawn along the white matter of the internal capsule using the TE20 image (A). The medial and posterior portions bordering CSF are defined using the TE90 image (B).
C: Putamen (P) and globus pallidus (G): the slice containing the anterior commissure and the slice immediately superior to it (C) are chosen.
D: Hippocampus (Hip): the slices containing the largest area of anterior hippocampus are chosen. The anterior Hip measure is limited posteriorly by drawing a horizontal line at the level of the cerebral peduncle to exclude any tissue posterior to that line.
Transverse relaxation times (T2) were calculated for each voxel by an automated algorithm from the two signal intensities (TE = 20 & 90) of the dual spin-echo sequence to produce gray-scale encoded T2 maps of the brain (Bartzokis et al., 1993). The T2 measures were extracted using an Apple Macintosh-configured image analysis workstation. T2 data for each of the ROIs were obtained from contiguous pairs of slices. The relaxation rate (R2) was calculated as the reciprocal of T2 × 1000 milliseconds/second. For all regions except the two midline structures (GMW and SWM), the measures from left and right hemispheres were averaged. The average R2 of the two slices were the final measures used in the subsequent analyses. The FDRI measure was calculated as the difference in R2 (high field R2 - low field R2). Test-retest reliability for FDRI measures was very high with intraclass correlation coefficients ranging from .88 to .99 (p < .0023) (Bartzokis et al., 1993).
2.4. Data analysis
A multivariate analysis of covariance (MANOVA) was conducted on the FDRI measures in the three white matter regions: FWM, GWM, and SWM. The dependent variable was FDRI and the independent variable was hysterectomy. Age, education, MMSE, and racial composition did not differ significantly between the HYST groups (see Table 1a). In this restricted age-span, age was also not significantly correlated with FDRI in any of the regions (p>.14) and was therefore not introduced as a covariate in subsequent analyses. Student’s t-tests (two-tailed) were then conducted to examine the contribution of HYST to the FDRI for each of the white matter regions separately. For completeness, the five gray matter regions were also assessed using the same approach (Table 2).
Table 2.
Brain Iron in Women With Hysterectomies Before Menopause Versus Those Without
| Hysterectomy | |||||
|---|---|---|---|---|---|
| Region | HYST− (n=24) | HYST+ (n=15) | t | p | d' |
| FWM | 1.43 (.20) | 1.67 (.29) | −3.11 | 0.0036 | 1.02 |
| GWM | 1.08 (.35) | 1.19 (.36) | −0.92 | 0.36 | 0.30 |
| SWM | 0.87 (.47) | 1.10 (.35) | −1.60 | 0.12 | 0.53 |
| Hysterectomy | |||||
|---|---|---|---|---|---|
| Region | HYST− (n=24) | HYST+ (n=15) | t | p | d' |
| C | 2.27 (.53) | 2.51 (.44) | −1.46 | 0.15 | 0.48 |
| G | 4.42 (.95) | 4.70 (.62) | −1.01 | 0.32 | 0.33 |
| P | 2.97 (.71) | 3.09 (.61) | −0.52 | 0.61 | 0.17 |
| T | 1.07 (.35) | 1.23 (.24) | −1.55 | 0.13 | 0.51 |
| Hip | 0.75 (.22) | 0.76 (.30) | −0.19 | 0.85 | 0.06 |
HYST+: female subjects who had a premenopausal hysterectomy.
HYST−: female subjects who did not undergo hysterectomy.
Results are unadjusted FDRI means (standard deviation) of Student’s t-test analyses (df=37).
d’ = Cohen’s effect size (Cohen, 1988)
Female HYST+ and HYST− groups were then compared to the male group using Student’s t-tests (two-tailed) for each brain region.
3. Results
The MANOVA model indicated a significant effect of HYST on white matter brain iron (Wilks’ Lambda=.786, F=3.18, df=35, p=0.036). Student t-tests comparing the mean FDRI in each of the three white matter regions separately revealed significant group differences for FWM (t=−3.11, df=37, p=0.0036), with increased FDRI in the HYST+ group.
