Abstract
The lower extremities are important to performing physical activities of daily life. This study investigated lower extremity tissue composition, i.e. muscle and fat volumes, in young and older adults and the relative importance of individual tissue compartments to the physical function of older adults. A total of 43 older (age 78.3 ± 5.6 yr) and 20 younger (age 23.8 ± 3.9 yr) healthy men and women participated in the study. Older participants were further classified as either high- (HF) or low-functioning (LF) according to the Short Physical Performance Battery (SPPB). Magnetic resonance images were used to determine the volumes of skeletal muscle, subcutaneous fat (SAT), and intermuscular fat (IMAT) in the thigh (femoral) and calf (tibiofibular) regions. After adjusting for the sex of participants, younger participants had more femoral muscle mass than older adults (p < 0.001 for between group differences) as well as less femoral IMAT (p = 0.008) and tibiofibular IMAT (p < 0.001). Femoral muscle was the only tissue compartment demonstrating a significant difference between the two older groups, with HF participants having 31% more femoral muscle mass than LF participants (mean difference = 103.0 ± 34.0 cm3; p = 0.011). In subsequent multiple regression models including tissue compartments and demographic confounders, femoral muscle was the primary compartment associated with both SPPB score (r2 = 0.264, p= 0.001) and 4-meter gait speed (r2 = 0.187, p= 0.007). These data suggest that aging affects all lower extremity compartments, but femoral muscle mass is the major compartment associated with physical function in older adults.
Keywords: Aging, Sarcopenia, Older Adults, Disability, SPPB, IMAT
1. Introduction
Advancing age is a significant risk factor for the development of physical disability.[1, 2] While the etiology of disability is complex, age-related changes in body composition are considered important predictors of functional decline.[3, 4] Indeed, age-related declines in muscle mass – i.e. sarcopenia – and increases in adipose tissue mass each contribute to declines in physical function among the elderly.[4–6] While several studies have demonstrated associations between physical function and whole-body tissue composition, recent studies have largely focused on tissue changes in the lower extremities due to their importance in performing most daily tasks.[7–9]
Many of these studies have utilized dual energy x-ray absorptiometry (DEXA) to quantify total muscle and total adipose tissue. More recently however, increasing numbers of investigators have utilized either computed tomography (CT) or magnetic resonance imaging (MRI) to quantify tissue volumes and further divide adipose tissue depots into subcutaneous (SAT) and intermuscular (IMAT) compartments.[5, 10–13] However, these studies have almost exclusively utilized the thigh (femoral) region — thus data regarding age-related changes in the composition of the calf (tibiofibular) region and its importance to function are scarce. The paucity of data in this area is surprising given the importance of the plantar- and dorsi-flexor muscles to gait and balance.[14] Recent studies have demonstrated the importance of the tibiofibular muscles to the function of older patients with peripheral vascular disease.[15, 16] However, little information is available regarding the contribution of these muscles to the function older adults without overt vascular disease. Over 15 years ago, Posner and colleagues [17] reported that calf muscle strength was positively associated with the ability to perform activities of daily living (ADLs), including a gait and balance test. However, this group did not collect any measure of tissue composition. To our knowledge, Lauretani and colleagues [18] are the only group to report age-related changes in tissue composition of the tibiofibular region. Comparing persons in their 20’s to those between ages 75–85, these investigators reported relatively modest declines in calf muscle cross-sectional area of 0.33 cm2/year for men and 0.14 cm2/year for women and that this measure was a weak and inconsistent predictor of mobility limitation. However, these findings have not been either supported or refuted by subsequent study. Moreover, this study did not utilize any measure of muscle fat infiltration, an important contributor to functional performance.[5, 11]
Therefore, the objective of the present study was to add to the literature regarding the relationship between lower extremity tissue composition and physical performance in older adults. Further information in this area is important for continuing to optimize strategies to maintain physical function and prevent disability during advanced age. For example, one study previously showed that exercise increases muscle and decreases IMAT in the thigh region of older adults.[13] More recently, it was shown that the addition of diet-induced weight loss was more efficacious for inducing these changes in overweight to moderately obese older adults.[19] No study to date has examined the efficacy of such interventions on the tissue composition of the tibiofibular region. In our opinion, this fact is likely because the importance of this region to functional performance is largely unknown.
