Abstract
Low-density lipoprotein receptor–related proteins 5 and 6 (Lrp5 and Lrp6) are co-receptors of Wnt ligands and play important roles in Wnt/β-catenin signal transduction. Mice homozygous for a germline deletion of Lrp6 die at birth with several associated defects, while Lrp5-deficient mice are viable. Here we conditionally deleted Lrp5 and/or Lrp6 in the mouse gut (gut−/−) by crossing mice carrying floxed alleles of Lrp5 and Lrp6 to a strain expressing Cre recombinase from the villin promoter (villin-Cre). The changes in morphology, differentiation and Wnt signal transduction were validated using immunohistochemistry and other staining. Consistent with observations in mice carrying a homozygous germline deletion in Lrp5, intestinal development in Lrp5gut−/− mice was normal. In addition, mice homozygous for villin-Cre–induced deletion of Lrp6 (Lrp6gut−/−) were viable with apparently normal intestinal differentiation and function. However, mice homozygous for villin-Cre inactivated alleles of both genes (Lrp5gut−/−;Lrp6gut−/−) died within one day of birth. Analysis of embryonic Lrp5gut−/−;Lrp6gut−/− intestinal epithelium showed a progressive loss of cells, an absence of proliferation, and a premature differentiation of crypt stem/precursor cells; no notable change in differentiation was observed in the embryos lacking either gene alone. Further immunohistochemical studies showed that expression of the Wnt/β-catenin target, cyclin D1, was specifically reduced in the intestinal epithelium of Lrp5gut−/−;Lrp6gut−/− embryos. Our data demonstrate that Lrp5 and Lrp6 play redundant roles in intestinal epithelium development, and that Lrp5/6 might regulate intestinal stem/precursor cell maintenance by regulating Wnt/β-catenin signaling.
Keywords: Lrp5, Lrp6, Wnt/β-catenin signaling, intestine, epithelium, embryonic development
Introduction
Wnt signaling plays a key role in many stages of development, such as generation of tissues and cell types and the regulation of cell polarity and movement [Clevers, 2006; He et al., 2004]. The intestinal epithelium is a self-renewing tissue in which the intervillus regions consist of a group of undifferentiated and actively dividing cells with stem cell properties that will eventually invaginate into the mucosa to form crypts and villi [Scoville et al., 2008]. It has been well established that Wnt/β-catenin signaling plays key roles in intestine homeostasis and the maintenance of intestinal stem cells [Clevers, 2006; Pinto et al., 2003].
The β-catenin-dependent pathway is often referred as the “canonical pathway”, and it requires Lrp5 or Lrp6 as a co-receptor for Wnt ligands. Lrp5 and Lrp6 are a subclass of the low-density lipoprotein receptor family and have 71% homology. Lrp5 and Lrp6 share a structure that contains a large extracellular domain (ECD, about 1600 amino acids), a 22-amino-acid single-pass transmembrane domain, and a relatively short (207 amino acids) cytoplasmic domain [Williams and Insogna, 2009]. The large amount of research on the roles of Lrp5 and Lrp6 in development has indicated that Lrp6 might be more developmentally critical than Lrp5. Homozygous Lrp6 mutant mice show embryonic defects and die at birth, while Lrp5-deficent mice are viable although with a low-bone-mass phenotype [Holmen et al., 2004; Kelly et al., 2004].
In the intestine, Lrp5 and Lrp6 have been mapped to localize exclusively in proliferative epithelial cells [Gregorieff et al., 2005], but the specific roles of Lrp5 and Lrp6 in the intestinal epithelium have not been clearly defined. Here we compare the functions of Lrp5 and Lrp6 in the development of intestinal epithelium, using a gut-targeting mouse model. We find that while deletion of either gene alone does not result in a significant phenotype, concomitant deletion of both genes leads to severe defects in intestinal differentiation and neonatal lethality.
Results
Generation of mice carrying conditional deletions of Lrp5 and/or Lrp6 in the intestinal epithelium