When corrected for multiple comparisons (Bonferroni) and multiplied by 3 (corresponding to the 3 regions analyzed), the p value in the frontal white matter region remained statistically significant (p=.01). The FDRI values were consistently elevated in the HYST+ group for all three regions, but the group comparisons did not reach statistical significance for the GWM and SWM. These results are summarized in Table 2 below.
When the analysis was repeated including age and race as covariates in addition to HYST status the results in Table 2 were not meaningfully changed and specifically, the FWM region group difference remained highly significant (p=.008).
In the HYST+ women, “years since hysterectomy” was not significantly correlated with FDRI in any of the regions (p>.13). Because of the confounding effect of age within “years since hysterectomy” (i.e. subjects with greater years since hysterectomy will tend to be older) we performed an additional analysis controlling for age, and found similar results: after controlling for age, “years since hysterectomy” was not significantly correlated with FDRI in any of the regions (p>.22).
FDRI was also elevated in the HYST+ group in all five GM regions, but the group comparisons did not reach statistical significance (Table 2). Unlike the WM regions, results of MANOVA analysis to test for an overall Hysterectomy effect did not reach significance when combining the five GM regions: p=.39 (Wilks' Lambda=.859, F=1.08, df=33).
Comparisons between the two groups of women and the group of men are shown below in Table 3 and Figure 2.
Table 3.
Brain Iron in Women With Hysterectomies Before Menopause and Those Without Versus Men
| Female | Male | HYST− vs. Male (df=76) | HYST+ vs. Male (df=67) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | HYST− (n=24) | HYST+ (n=15) | (n=54) | t | p | d' | t | p | d' |
| FWM | 1.43 (.20) | 1.67 (.29) | 1.65 (.28) | −3.47 | 0.0009 | 0.80 | 0.29 | 0.77 | 0.07 |
| GWM | 1.08 (.35) | 1.19 (.36) | 1.23 (.33) | −1.85 | 0.068 | 0.42 | −0.45 | 0.65 | 0.11 |
| SWM | 0.87 (.47) | 1.10 (.35) | 1.04 (.42) | −1.61 | 0.11 | 0.37 | 0.44 | 0.66 | 0.11 |
| Female | Male | HYST− vs. Male (df=76) | HYST+ vs. Male (df=67) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | HYST− (n=24) | HYST+ (n=15) | (n=54) | t | p | d' | t | p | d' |
| C | 2.27 (.53) | 2.51 (.44) | 2.56 (.47) | −2.40 | 0.019 | 0.55 | −0.36 | 0.72 | 0.09 |
| G | 4.42 (.95) | 4.70 (.62) | 4.71 (.77) | −1.43 | 0.16 | 0.33 | −0.05 | 0.96 | 0.01 |
| P | 2.97 (.71) | 3.09 (.61) | 3.13 (.63) | −0.97 | 0.34 | 0.22 | −0.22 | 0.83 | 0.05 |
| T | 1.07 (.35) | 1.23 (.24) | 1.33 (.32) | −3.27 | 0.0016 | 0.75 | −1.17 | 0.25 | 0.29 |
| Hip | 0.75 (.22) | 0.76 (.30) | 0.79 (.27) | −0.73 | 0.47 | 0.17 | −0.38 | 0.71 | 0.09 |
HYST+: female subjects who had a premenopausal hysterectomy.
HYST−: female subjects who did not undergo hysterectomy.
Results are unadjusted FDRI means (standard deviation) of Student’s t-test analyses.
d’ = Cohen’s effect size (Cohen, 1988).