In this study, we compared femoral and tibiofibular muscle, SAT, and IMAT volumes between young adults and older persons classified as either high- or low-functioning using a standardized test – the Short Physical Performance Battery (SPPB). We hypothesized that advanced age would be associated with less skeletal muscle mass and more fat (SAT, IMAT, or both) within both the femoral and tibiofibular regions. Furthermore, we aimed to evaluate the relationship of these tissue compartments to the overall physical function of older adults.
2. Methods
2.1 Participants
Sedentary men and women over 70 years of age or between the ages of 18 and 35 years were recruited to participate in this study. The exclusionary criteria were a history of smoking in the prior 12 months, regular performance of structured exercise, active treatment for cancer or history of cancer in prior 3 years, congestive heart failure NYHA Class III or IV, previous stroke with upper and/or lower extremities involvement within prior 6 months, peripheral vascular disease Fontaine Class III/IV, history of life-threatening cardiac arrhythmias, stroke, cognitive impairment (i.e., Mini-Mental State Examination score ≤23), severe neurological disorders including Parkinson’s disease, renal disease requiring dialysis, lung disease requiring chronic steroid treatment, lower extremity amputation, severe osteoarthritis interfering with physical function, complicated diabetes, inflammatory diseases such as active rheumatoid arthritis, vasculitis, autoimmune disorders, and inflammatory bowel disease, and life-threatening illnesses with an estimated life expectancy less than 1 year. Prior to enrollment in the study, all participants provided written informed consent based on documents approved by the University of Florida Institutional Review Board.
2.2 Screening Procedures
Recruitment was performed by the Recruitment Core of the University of Florida Claude D. Pepper Older Americans Independence Center. Participants were recruited through a variety of methods including media articles, direct mailings, newspaper announcements, and presentations to community groups. Following telephone screening, potentially eligible persons were invited to attend a screening visit during which the purposes and procedures of the study were explained and informed consent was obtained. After the participant provided consent, a general assessment was performed that included questionnaires to collect information regarding medical history, cognitive function (i.e. Mini-Mental State Examination), and self-reported disability, as well as a brief physical exam to determine participant height, weight, and body mass index (BMI).
2.3 Short Physical Performance Battery
To assess physical function, the SPPB test was performed. The SPPB test is scored on a scale of 0–12, with higher scores indicating a higher level of function.[20] Older participants in this study were stratified into high- and low-functioning groups based on their performance on the SPPB. Participants were considered high-functioning if they scored ≥ 11 on the SPPB, while low-functioning participants were included if they scored ≤ 7. We chose the lower cut-off because of its clinical relevance as SPPB scores ≤ 7 are associated with adverse outcomes including disability, hospitalization, and mortality.[21–23]
The SPPB is composed by three subtasks: usual gait speed, standing balance and chair stand tests. Usual gait speed was measured as the best performance (in meter/second) of two trials on a four meter (4m) course at the subject’s self-selected pace. For the standing balance test, participants were asked to stand in three progressively more difficult positions for 10 seconds each: a side-by-side feet standing position, a semi-tandem position and a full tandem position. For the chair stand test, participant were asked to perform 5 repetitions of standing up and sitting down from a chair without using hands and the performance was timed. In addition to recording the absolute time taken to perform the chair stand and 4m walk portions of the SPPB, each of the three tasks were categorized into a five-level score, with zero representing inability to do the test and four representing the highest level of performance. Gait speed was also determined from the walk time during the 4m test.