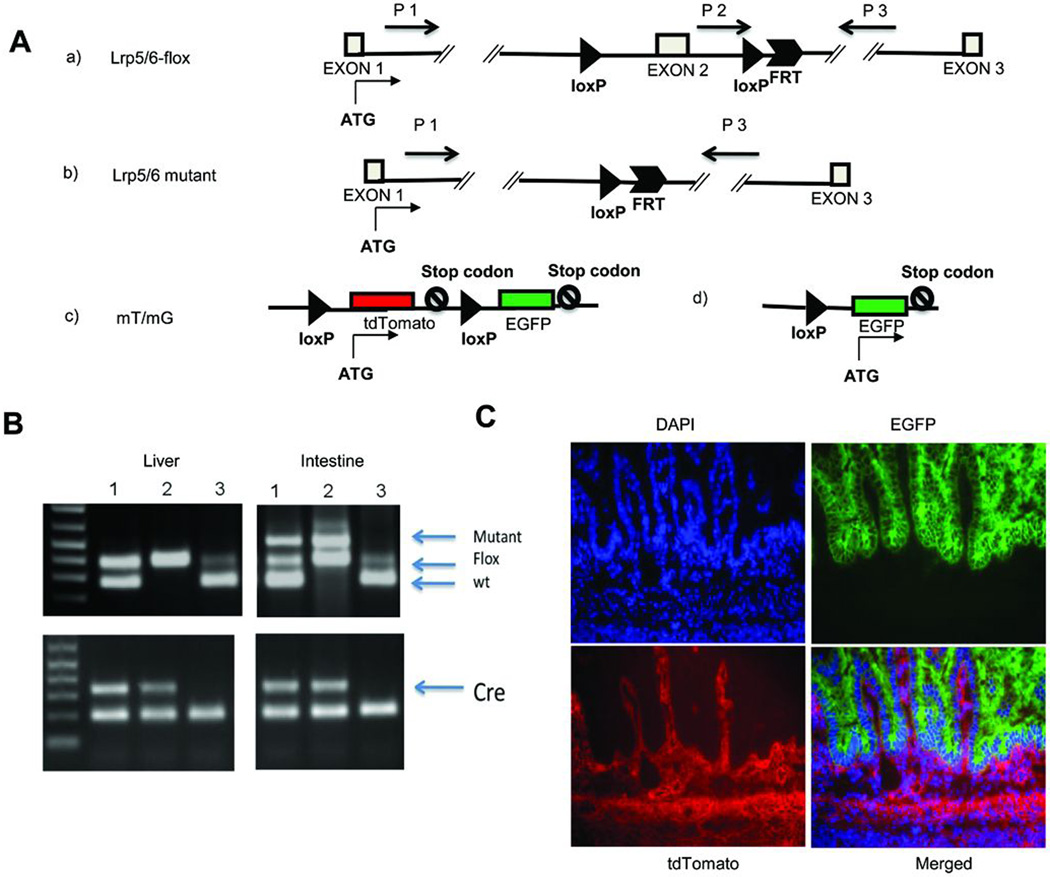
To address the importance of Lrp5 and Lrp6 during intestinal development, we generated three kinds of mouse models having gut-specific Lrp5/6 disruption by crossing villin-Cre transgenic mice with mice carrying conditional alleles of Lrp5 (Lrp5flox/flox), Lrp6 (Lrp6flox/flox), or both (Fig. 1A). To confirm the specificity of Cre recombinase expression driven by villin-Cre in the intestinal epithelium [el Marjou et al., 2004], we performed allele-specific PCR (Fig. 1B). We also crossed the villin-Cre transgenic mouse with an mT/mG reporter mouse [Muzumdar et al., 2007] to generate a mouse carrying both transgenes (Cre and mT/mG). In the absence of exposure to cre recombinase, tissues in the mT/mG mouse express the Tomato red fluorescent protein. In mice carrying both transgenes, the mT cassette is deleted in the Cre-expressing tissue, allowing expression of the enhanced green fluorescent protein (EGFP/mG) cassette located just downstream. The small intestine from a doubly transgenic embryonic day 17.5 (E17.5) embryo showed EGFP fluorescence (green) in all the epithelial cells and tdTomato fluorescence (red) in other regions (Fig. 1C), consistent with villin-Cre being specifically expressed within the intestinal epithelium. We found villin-Cre expressed in the E17.5 large intestine epithelium (Supplemental Fig. 1A) and in E14.5 intestinal epithelium (Supplemental Fig. 1B) as well.
FIGURE 1. Conditional inactivation of Lrp5 and/or Lrp6 in intestinal epithelium.
(A) Schematic diagram of loxP-flanked alleles of the Lrp5 and Lrp6 genes. LoxP sites are indicated by dark triangles. An FRT site remains from the FLP-mediated removal of the neomycin-resistance cassette used for positive selection to identify homologous recombination (see Methods). Primers used for the allele-specific PCR in panel B are indicated by black arrows.
(B) Allele-specific PCR confirms specificity of Cre activity for intestine tissue. The Cre-mediated recombination was verified using DNA isolated from the liver and intestine of villin-Cre;Lrp5-flox mice with the genotype TG/+, flox/+ (sample 1), TG/+, flox/flox (sample 2), and +/+, flox/+ (sample 3). The upper panels show positions of the wild type (wt), unrecombined floxed allele (Flox), and deleted allele (Mutant). The lower panels confirm the presence of the villin-Cre transgene in the DNA samples from these mice (samples 1, 2, and 3). The wt and flox bands are amplified with the primers P2 and P3 (panel A.a), and the mutant band is amplified with the primers P1 and P3 (panel A.b). (C) Mice carrying both the villin-Cre transgene and the mT/mG reporter were generated to confirm the tissue specificity of Cre-mediated deletion. The schematic diagram of the mT/mG allele (see Panel A.c) emphasizes that tdTomato expression is present prior to Cre-mediated recombination. A section of the small intestine was prepared for analysis. After exposure to Cre recombinase (see Panel A.d), tdTomato expression is eliminated and EGFP expression is induced. DAPI stains nuclei, EGFP shows cells in which Cremediated recombination has occurred at some point in differentiation, and tdTomato indicates the absence of Cre activity. Note that EGFP expression is limited exclusively to the epithelial portion of the small intestine.
We recovered the expected Mendelian ratio of offspring in villin-Cre TG/+;Lrp5flox/flox (Lrp5gut−/−) and villin-Cre TG/+;Lrp6flox/flox (Lrp6gut−/−) mice, and no differences were noted relative to wild-type littermates in their size or body weight during development or in adulthood (data not shown). However, mice homozygous for the conditional deletion of both genes (Lrp5/6gut−/- died within one day of birth (Supplemental Fig. 2). Analysis of the mice during necropsy found milk in their stomachs, indicating that the neonatal lethality was not associated with either a failure to nurse or maternal neglect.