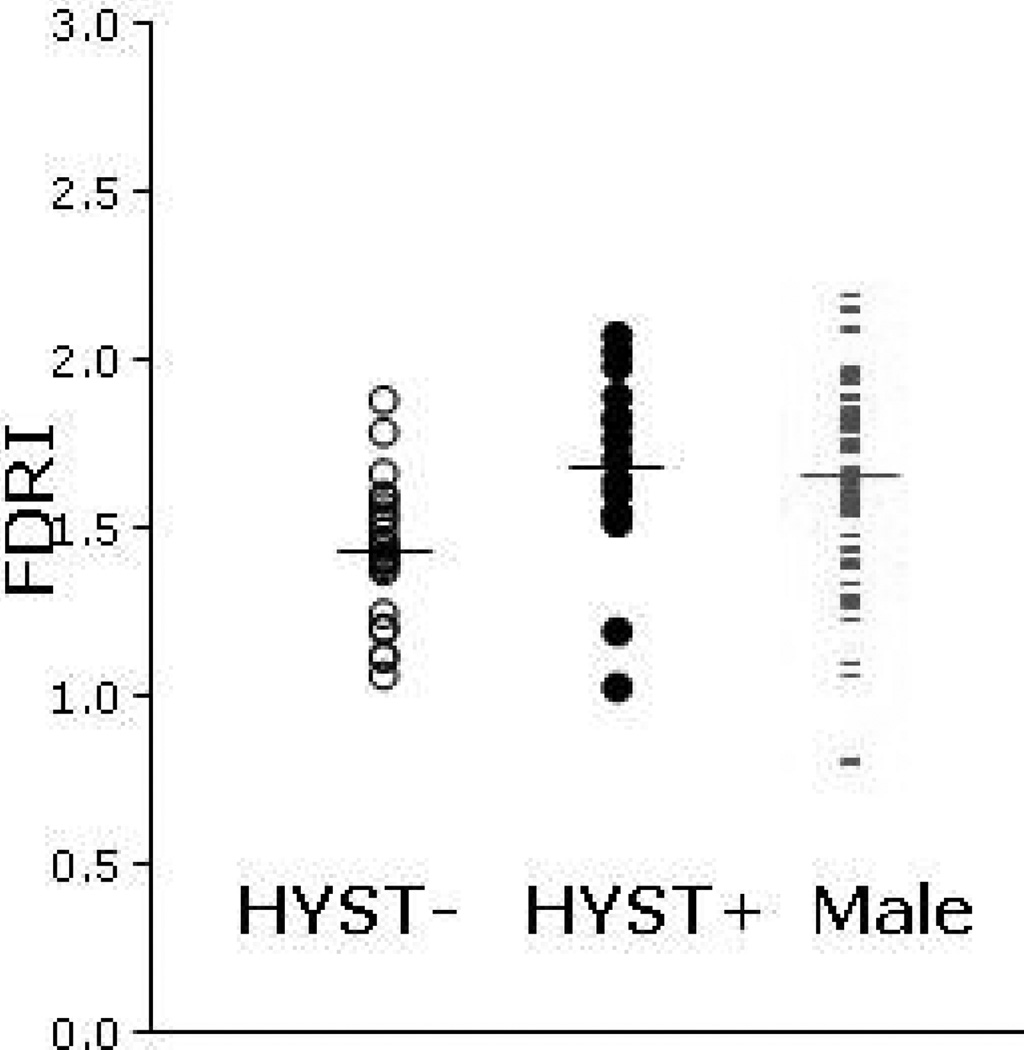
Figure 2. Frontal Lobe White Matter Iron in Healthy Women With Hysterectomies Before Menopause (HYST+), Those Without (HYST−), and Matched Men.
Brain iron (FDRI) in frontal lobe white matter (FWM) of HYST+ women is increased compared to HYST− women and is similar to that of men. Horizontal lines represent mean values for each group. Only two HYST+ women have values below the mean of the HYST− women and vice-versa. Group mean values and statistics for group differences are presented in Tables 2 and 3.
When the above analyses was repeated including age and race as covariates in addition to HYST status the results in Table 3 were not meaningfully changed; specifically, all gender differences remained significant (HYST− vs. HYST+: FWM p=.008; HYST− vs. Male: FWM p=.0005, C p=.016, T p=.0013), while in the HYST+ vs. Male comparison none of the differences were significant.