2.4 Magnetic Resonance Imaging
T1-weighted magnetic resonance imaging (MRI) was used to quantify tissue volumes of the femoral and tibiofibular regions of the dominant leg. Images were obtained using a Phillips 3.0 Tesla magnet (Philips Medical Systems, Bothell, WA). Three dimensional (3D) data were collected using a fast gradient-echo sequence, with TR=100 ms, TE=10ms, flip angle of 30 and a chemically-selective fat suppression was utilized. Images were acquired with an encoding matrix of 256×256, field of view of 16–24 cm and 7 mm slice thickness. The femoral images were collected using the body coil while tibiofibular images were collected using an 8-channel sensitivity encoding, receive-only extremity coil. All images were exported and analyzed on a separate workstation. For image analyses, 11 contiguous axial slices (10-mm thickness) were selected beginning at the maximal cross-sectional area of the thigh and five continuous axial slices were selected beginning at the maximal cross-sectional area of the tibiofibular region. For each region, muscle, SAT, and IMAT were measured volumetrically.
Images were analyzed using the freely-available software program MIPAV (version 1.3; Medical Image Processing, Analysis and Visualization, Center for Information Technology, National Institutes of Health, Bethesda, MD http://mipav.cit.nih.gov). To quantify muscle fat infiltration, IMAT was defined as the visible low-signal intensity pixels between muscle groups and within muscle fascia. The image segmentation process was performed using a non-parametric, non-uniform intensity normalization (N3) algorithm that corrects smoothly varying shading caused by poor radiofrequency coil uniformity or gradient-driven eddy currents.[24] This step is essential for subsequent analyses that assume images are homogeneous. Next, a volume of interest was created for bone and the fascia lata. The technical error of drawing the fascia lata border, separating IMAT and SAT, in our laboratory is CV=0.26% (n=10). Once the bone and the SAT were removed, muscle and IMAT were segmented using pixel signal intensities. Choosing a pixel intensity that uniquely identifies muscle and adipose tissue is a subjective process and a major drawback in traditional MR image processing. Therefore we chose to use automated pixel clustering using fuzzy-c-means. This process clusters pixels on a membership function determined by a specified number of classes (e.g. muscle, adipose tissue and background). The function compares pixel intensities to a membership value of 0 or 1. If the pixel intensity is close to the centroid (median point) of the specified class then it receives a score of 1. Pixel intensities far from the centroid receive values close to zero. Both the membership function and centroids are derived through an iterative process. Comparisons between hand-traced and fuzzy-c-mean segmentation show strong correlations for muscle (r = 0.99) and adipose tissue (r = 0.93) segmentation. More importantly, this approach removes subjectivity by clustering pixels algorithmically thus improving reproducibility for identifying muscle and adipose tissue pixels.
2.5 Statistical Analyses
Data were analyzed using SPSS software (version 19 for Windows, Chicago, IL) and an alpha level of 0.05 was set to determine statistical significance. Data were initially analyzed for normality of distribution and homogeneity of variance and descriptive statistics were then calculated. Continuous descriptive variables were analyzed by analysis of variance (ANOVA), and categorical descriptive variables by the chi-square test. Measures of disability and functional status were only collected for older participants and thus were analyzed by independent samples t-test. Tissue volumes for femoral and tibiofibular regions were analyzed by one-way analysis of covariance (ANCOVA) with group as the independent factor and sex as a covariate. Additionally, to account for differences in limb size, tissue volumes were also analyzed by ANCOVA as a percentage of total region volume. All post-hoc analyses were conducted using Boneferroni’s correction for multiple comparisons.
Pearson’s correlation coefficient (r) was determined to assess associations between tissue compartments and gait speed. Because SPPB was non-normally distributed, the correlation of SPPB to tissue compartments was determined using Spearman’s rank correlation coefficient (ϱ). Finally, using the stepwise procedure, linear regression models were created using absolute tissue volumes to predict SPPB and 4m gait speed due to the established clinical relevance of these outcomes. The stepwise procedure computes a series of F tests to determine the optimal model; variables were entered into the model at p ≤ 0.05 and removed from the model at p ≥ 0.10. Age and sex as well as muscle, IMAT, and SAT for both the femoral and tibiofibular regions were considered for inclusion in the models. To account for differences in limb size, the total volume of the femoral and tibiofibular images was also included.