Inactivation of both Lrp5 and Lrp6 causes defects in embryonic small intestine epithelium
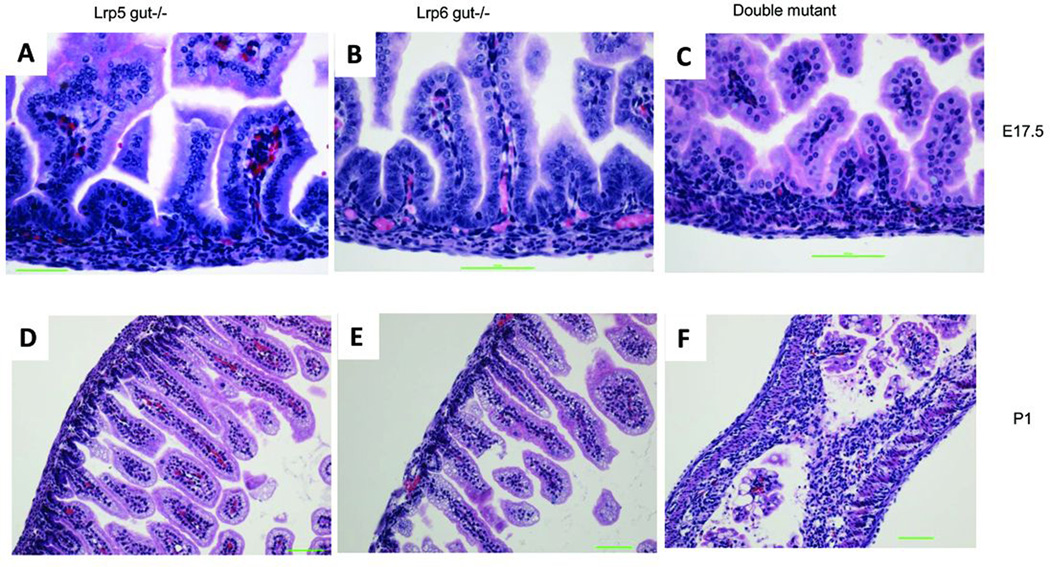
Because mouse intestinal tissue architecture is established during mid to late gestation, we collected embryos from E14.5 to post-natal day 1 (P1) and stained formalin-fixed paraffin-embedded sections of the intestines with hematoxylin and eosin (H&E) to look for the histological differences. At E14.5, no obvious differences were noted among genotypic classes (Supplemental Fig. 3A, B, C). At E17.5, the double mutant (Lrp5gut−/−;Lrp6gut−/−) (Fig. 2C) showed fewer cells in both the villus and crypt regions of the small intestine than did the other genotypes. The defect was even more pronounced in newborn double-mutant pups (Fig. 2F). Compared with wild type, no obvious histological changes were observed in Lrp5gut−/− (Fig. 2A, D), Lrp6gut−/− (Fig. 2B, E), Lrp5gut−/−;Lrp6gut+/− (data not shown), and Lrp5gut+/−;Lrp6gut−/− (data not shown) small intestine epithelia throughout the embryonic period.
FIGURE 2. Development of the small intestine in Lrp5/6gut−/− mice.
H&E-stained FFPE sections of small intestine from Lrp5gut−/− (left column), Lrp6gut−/− (middle), and double-mutant mice (right) at E17.5 (A–C), and P1 (D–F). No obvious differences were observed between single-mutant mice and wild-type mice (not shown) at any age. At E17.5, a significantly reduced number of cells were found in the villus and intervillus regions of double-mutant mice (C versus A and B). In newborn animals, the number of cells in the double-mutant mucosal epithelium (F versus D and E) was even more dramatically reduced. Scale bars indicate 50 µm.
We further investigated epithelial cell proliferation by immunohistochemical analysis using the nuclear proliferation marker Ki67. At E14.5, epithelial cells were robustly proliferating and no differences were observed among the genotypes (Supplemental Fig. 3D, E, F). At E17.5, Ki67+ cells were almost completely absent from Lrp5gut−/−;Lrp6gut−/− epithelium (Fig. 3C), but were clearly observed in the other genotypes (Fig. 3A,B). The proliferation of mesenchymal cells in the submucosa and other cells underneath was not affected in the double mutants at E17.5 (Fig. 3C), consistent with the specificity of Cre expression. We found a similar result in the P1 Lrp5gut−/−;Lrp6gut−/− intestine (Fig. 3F), and in Lrp6gut−/− intestinal epithelium, a mild suppression of proliferation at P1 (Fig. 3E). Interestingly, we found no increase of apoptosis in the intestinal epithelium of any genotype at E17.5 (Supplemental Fig. 4).
FIGURE 3. Absence of proliferating cells in Lrp5/6gut−/− small intestine epithelium.
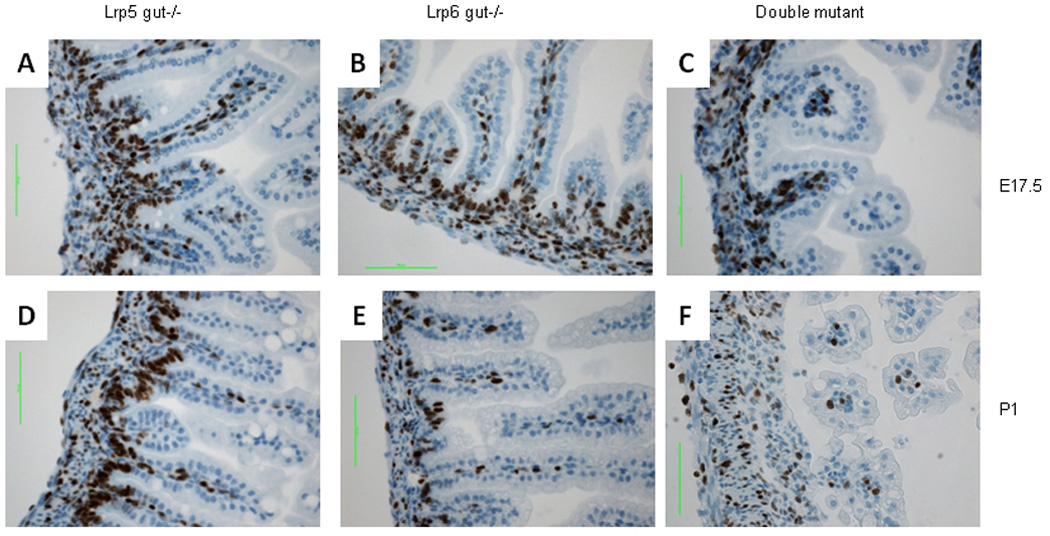
Ki67-stained FFPE sections of small intestine from Lrp5gut−/− (left column), Lrp6gut−/− (middle), and double-mutant mice (right) at E17.5 (A–C), and P1 (D–F). At E17.5, proliferating epithelial cells were in the base-of-crypt and intervillus regions of Lrp5gut−/− (A) and Lrp6gut−/− (B) small intestine, while proliferation of intestinal epithelium in the double mutant was almost completely absent (C). Note the positive staining in the underlying stromal layer in all embryos. At P1, Lrp5gut−/− (D) and wild-type (not shown) intestinal epithelia were indistinguishable. The epithelium from Lrp6gut−/− had slightly fewer proliferating cells (E), and the reduction was even more dramatic in the double-mutant small intestine (F). Scale bars indicate 50 µm.