4. Discussion
The data suggest that women who had hysterectomies before menopause will likely have significantly higher FWM iron levels than women who did not (Table 2 and Figure 2). Brain iron levels were also numerically higher in the remaining seven regions among women who had a hysterectomy, although, given the small sample size, none of these differences achieved statistical significance. Women begin their post-menopausal years in a state of relative peripheral iron deficiency compared to men and their iron levels increase for the first 15–20 years after menopause without fully “catching up” to those in men (Whitfield et al., 2003). Since peripheral iron levels can influence brain iron accumulation (Bartzokis et al., 2007b; House et al., 2010; Li et al., 2010), these peripheral iron increases could manifest in the brain. Our results support the hypothesis that the premature cessation of menstruation through hysterectomy eliminated a source of iron loss in those women at an earlier age, leading to an earlier increase in brain iron than in women who did not have hysterectomies. Comparing the two groups of women to men makes this contrast even more apparent, with HYST− women having lower ferritin iron than men in frontal lobe WM as well as two other regions, while HYST+ did not differ from men in any region (Table 3 and Figure 2).
The most striking hysterectomy-related differences as well as the most striking gender-related differences in brain iron (Bartzokis et al., 2007b) were observed in FWM. In WM, oligodendrocytes represent the vast majority of cells (>85%, as observed in monkeys (Peters and Sethares, 2002)). Age-related changes in ferritin iron levels in WM regions probably closely reflect changes in oligodendrocyte and myelin density, since the remaining tissue is composed primarily of axonal cytoplasm and astrocytes, both of which have very low ferritin and iron levels (Dwork, 1995; Erb et al., 1996; Hill and Switzer, 1984). Oligodendrocytes, on the other hand, are enriched in iron (LeVine and Macklin, 1990) and have the highest iron and ferritin content of all brain cell types (Connor and Menzies, 1996; Dwork, 1995; Erb et al., 1996). Oligodendrocytes are unique in their capacity to obtain iron directly through binding ferritin (Hulet et al., 2002), and may be directly involved in brain iron regulation (Gerber and Connor, 1989). A large portion (as much as 70%) of brain iron is associated with myelin (de los Monteros et al., 2000; Francois et al., 1981) that contains ferritin (Quintana et al., 2006), and ferritin mRNA (Gould et al., 2000) is expressed at myelination onset (Sanyal et al., 1996).
Of the three white matter regions examined, frontal lobe white matter myelinates last (continuing to myelinate into the late fifth decade of life) (Bartzokis et al., 2004a; Kemper, 1994; Meyer, 1981; Yakovlev and Lecours, 1967) and, at older ages, it is also most vulnerable to accelerated age-related myelin breakdown and loss (Bartzokis et al., 2004a; Marner et al., 2003) (reviewed in Bartzokis, 2011). Myelination as well as the remyelination that occurs in the process of repairing broken-down myelin is triggered only when adequate iron amounts accumulate in oligodendrocytes (Sow et al., 2006). Thus, the higher FWM ferritin iron in women that had hysterectomies suggests that regions actively engaged in repair of age-related myelin breakdown may be differentially acquiring the additional oligodendrocyte iron needed for such repair processes (Bartzokis et al., 2011).