3. Results
A total of 63 persons participated in the study, including 43 older and 20 young men and women. The majority of the sample was Caucasian (n= 57) and was nearly evenly split between males (n= 33) and females (n= 30). No significant differences were observed for height, weight, BMI, or sex composition of the three groups. However, older participants in LF were on average 5 years older than those in the HF group (Table 1). In accordance with their performance on the SPPB, LF participants reported significantly more limitations in performing activities of daily living than did the HF group (p < 0.001). Moreover, average gait speed of the HF group was more than 0.4 m/sec greater than that of the LF group, indicating a clinically meaningful difference.[25]
Table 1.
Descriptive characteristics and measures of physical function
| Young (n=20) | HF (n=25) | LF (n=18) | P-value for group difference | |
|---|---|---|---|---|
| Age, years | 23.8 ± 3.9a | 76.4 ± 5.7b | 81.1 ± 4.1c | < 0.001* |
| Height, m | 1.7 ± 0.1a | 1.7 ± 0.1a | 1.6 ± 0.1a | 0.128 |
| Weight, kg | 73.2 ± 13.7a | 75.6 ± 16.3a | 76.0 ± 12.7a | 0.801 |
| BMI, kg/m2 | 25.0 ± 5.1a | 26.6 ± 3.7a | 28.1 ± 4.3a | 0.093 |
| Female, % (n) | 50.0 (10)a | 36.0 (9)a | 61.1 (11)a | 0.258 |
| MMSE score | 29.2 ± 1.0a | 28.5 ± 1.7a | 27.9 ± 1.7a | 0.071 |
| Co-morbid conditions† | 0.1 ± 0.2a | 0.4 ± 0.6a,b | 0.7 ± 0.7b | 0.003* |
| Self-reported ADL limitations, # | N/A | 0.8 ± 1.7 | 6.3 ± 4.6 | < 0.001* |
| SPPB Total Score | N/A | 11.4 ± 0.5 | 6.1 ± 1.3 | < 0.001* |
| Chair stands sub-score | N/A | 3.4 ± 0.5 | 0.8 ± 0.6 | < 0.001* |
| Balance test sub-score | N/A | 4.0 ± 0.0 | 2.6 ± 1.3 | < 0.001* |
| 4m walk test sub-score | N/A | 4.0 ± 0.2 | 2.7 ± 0.8 | < 0.001* |
| Gait speed (m/sec) | N/A | 1.10 ± 0.19 | 0.67 ± 0.13 | < 0.001* |
Values are mean ± standard deviation unless otherwise noted.
Symbols (i.e. a, b, or c) indicate homogenous subsets. Groups sharing a common symbol are not significantly different at α = 0.05.
HF = High-functioning; determined by a score ≥ 11 on the Short Physical Performance Battery (SPPB)
LF = Low-functioning; determined by a score ≤ 7 on the SPPB
MMSE = Mini-Mental State Examination
N/A = not applicable
Includes prior myocardial infarction or stroke, diabetes, chronic lung disease, and osteoarthritis
Note: Young participants did not perform the Short Physical Performance Battery nor complete the disability questionnaire.