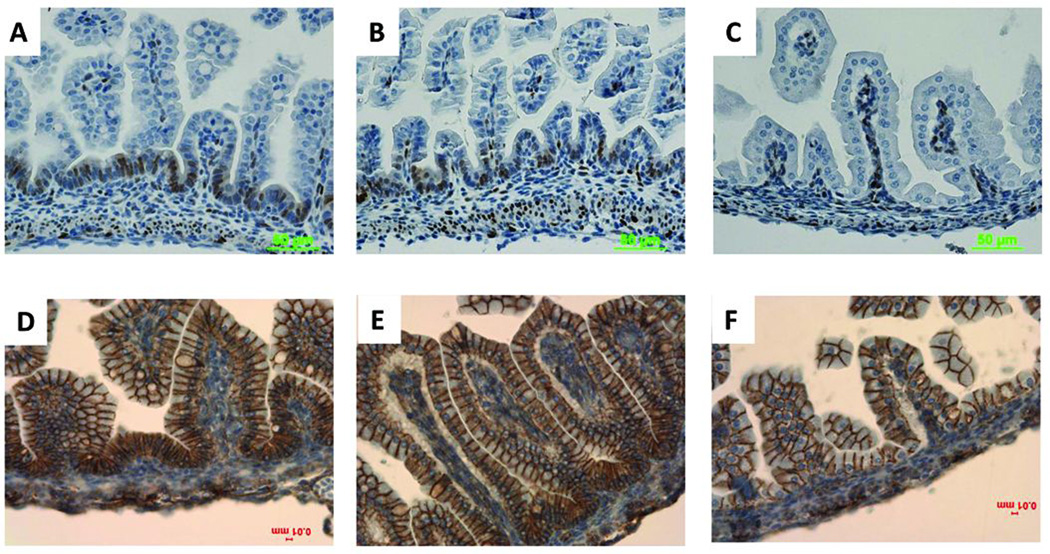
To trace the Wnt/β-catenin signaling change after Lrp5/6 depletion in the intestinal epithelium, we used immunohistochemistry to detect the expression of cyclin D1, a Wnt/β-catenin target gene within the intestinal epithelium [Tetsu and McCormick, 1999]. At E14.5, no cyclin D1 staining was detectable in the intestinal epithelium of any genotype (data not shown). At E17.5, no cyclin D1 staining was observed in the double-mutant small intestine epithelium (Fig. 4C), but robust cyclin D1 staining was seen in other genotypes (Fig. 4A, B). The expression of β-catenin itself (Fig.4F) and of CD44 (which is both a Wnt target gene and a presumable crypt progenitor cell marker) (Supplemental Fig. 5) was also inhibited in the crypt base and the intervillus regions of the double mutant relative to the single-mutant small intestine epithelium at E17.5.
FIGURE 4. Wnt/β-catenin signaling is inhibited in Lrp5/6gut−/− small intestine epithelium.
At E17.5, cyclin D1 was robustly expressed in Lrp5gut−/− (A), Lrp6gut−/−(B), and wild-type (not shown) intervillus regions of small intestine epithelium; it was undetectable in the double-mutant small intestine epithelium (C). Compared with Lrp5gut−/− (D) and Lrp6gut−/−(E), the expression of cytoplasmic and nuclear β-catenin in double-mutant small intestine epithelium was notably inhibited (F). Scale bars indicate 50 µm or 10 µm.
Because Wnt/β-catenin signaling plays a crucial role in maintaining intestinal stem cells, the inactivation of Lrp5/6 may inhibit intestinal stem cell self-renewal by removing Wnt receptors and blocking the Wnt/β-catenin signaling pathway. Intestinal epithelium renewal requires the division of multipotent stem cells followed by the differentiation of daughter cells into specialized absorptive and secretory cells. We used NBT/BCIP, Grimelius, and alcian blue staining to identify enterocytes, enteroendocrine cells, and goblet cells, respectively, all of which are differentiated cells occupying the villi of the small intestine.
Alkaline phosphatase (ALP) activity (shown by NBT/BCIP staining) is a characteristic of enterocytes in the villus, and we observed ALP activity in only the villus region of the wild-type small intestine (Fig. 5A). In the double-mutant small intestine, ALP activity was considerably enhanced in the entire small intestine epithelium. More importantly, ALP activity was also detected at the bottom of crypts that are supposed to be occupied by stem/progenitor cells that have no ALP activity (Fig. 5D). That ALP activity suggests that the stem/progenitor cells are inappropriately differentiated in the absence of both Lrp5 and Lrp6. The number of enteroendocrine cells (Grimelius-positive cells) was dramatically reduced in the E17.5 double-mutant small intestine (Fig. 5E), which could be due to the lack of the intestinal stem cells from which this differentiated cell type originates. However, the number of goblet (alcian blue–positive) cells was not reduced in the double-mutant small intestine (Fig. 5F), which might be due to differentiated goblet cells retaining proliferation ability [Paulus et al., 1993].