On the other hand, prevalent genes associated with increased peripheral iron levels may increase brain GM iron and risk of AD (Bartzokis et al., 2010; Lehmann et al., 2010) and elevated GM iron levels are observed in preclinical stages of AD (Bartzokis et al., 2011; Smith et al., 2010). These studies suggest an accelerated trajectory of brain iron accumulation may be occurring in GM during the transition from healthy aging into preclinical stages and eventually dementia (Bartzokis, 2011). We note that for the comparison of HYST+ vs. HYST− in the current study (Table 2) the effect size for caudate and thalamus ferritin iron was substantial (d’=.5) but did not reach significance due to our small sample size. As expected from our prior study comparing men and women, FWM, caudate, and thalamus differed significantly when HYST− women were compared to men. These differences were not evident in comparisons between HYST+ women and men (Table 3). This suggests that our previously reported gender differences in brain iron are likely underestimated since they included women with hysterectomies (Bartzokis et al., 2007b).. Only larger prospective studies will be able to clarify whether the hysterectomy-associated increase in FWM ferritin iron we observed benefits the increasing need for myelin repair that occurs in old age or helps accelerate iron accumulation-related toxicity and emergence of degenerative disorders such as AD and PD. Animal studies suggest that brain iron undergoes substantial translocation between regions (Dwork et al., 1990; Dwork, 1995). A reanalysis of our large cross-sectional sample (Bartzokis et al., 2007b) using statistical path modeling supports this possibility and suggests that iron may be translocated from WM regions to subcortical and hippocampus regions (unpublished data).
Important limitations need to be considered before further interpretation of these data. First, women who undergo hysterectomy have been shown to differ from women in the general population (e.g. in socioeconomic status and in race) (Brett, 2005), so caution should be used when extrapolating these results to the general population. Second, in cross-sectional studies, interpretation of age-related differences as “changes” or “cause and effect” must be made with caution, and confirmatory prospective studies are needed (Schaie et al., 2004). Third, peripheral iron measures and detailed information on blood loss during life and other environmental influences such as iron supplementation and HRT that may affect brain iron could have influenced the results but were not available. Fourth, the large amount of time lapsed between menopause/hysterectomy and MRI scan may have mitigated against the detection of differences between the two groups of women. Fifth, we assessed a very limited number of white matter regions based on a priori hypothesis regarding late-myelinating FWM, however, based on these promising initial results, future assessments of additional WM regions are warranted. Finally, although reproducible and very highly correlated with post mortem iron levels (Bartzokis et al., 2007b), the FDRI measure specifically quantifies ferritin iron load which may be only indirectly related to the amount of free iron or other transition metals that may be more directly associated with toxicity (Lavados et al., 2008; Rajendran et al., 2009).
Consistent with evidence that brain iron is responsive to peripheral iron status (Bartzokis et al., 2007b; Beard and Connor, 2003; Borten et al., 2004; House et al., 2010; Li et al., 2010; Pinero et al., 2000), the hysterectomy-related differences in brain iron suggest that higher peripheral iron levels could in turn lead to increased brain iron levels. Thus, it seems possible to manipulate brain iron in specific at risk groups for therapeutic purposes (Bartzokis et al., 2011). For example, age-related degenerative brain diseases such as AD can have increased brain iron levels even in preclinical stages (Bartzokis et al., 2011; Lavados et al., 2008; Smith et al., 2010) and elevated brain iron may lower the age of onset (Bartzokis et al., 2007a), especially in men (Bartzokis et al., 2004b). Since men have consistently higher peripheral iron levels than women (Whitfield et al., 2003), reducing brain iron may help counteract the negative effects of aging in the brain, by reducing the iron available to catalyze free radical reactions (Smith et al., 1997) (reviewed in Bartzokis, 2011; Kell, 2009).
Recently, there are indications that reducing body stores of iron, through pharmacologic means (Mandel et al., 2008; Zecca et al., 2008) or relatively simple actions such as phlebotomy (e.g. blood donation) (Engberink et al., 2008; Salonen et al., 1995) or exposure to natural iron-chelation substances such as curcumin (Jiao et al., 2006) or green tea (Srichairatanakool et al., 2006), may have positive consequences on degenerative brain diseases. The advent of in vivo neuroimaging methods that can assess tissue ferritin iron deposits on a regional basis with high specificity provides the means to prospectively examine the impact of age-related changes in iron on trajectories of cognitive decline into neurodegenerative disease (Bartzokis et al., 2011; Lavados et al., 2008; Smith et al., 2010). These methods could be used to measure iron accumulation as well as target emerging therapeutic interventions in high-risk groups identified by MRI, genetic, and clinical biomarkers, years before clinical manifestations of disease. Early intervention in higher-risk sub-groups may make it possible to increase effectiveness of treatments, decrease the need for later more aggressive approaches, and may identify heretofore unexplored opportunities for primary prevention of the exponentially increasing burden of age-related neurodegenerative diseases (Bartzokis, 2011).