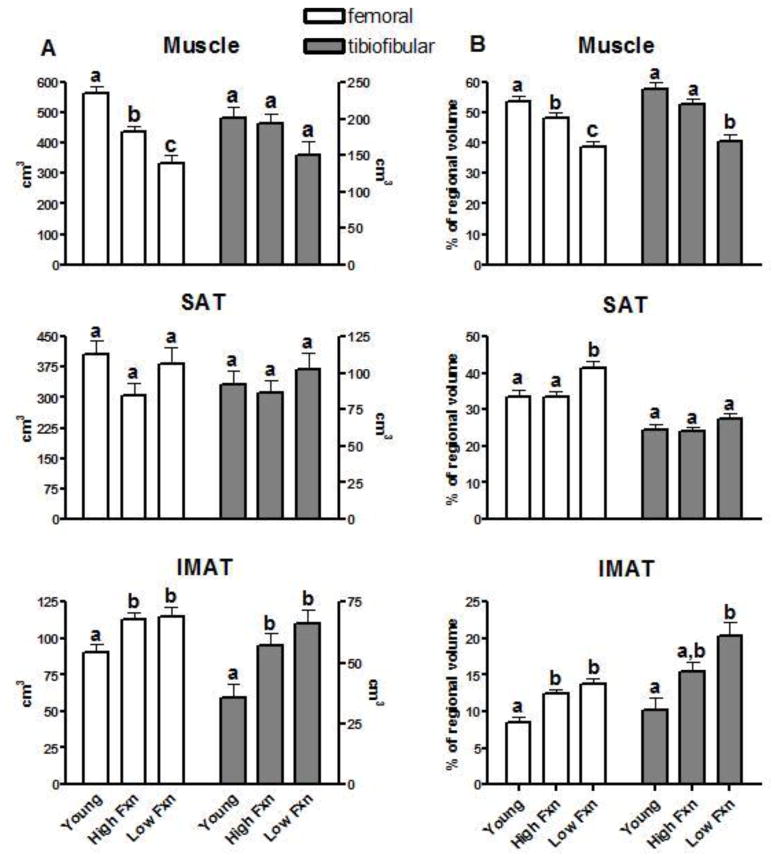
Tissue composition data via MRI are available for 58 of the 63 study participants (Young, n= 19; HF, n=24; LF, n=15). MRI data were not available for 5 participants due to equipment difficulties and/or poor image quality. In general, young participants had more muscle mass and less IMAT (in both regions) than older participants, while the HF group displayed the least SAT among the three groups (Figure 1). Within the femoral region, a significant difference was observed across the three groups for IMAT (p = 0.008), and muscle (p < 0.001) volumes (Figure 2A). Both age and functional status were associated with differences in femoral muscle mass, while only age was associated with differences in femoral IMAT. A trend (p = 0.076) toward significance was observed across groups for the femoral SAT compartment as the HF displayed the least SAT. However when expressed relative to the total volume of the femoral region, a significant effect was observed across groups (p = 0.002) as SAT of the LF group composed a larger percentage of the region volume compared to young (p = 0.005) and HF (p = 0.003) groups (Figure 2B).
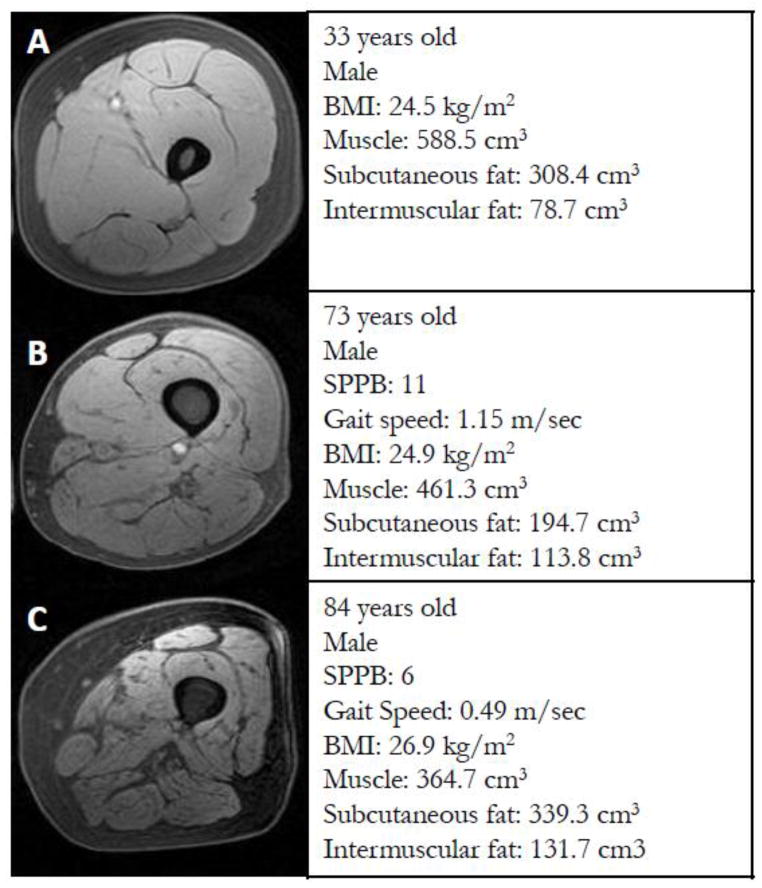
Figure 1.