FIGURE 5. Impact of Lrp5/6 inactivation on small intestine differentiation.
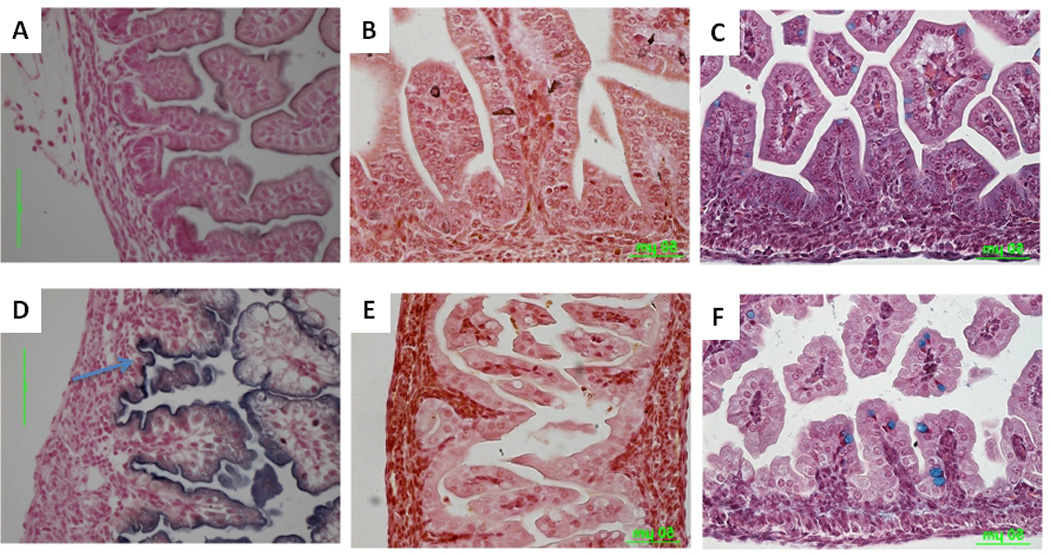
FFPE sections of small intestine from E17.5 wild-type (A–C) or double mutants (D–F) stained with NBT/BCIP to detect enterocyte-specific ALP activity (A,D), Grimelius (enteroendocrine cell–specific) (B,E), or alcian blue (goblet cell–specific)(C,F). NBT/BCIP staining of the epithelium was much stronger in the double mutant (D) than in the wild type (A). Note strong staining in the intervillus region of the double mutant (arrow). Enteroendocrine cells were almost completely absent in double-mutant intestine (E) relative to wild-type intestine (B). Goblet cells were present in both wild-type (C) and double-mutant (F) intestine. Scale bars indicate 50 µm.
Inactivation of both Lrp5 and Lrp6 inhibits large intestine epithelium after birth
The large intestine of all genotypes, including the double mutant, showed little morphologic change during the embryonic period (data not shown). Ki67 staining showed that all genotypes including wild-type, single-mutant (picture not shown) and double-mutant large- intestine epithelial cells, were robustly proliferating at E17.5 (Fig. 6B and E). However, cyclin D1 showed a notable decrease in double-mutant large intestine epithelium at E17.5 (Fig. 6D).
FIGURE 6. Wnt/β-catenin signaling is inhibited in Lrp5/6gut−/− large intestine epithelium.
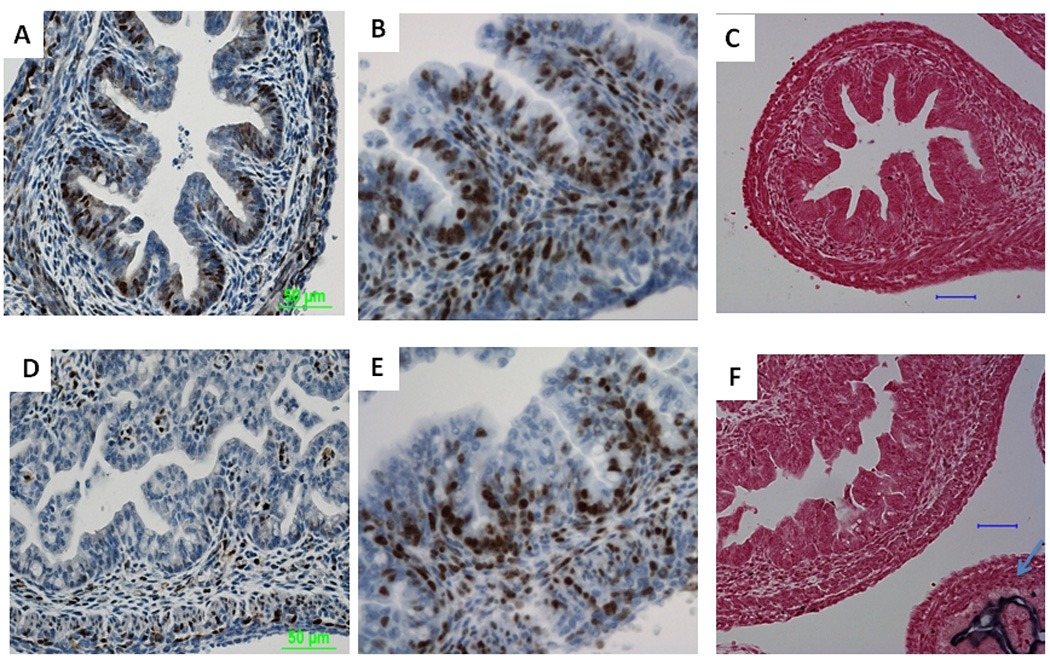
At E17.5, wild-type large intestine epithelium expressed robust amounts of cyclin D1 (A), while the double-mutant showed strongly inhibited cyclin D1 expression (D). Ki67 staining showed that there was no notable inhibition of proliferation in the double mutant (E) compared with the wild-type control (B). Neither was there a significant change in ALP activity (C vs. F). Note the strong NBT/BCIP staining in the double-mutant small intestine epithelium (F, arrow). Scale bars indicate 50 µm.