Acknowledgements
This work was supported in part by NIH grants (MH 0266029; AG027342), the Department of Veterans Affairs, and the RCS Alzheimer’s Foundation.
George Bartzokis has consulted for and received funding from Janssen Pharmaceutical Inc. and Novartis. Lori L. Altshuler received honoraria from Abbott Laboratories, Bristol-Meyers Squibb, Forest Laboratories and GlaxoSmithKline; is currently on an advisory board for Forest Laboratories; and has been on the speaker’s bureaus for Forest Laboratories, GlaxoSmithKline and Astra Zeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
This data was presented in part as an abstract and published as a Hot Topic at the Annual Meeting of the Society for Neuroscience, November 15, 2010, San Diego, CA.
Disclosure Statement
All other authors declare that they have no conflicts of interest.
REFERENCES
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the state of Florida brain bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Aravagiri M, Oldendorf WH, Mintz J, Marder SR. Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magn.Reson.Med. 1993;29:459–464. doi: 10.1002/mrm.1910290406. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Markham CH, Marmarelis PZ, Treciokas LJ, Tishler TA, Marder SR, Mintz J. MRI evaluation of brain iron in earlier- and later-onset Parkinson's disease and normal subjects. Magn Reson Imaging. 1999;17:213–222. doi: 10.1016/s0730-725x(98)00155-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004a;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin I-S, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. In: LeVine S, Connor J, Schipper H, editors. Redox-active Metals in Neurological Disorders. Ann N Y Acad Sci. New York: 2004b. pp. 224–236. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in Alzheimer's disease. Alzheimer's & Dementia: the Journal of the Alzheimer's Association. 2007a;3:122–125. doi: 10.1016/j.jalz.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007b;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Peters DG, Kosenko A, Barrall KA, Finn JP, Villablanca P, Laub G, Altshuler LL, Geschwind DH, Mintz J, Neely E, Connor JR. Prevalent iron metabolism gene variants associated with increase brain ferritin iron in healthy older men. Journal of Alzheimer's Disease. 2010;20:333–341. doi: 10.3233/JAD-2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Peters DG, Amar CP, Tishler TA, Finn JP, Villablanca P, Altshuler LL, Mintz J, Neely E, Connor JR. Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology. 2011;36:1375–1384. doi: 10.1038/npp.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron Status and Neural Functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Borten O, Liberman A, Tuchweber B, Chevalier S, Ferland G, Schipper HM. Effects of dietary restriction and metal supplementation on the accumulation of iron-laden glial inclusions in the aging rat hippocampus. Biogerontology. 2004;5:81–88. doi: 10.1023/B:BGEN.0000025071.78517.3a. [DOI] [PubMed] [Google Scholar]
- Brett KM. Can hysterectomy be considered a risk factor for cardiovascular disease? Circulation. 2005;111:1456–1458. doi: 10.1161/01.CIR.0000161141.92300.F3. [DOI] [PubMed] [Google Scholar]
- Bulte JW, Miller GF, Vymazal J, Brooks RA, Frank JA. Hepatic hemosiderosis in non-human primates: quantification of liver iron using different field strengths. Magn.Reson.Med. 1997;37:530–536. doi: 10.1002/mrm.1910370409. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- de los Monteros AE, Korsak RA, Tran T, Vu D, de Vellis J, Edmond J. Dietary iron and the integrity of the developing rat brain: a study with the artificially-reared rat pup. Cell Mol Biol (Noisy-le-grand) 2000;46:501–515. [PubMed] [Google Scholar]
- Dwork AJ, Lawler G, Zybert PA, Durkin M, Osman M, Willson N, Barkai AI. An autoradiographic study of the uptake and distribution of iron by the brain of the young rat. Brain Res. 1990;518:31–39. doi: 10.1016/0006-8993(90)90950-g. [DOI] [PubMed] [Google Scholar]