Representative magnetic resonance images of the femoral region collected at 3T from A) young B) high-functioning older, and C) low-functioning older study groups.
Figure 2.
Volumes of skeletal muscle, subcutaneous adipose tissue (SAT), and intermuscular adipose tissue (IMAT) in the femoral and tibiofibular regions expressed A) in absolute terms (cm3) and B) as a percentage (%) of total region volume. Columns represent group sex-adjusted means, error bars indicate standard error. Groups sharing a common symbol (i.e. a, b, or c) were not significantly different at α = 0.05. High Fxn = High-functioning older adults; Low Fxn = Low-functioning older adults. Functional status of older adults was determined based on the participants’ score on the Short Physical Performance Battery (SPPB).
With regard to absolute tissue volumes in the tibiofibular region, a significant effect across the three groups was observed for only IMAT (p = 0.001). This group effect was associated only with age as no significant difference was observed between HF and LF groups. A trend toward significance across groups (p = 0.083) was observed for tibiofibular muscle due to a lower group mean in the LF group. After correcting for the total volume of the tibiofibular region, this effect was significant (p < 0.001) as muscle of the LF group composed a lesser percentage of the region volume compared to the young (p < 0.001) and HF (p = 0.001) groups.
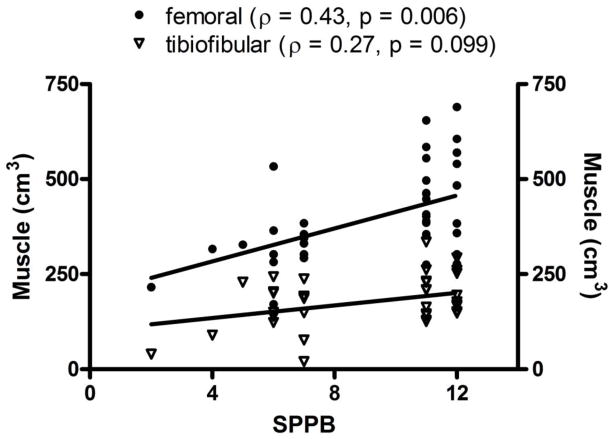
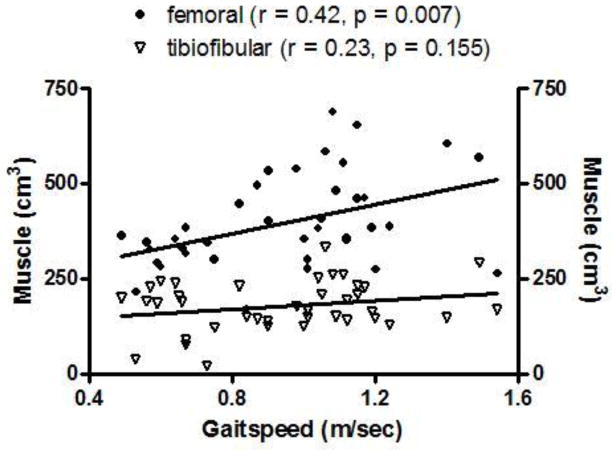
Femoral muscle mass was the only tissue compartment significantly correlated with SPPB score (ϱ = 0.43, p = 0.006, Figure 3). A trend was observed, however, for tibiofibular muscle mass (ϱ = 0.27, p = 0.099) (Figure 3). Likewise, femoral muscle was the only compartment significantly associated with gait speed (r = 0.43, p = 0.0007). Tibiofibular muscle mass was only modestly associated with gait speed (r = 0.23, p = 0.155) (Figure 4). In agreement with the findings of the correlation analyses, stepwise regression models indicated that femoral muscle was the only significant contributor to the final models of both SPPB and gait speed (Tables 2a and 2b). These analyses suggest that femoral muscle volume explains approximately 26% of the variance in SPPB score and 19% of the variance in gait speed.