Little ALP activity was observed in E17.5 double-mutant large intestine (Fig. 6F), but it was obvious in most of the double-mutant large intestine epithelium of one-day-old pup (Fig. 7D), which indicates that the crypt cells in the double-mutant possess the property of villus cells at 1 day of age. In one-day-old pup, we also noticed fewer epithelial cells in the double-mutant large intestine, and epithelial proliferation was inhibited as shown by Ki67 staining (Fig. 7C). We saw no obvious change in all other genotypes’ large intestines including wild-type and single-mutant (pictures not shown).
FIGURE 7. Lrp5/6gut−/− large intestine epithelium shows reduced proliferation and increased crypt cell differentiation at P1.
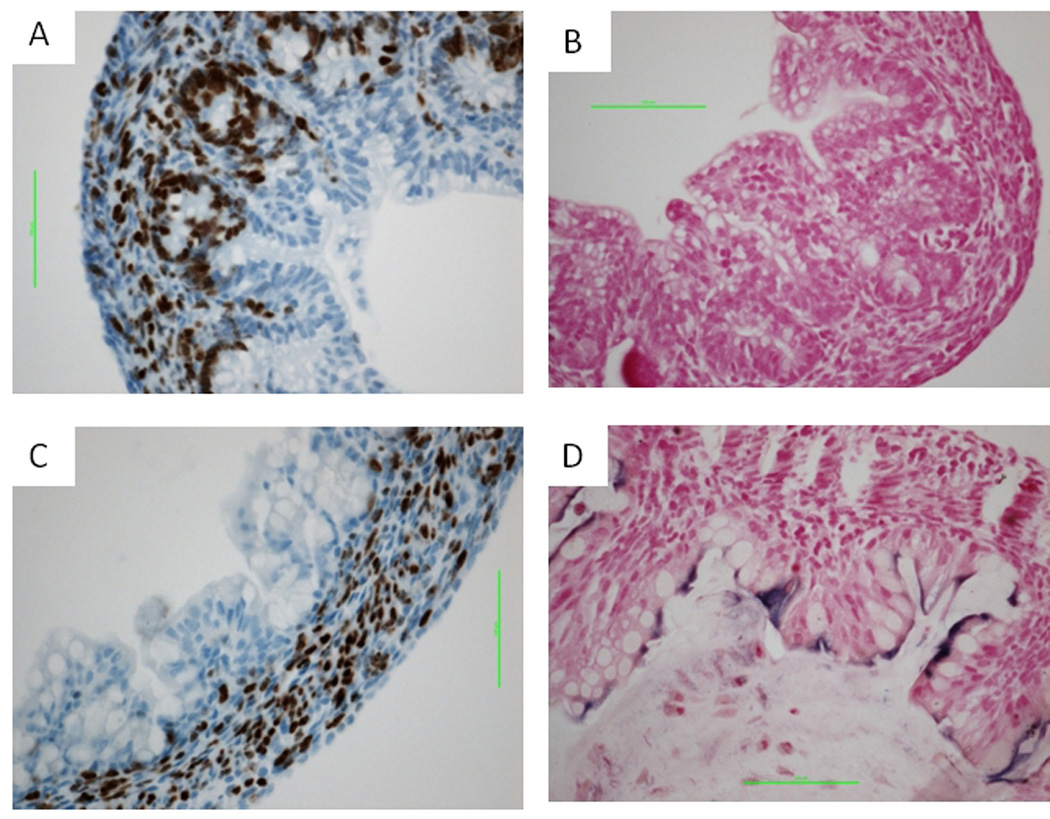
Relative to wild-type controls (A, B) at 1 day after birth, double-mutant large intestine epithelium showed growth inhibition (C) and increased ALP activity (D). Scale bars indicate 50 µm.
Discussion
Wnt signaling through the canonical β-catenin pathway plays an essential role in development. In the canonical pathway, Wnt binds to a receptor complex that includes a frizzled member and the either Lrp5 or Lrp6, which results in the phosphorylation of the cytoplasmic tail of Lrp5 or Lrp6. This signal transduction ultimately leads to the stabilization and nuclear localization of β-catenin, binding of β-catenin to a member of the LEF/TCF family of DNA-binding proteins, and subsequent regulation of target gene transcription [He et al., 2004].
Dysregulation of Wnt signaling is highly associated with the development of colon cancer. This association was first made when patients with an inherited susceptibility to early onset colon cancer associated with the formation of numerous colon polyps, Familial adenomatous polyposis, were found to have mutations in the Adenomatous polyposis coli (APC) gene [Fearon and Vogelstein, 1990; Nishisho et al., 1991]. The protein product of the APC gene encodes a protein required for the normal degradation of β-catenin. Subsequent work has found that approximately 85% of sporadically occurring colon tumors have loss of APC function. In addition, other colon tumors have activating mutations in the β-catenin [Sparks et al., 1998].