- Dwork AJ. Effects of diet and development upon the uptake and distribution of cerebral iron. J.Neurol.Sci. 1995;(134 Suppl):45–51. doi: 10.1016/0022-510x(95)00207-i. [DOI] [PubMed] [Google Scholar]
- Engberink MF, Geleijnse JM, Durga J, Swinkels DW, de Kort WL, Schouten EG, Verhoef P. Blood donation, body iron status and carotid intima-media thickness. Atherosclerosis. 2008;196:856–862. doi: 10.1016/j.atherosclerosis.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Erb GL, Osterbur DL, LeVine SM. The distribution of iron in the brain: a phylogenetic analysis using iron histochemistry. Brain Res Dev Brain Res. 1996;93:120–128. doi: 10.1016/0165-3806(96)00020-x. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Jacques PF, Tucker KL, Massaro JM, D'Agostino RB, Sr, Wilson PW, Wood RJ. Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores. Am J Clin Nutr. 2001;73:638–646. doi: 10.1093/ajcn/73.3.638. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Carney JM. The role of metal ions in oxidative processes and aging. Toxicol.Ind.Health. 1993;9:197–214. doi: 10.1177/0748233793009001-214. [DOI] [PubMed] [Google Scholar]
- Francois C, Nguyen-Legros J, Percheron G. Topographical and cytological localization of iron in rat and monkey brains. Brain Res. 1981;215:317–322. doi: 10.1016/0006-8993(81)90510-2. [DOI] [PubMed] [Google Scholar]
- Gerber MR, Connor JR. Do oligodendrocytes mediate iron regulation in the human brain? Ann Neurol. 1989;26:95–98. doi: 10.1002/ana.410260115. [DOI] [PubMed] [Google Scholar]
- Gould RM, Freund CM, Palmer F, Feinstein DL. Messenger RNAs located in myelin sheath assembly sites. J Neurochem. 2000;75:1834–1844. doi: 10.1046/j.1471-4159.2000.0751834.x. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hill JM, Switzer RC. The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Milward EA, Bruce DG, Olynyk JK. Relationship between brain R(2) and liver and serum iron concentrations in elderly men. Magn Reson Med. 2010;63:275–281. doi: 10.1002/mrm.22263. [DOI] [PubMed] [Google Scholar]
- Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- Hulet SW, Menzies S, Connor JR. Ferritin binding in the developing mouse brain follows a pattern similar to myelination and is unaffected by the jimpy mutation. Dev Neurosci. 2002;24:208–213. doi: 10.1159/000065704. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson Jt, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert M, Knoefel J, editors. Clinical Neurology of Aging. 2nd ed. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2004;13:1–198. [PubMed] [Google Scholar]
- Lavados M, Guillon M, Mujica MC, Rojo LE, Fuentes P, Maccioni RB. Mild cognitive impairment and Alzheimer patients display different levels of redox-active CSF iron. J Alzheimers Dis. 2008;13:225–232. doi: 10.3233/jad-2008-13211. [DOI] [PubMed] [Google Scholar]
- Lehmann DJ, Schuur M, Warden DR, Hammond N, Belbin O, Kolsch H, Lehmann MG, Wilcock GK, Brown K, Kehoe PG, Morris CM, Barker R, Coto E, Alvarez V, Deloukas P, Mateo I, Gwilliam R, Combarros O, Arias-Vasquez A, Aulchenko YS, Ikram MA, Breteler MM, van Duijn CM, Oulhaj A, Heun R, Cortina-Borja M, Morgan K, Robson K, Smith AD. Transferrin and HFE genes interact in Alzheimer's disease risk: the Epistasis Project. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Lepine LA, Hillis SD, Marchbanks PA, Koonin LM, Morrow B, Kieke BA, Wilcox LS. Hysterectomy surveillance--United States, 1980–1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]
- LeVine SM, Macklin WB. Iron-enriched oligodendrocytes: a reexamination of their spatial distribution. J Neurosci Res. 1990;26:508–512. doi: 10.1002/jnr.490260415. [DOI] [PubMed] [Google Scholar]
- Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MB. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG) J Alzheimers Dis. 2008;15:211–222. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Meyer A. Paul Flechsig's system of myelogenetic cortical localization in the light of recent research in neuroanatomy and neurophysiology part II. Can J Neurol Sci. 1981;8:95–104. doi: 10.1017/s0317167100042980. [DOI] [PubMed] [Google Scholar]
- Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- Morris CM, Candy JM, Oakley AE, Bloxham CA, Edwardson JA. Histochemical distribution of non-haem iron in the human brain. Acta.Anat.(Basel) 1992;144:235–257. doi: 10.1159/000147312. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–263. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Arranz R, Patino C. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer's disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. 2006;153:42–54. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Rajendran R, Minqin R, Ynsa MD, Casadesus G, Smith MA, Perry G, Halliwell B, Watt F. A novel approach to the identification and quantitative elemental analysis of amyloid deposits--insights into the pathology of Alzheimer's disease. Biochem Biophys Res Commun. 2009;382:91–95. doi: 10.1016/j.bbrc.2009.02.136. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Korpela H, Nyyssonen K, Porkkala E, Tuomainen TP, Belcher JD, Jacobs DRJ, Salonen R. Lowering of body iron stores by blood letting and oxidation resistance of serum lipoproteins: a randomized cross-over trial in male smokers. J.Intern.Med. 1995;237:161–168. doi: 10.1111/j.1365-2796.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Sanyal B, Polak PE, Szuchet S. Differential expression of the heavy-chain ferritin gene in non-adhered and adhered oligodendrocytes. J Neurosci Res. 1996;46:187–197. doi: 10.1002/(SICI)1097-4547(19961015)46:2<187::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Caskie G. The Seattle longitudinal study: Relationship between personality and cognition. Aging, Neuropsychology, & Cognition. 2004;11:304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow A, Lamant M, Bonny JM, Larvaron P, Piaud O, Lecureuil C, Fontaine I, Saleh MC, Garcia Otin AL, Renou JP, Baron B, Zakin M, Guillou F. Oligodendrocyte differentiation is increased in transferrin transgenic mice. J Neurosci Res. 2006;83:403–414. doi: 10.1002/jnr.20741. [DOI] [PubMed] [Google Scholar]
- Srichairatanakool S, Ounjaijean S, Thephinlap C, Khansuwan U, Phisalpong C, Fucharoen S. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin. 2006;30:311–327. doi: 10.1080/03630260600642666. [DOI] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: The role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Turnbull S, Tabner BJ, Brown DR, Allsop D. Generation of hydrogen peroxide from mutant forms of the prion protein fragment PrP121–231. Biochemistry. 2003;42:7675–7681. doi: 10.1021/bi030036e. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Brooks RA, Baumgarner C, Tran V, Katz D, Bulte JW, Bauminger R, Di Chiro G. The relation between brain iron and NMR relaxation times: An in vitro study. Magn.Reson.Med. 1996a;35:56–61. doi: 10.1002/mrm.1910350108. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Zak O, Bulte JW, Aisen P, Brooks RA. T1 and T2 of ferritin solutions: effect of loading factor. Magn.Reson.Med. 1996b;36:61–65. doi: 10.1002/mrm.1910360111. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Treloar S, Zhu G, Powell LW, Martin NG. Relative importance of female-specific and non-female-specific effects on variation in iron stores between women. Br J Haematol. 2003;120:860–866. doi: 10.1046/j.1365-2141.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Boston: Blackwell Scientific Publications; 1967. [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J Neurochem. 2008;106:1866–1875. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Cui Y, Chen J, Lu M, Elias SB, Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]