Figure 3.
Scatterplot demonstrating the linear relationships, including Spearman’s correlation coefficient, between lower extremity skeletal muscle volumes and score on the Short Physical Performance Battery among high- and low-functioning older adults.
Figure 4.
Scatterplot demonstrating the linear relationships, including Pearson’s correlation coefficient, between lower extremity skeletal muscle volumes and self-selected gait speed among high- and low-functioning older adults.
Table 2a.
Stepwise regression model of factors predicting SPPB score in older adults
| Beta | t | Prob (t) | R2 | Adjusted R2 | F | Prob (F) | |
|---|---|---|---|---|---|---|---|
| Significant to final model | |||||||
| Femoral muscle | 0.264 | 0.243 | 12.90 | 0.001 | |||
| Excluded Variables | |||||||
| Age | −0.279 | −1.86 | 0.071 | ||||
| Sex | 0.196 | 1.079 | 0.288 | ||||
| Limb Volume | −0.126 | −0.81 | 0.425 | ||||
| Femoral IMAT | −0.161 | −1.12 | 0.272 | ||||
| Femoral SAT | −0.146 | −0.96 | 0.341 | ||||
| Tibiofibular Muscle | 0.208 | 1.31 | 0.199 | ||||
| Tibiofibular IMAT | −0.214 | −1.52 | 0.138 | ||||
| Tibiofibular SAT | −0.021 | −0.138 | 0.891 |
SPPB = Short Physical Performance Battery
Table 2b.
Stepwise regression model of factors predicting usual gait speed in older adults
| Beta | t | Prob (t) | R2 | Adjusted R2 | F | Prob (F) | |
|---|---|---|---|---|---|---|---|
| Significant to final model | |||||||
| Femoral muscle | 0.187 | 0.165 | 8.30 | 0.007 | |||
| Excluded Variables | |||||||
| Age | −0.274 | −1.73 | 0.093 | ||||
| Sex | 0.335 | 1.81 | 0.080 | ||||
| Limb Volume | −0.118 | −0.72 | 0.478 | ||||
| Femoral IMAT | −0.117 | −0.77 | 0.447 | ||||
| Femoral SAT | −0.102 | −0.64 | 0.530 | ||||
| Tibiofibular Muscle | 0.077 | 0.45 | 0.653 | ||||
| Tibiofibular IMAT | −0.139 | −0.92 | 0.362 | ||||
| Tibiofibular SAT | −0.015 | −0.09 | 0.928 |
4. Discussion
This cross-sectional study of lower extremity tissue composition and functional performance found that older adults classified as LF according to the SPPB have significantly less muscle mass in the femoral region than their HF peers and young adults. In contrast, we did not observe significant differences among groups for the absolute muscle volume in the tibiofibular region. We also observed age-related differences for IMAT in both the femoral and tibiofibular regions, but these differences did not exist between the functionally-distinct groups of older adults. No significant differences existed between groups for SAT in either region unless adjustments were made for total region volume. When all compartments were considered together using multiple regression, muscle mass of the femoral region was the primary predictor of physical performance among the older participants.