Much effort has been devoted to studying the function of Lrp5 and Lrp6 in development. Lrp5−/− mice show normal development but have low bone mass and some metabolic abnormalities [Kato et al., 2002; Magoori et al., 2003]. Lrp6−/− mice are perinatal lethal and exhibit other abnormalities including mid/hindbrain defects [Pinson et al., 2000]. Lrp5−/−;Lrp6−/− double-mutant mice fail to establish a primitive streak and die during gastrulation, which is much earlier than Lrp6−/− mice [Kelly et al., 2004]. All these studies suggest that Lrp6 is more crucial than Lrp5 in embryogenesis and that Lrp5 and Lrp6 have a functional redundancy.
In this work, we have carefully examined the morphology, cell proliferation, cell differentiation, and Wnt/β-catenin signaling of intestinal epithelium in Lrp5gut−/−, Lrp6gut−/−, and Lrp5gut−/−;Lrp6gut−/− embryos and neonates. The Lrp5gut−/−;Lrp6gut−/− (double-mutant) intestinal epithelium underwent unregulated differentiation associated with decreased proliferation, which eventually led to neonatal death. Surprisingly, we did not see a defect in the Lrp6gut−/− or Lrp5gut+/−;Lrp6gut−/− embryo. These observations provide another piece of evidence for the compensatory roles of Lrp5 and Lrp6 in the development of intestinal epithelium, and they imply that only one allele of either Lrp5 or Lrp6 is sufficient for Wnt signal transduction in the gut.
Comparing our result with several previous studies on Wnt signaling components in the gut, we found some subtle differences. Korinek [Korinek et al., 1998] showed that homozygosity for a germline null mutation in Tcf-4 (Tcf4−/−) caused a developmental defect in the small intestine. However, the Tcf4−/− mice showed no obvious defect in the large intestine, which, the authors explained, might be due to the redundant function of other Tcf family members. Our observation on Lrp5gut−/−;Lrp6gut−/− large intestine suggests that the large intestine might not be as sensitive as the small intestine to Wnt/β-catenin signaling inhibition, and Lrp5gut−/−;Lrp6gut−/− large intestine only shows proliferation inhibition and differentiation after birth. This also could be a reason that there was no phenotype in the large intestine of Tcf4−/− mice.
In a tamoxifen-inducible β-catenin gene ablation model using adult mice, increasing numbers of epithelial cells underwent apoptosis after β-catenin was ablated in the intestinal epithelium [Fevr et al., 2007]. However, we did not observe an increase of apoptosis in the Lrp5gut−/−;Lrp6gut−/− intestine, nor was an increase observed in the Tcf-4−/− model [Korinek et al., 1998]. One reason could be that intestinal stem cells at different stages require different Wnt/β-catenin signaling in terms of signal intensity and signal location. The TOP-GAL mouse model showed that Wnt signaling intensity and location in the intestinal epithelium changed time-dependently from the embryonic period to the adult period [Kim et al., 2007]. On the other hand, it could be that the β-catenin gene ablation model inhibited canonical Wnt signaling in a more acute and complete manner.
Aside from the direct control of intestinal cell differentiation, there are additional reasons why linking both Lrp5 and Lrp6 function to this process is important. One example is the recent report that Lrp5 expression within the intestine regulates the expression of serotonin, which is subsequently released into the serum and ultimately controls bone mass [Yadav et al., 2008]. Another example is the examination of gene expression signatures in ulcerative colitis, which revealed increased levels of both Lrp5 and Lrp6 in disease-associated samples [Watanabe et al., 2007]. In addition, interferon-gamma–induced intestinal inflammation was associated with changes in LRP6 function [Nava et al., 2010]. Taken together, these studies emphasize the importance of understanding the specific functions of Lrp5 and Lrp6 during intestinal development and homeostasis.
In summary, our study suggests a redundant function between Lrp5 and Lrp6 in intestinal development. The presence of at least one allele of either gene is required for normal development, and the two genes appear to be functionally redundant in this context.
Methods
Mouse Crosses
Villin-Cre mice, in which cre recombinase is expressed with high specificity and penetrance to the intestinal epithelium, were crossed in several steps with homozygous conditional mutants carrying modified Lrp5 and/or Lrp6 alleles to generate villin-Cre;Lrp5-flox (Lrp5gut−/−), villin-Cre-Lrp6-flox (Lrp6gut−/−), or villin-Cre-Lrp5-flox-Lrp6-flox (Lrp5gut−/−;Lrp6gut−/−) progenies, which were used in subsequent matings. All experiments performed were in compliance with the guiding principles of the “Care and Use of Animals” and approved prior to use by the Van Andel Research Institute Institutional Animal Care and Use Committee (Approval ID#:VARI10-04-015). Animals were monitored on a daily basis by trained staff members of the VARI vivarium and any animals showing signs of illness or distress were humanely euthanized to minimize suffering. Euthanasia of animals for distress or the required timepoints was carried out following the recommendations of the 2000 American Veterinary Medical Association Panel Report on Euthanasia. Villin-Cre and mT/mG transgenic mouse strains were obtained from the Jackson Laboratories.
Generation of Lrp5-flox Mice
To create a conditional Lrp5 loss-of-function allele, we performed homologous recombination in the CJ7 ES cell line [Swiatek and Gridley, 1993]. The targeting vector consisted of a 5’ targeting arm (about 6.7 kb) containing genomic DNA from Lrp5 intron 1 extending into intron 2 and a 3’ targeting arm containing about 2.0 kb of genomic DNA extending from Lrp5 intron 2. We placed LoxP sites in the same orientation about 1600 bp upstream of exon 2 and about 800 bp downstream of exon 2. In order to select for correctly targeted clones, we included an FRT-flanked neomycin selection cassette (neo-cassette) in the 5’ targeting arm that is transcribed in reverse orientation to the Lrp5 transcript, and we included a Diptheria toxin selection cassette at the end of the 3’ targeting arm. We electroporated and selected ES cells using standard methods. Homologous recombinants were sought by a PCR-based strategy, and we confirmed correct targeting by Southern blot using 5’ and 3’ flanking probes. We also confirmed that the LoxP sites were retained within correctly targeted clones by PCR amplification and sequencing. Correctly targeted ES cells were used to generate floxed mice. The neo cassette was removed by breeding mice with FLPeR mice, a 129-derived strain that broadly expresses flippase driven by the ROSA26 locus [Farley et al., 2000].
Generation of Lrp6-flox Mice
A similar strategy was applied to generate Lrp6-flox mice (Fig. 1A).
Genotyping Analysis
DNA was prepared from tail biopsies using an AutoGenprep 960 automated DNA isolation system. PCR-based strategies were then used to genotype these mice (details available upon request).
Histology and Immunohistochemistry
Fresh intestinal tissues for immunohistochemistry and hematoxylin and eosin (H&E) staining were fixed in 4% paraformaldehyde overnight in 4 °C, embedded in paraffin, and sectioned (5 µm). Immunohistochemistry was performed using Vector ABC and DAB kits according to the manufacturer’s recommendations (Vector Laboratories). The following primary antibodies were used: Ki67 (Dako), Cyclin D1 (Cell Signaling), CD44 (BD Biosciences), and β-catenin (Cell Signaling). The other staining reagents used were Alcian Blue (Sigma-Aldrich), Grimelius staining kit (American MasterTech), and NBT/BCIP (Roche). In some cases, immunohistochemistry was completed using the Ventana Discovery System (Tucson, AZ).
Supplementary Material
(A) Large intestine frozen sections from an E17.5 embryo carrying both the villin-Cre transgene and the mT/mG reporter (villin-Cre TG/+;mT/mG KI/+). (B) E14.5 villin-Cre TG/+;mT/mG KI/+ embryo intestine. Green marks the area where Cre-mediated recombination takes place.
For villin-cre; Lrp5-flox mice, TG/+, flox/flox males were crossed with +/+, flox/flox females. For villin-cre; Lrp6-flox mice, +/+, flox/flox males were crossed with TG/+, flox/+ females. And for villin-cre; Lrp5-flox; Lrp6-flox mice, +/+, flox/flox, flox/flox males were crossed with TG/+, flox/flox, flox/+ females. The animals from each genotype are from two to three separate mate cages.
H&E-stained FFPE sections of small intestine from Lrp5gut−/− (left column), Lrp6gut−/− (middle), and double-mutant mice (right) at E14.5 (A–C). Ki67 IHC was performed using these sections (D–E). Scale bars indicate 50 µm.
The DNase I pre-treated section was used as a positive control. Scale bars indicate 10 µm.
Acknowledgements
We thank David Nadziejka for assistance with editing this manuscript. We also thank Robert Sigler for assistance with histological analysis and members of the VARI Laboratory of Analytical, Cellular, and Molecular Microscopy for technical assistance. In addition, we are grateful to Bryn Eagleson and the members of the VARI Vivarium staff for expert animal husbandry. We also thank past and present members of the Williams Laboratory for helpful discussions and assistance with genotyping. This work was supported by NIAMS/NIH grant AR053292 (to BOW) and by the Van Andel Research Institute.
Grant Support – This work was supported by NIH grant R01AR053293 to B.O.W. and by the Van Andel Research Institute.
Abbreviations
- ALP
alkaline phosphatase
- E
embryonic day
- GFP
green fluorescent protein
- Lrp
low density lipoprotein receptor-related protein
- P
post-natal day
Footnotes
Disclosures-No conflicts of interest exist.
Supplemental 5. FFPE sections of small intestine from Lrp5gut−/− (left) and double-mutant E17.5 embryos (right) stained for CD44. Scale bars indicate 10 µm.
References
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–11336. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, Skerra A, Li L, Parkos CA, Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Paulus U, Loeffler M, Zeidler J, Owen G, Potten CS. The differentiation and lineage development of goblet cells in the murine small intestinal crypt: experimental and modelling studies. J Cell Sci. 1993;106(Pt 2):473–483. doi: 10.1242/jcs.106.2.473. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kobunai T, Toda E, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Hata K, Kojima T, Yokoyama T, Konishi T, Okayama Y, Sugimoto Y, Oka T, Sasaki S, Ajioka Y, Muto T, Nagawa H. Gene expression signature and the prediction of ulcerative colitis-associated colorectal cancer by DNA microarray. Clin Cancer Res. 2007;13:415–420. doi: 10.1158/1078-0432.CCR-06-0753. [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Large intestine frozen sections from an E17.5 embryo carrying both the villin-Cre transgene and the mT/mG reporter (villin-Cre TG/+;mT/mG KI/+). (B) E14.5 villin-Cre TG/+;mT/mG KI/+ embryo intestine. Green marks the area where Cre-mediated recombination takes place.
For villin-cre; Lrp5-flox mice, TG/+, flox/flox males were crossed with +/+, flox/flox females. For villin-cre; Lrp6-flox mice, +/+, flox/flox males were crossed with TG/+, flox/+ females. And for villin-cre; Lrp5-flox; Lrp6-flox mice, +/+, flox/flox, flox/flox males were crossed with TG/+, flox/flox, flox/+ females. The animals from each genotype are from two to three separate mate cages.
H&E-stained FFPE sections of small intestine from Lrp5gut−/− (left column), Lrp6gut−/− (middle), and double-mutant mice (right) at E14.5 (A–C). Ki67 IHC was performed using these sections (D–E). Scale bars indicate 50 µm.
The DNase I pre-treated section was used as a positive control. Scale bars indicate 10 µm.