These findings are important for several reasons. First, this is the first study to directly compare the tissue compositions of the femoral and tibiofibular regions in young adults and older persons with contrasting levels of physical function. In recent years, investigators have developed sizable interest in the effects of age on lower extremity tissue composition due to the importance of the limbs in maintaining functional capabilities such as mobility. Numerous studies have demonstrated the importance of total lower extremity muscle and adipose tissue masses in the physical function of older adults.[4, 6, 7, 26–29] However, our data indicate that the compositions of the proximal and distal leg regions differ in their associations with age. Moreover, muscle mass of the femoral region appears to be a stronger contributor to physical function of older adults than does tibiofibular muscle. Knowledge of these differences is critical to optimizing interventions designed to maintain leg muscle and limit adipose tissue accumulation in older adults.
Our data demonstrate that the mass of the proximal leg muscles predicts physical function among older adults. Subsequently, interventions (e.g. exercise training) designed to maintain function in advanced age may be best served by preferentially targeting these muscle groups. Our results are in agreement with previous work by others who reported that thigh muscle mass was an important predictor of functional capacity, [5] but that calf muscle mass was a weak predictor of lower extremity performance in older adults.[18] Our findings are, however, in contrast with those of Posner et al. [17] who previously reported that calf muscle strength was a stronger predictor of functional task performance than strength of the quadriceps or hamstrings. The disparate results of previous studies and the present one may be at least partially because the relationship between muscle mass and muscle strength is not perfectly linear since motor neuron dysfunction and the infiltration of fat into muscle may impair muscle strength to a greater degree than muscle atrophy alone.[30]
The infiltration of fat into muscle, known as myosteatosis, is increasingly recognized as an important component of physical function as well as metabolic disease in the elderly.[5, 31–34] The findings of the present study are in agreement with previous observations which indicated that the quantity of lower-extremity IMAT increases significantly with advanced age.[12, 35] Delmonico et al. [25] reported 5 year increases in mid-thigh IMAT of 35–75% in men and 17–50% in women aged 70–79 years. Similarly, we observed age-related differences of over 20% in femoral IMAT. These differences were even more dramatic in the tibiofibular region as we observed age-related increases in IMAT of over 40%. Despite these remarkable age-related differences, these changes in IMAT were not associated with functional status. This finding is somewhat surprising given that previous studies have noted that fat infiltration of muscle was associated with declines in skeletal muscle strength, [31] future self-reported mobility limitations, [5] and risk of hospitalization.[10] It is possible that our small sample size limited our ability to detect differences in these compartments. However, this discrepancy may also be due to the fact that the previous cohort studies utilized computed tomography to determine muscle attenuation, a measure of fat infiltration that reflects intramuscular fat, rather than IMAT. It is possible that that intramuscular fat, or that which infiltrates within the myocyte, is more detrimental to muscle function than IMAT but more investigation is needed. Therefore, a simultaneous comparison of age-related changes in intra- and inter-muscular fat and their associations with functional performance is warranted.
An additional finding of our study is that, across both regions SAT does not differ between study groups. We speculate that aging per se may not have the same effects on SAT that it does on IMAT. Delmonico et al. [25] previously demonstrated that while over 5 years older adults experienced annual increases in IMAT of 29% (women) to 49% (men), they displayed 2% decreases in SAT. Moreover, this group noted that the changes in SAT were largely driven by changes in total body mass (i.e. those who lost weight lost SAT and vice versa). Our results are interesting from the standpoint that, though not statistically significant, the volume of SAT was smaller in the HF than young participants. This difference disappeared completely after adjusting for total femoral volume. Moreover, after this adjustment the SAT of the LF group constituted a significantly greater percentage of the femoral volume compared with the other two groups. These results indicate that measurements of SAT in the femoral region may be more meaningful if expressed in a manner that takes into account the volume of other tissues, particularly muscle. Further exploration is needed, however, to discover the true utility of this method.
The present study has several important strengths. Indeed, this study is among the first to directly compare age-related differences in muscle and adipose tissue compartments in both the femoral and tibiofibular regions. Moreover, ours is the first study to include each of these tissue compartments in assessing their relative importance to the functional performance of older adults. Despite its strengths, the present study is not without limitations. Most notable is the inherent limitation of the cross-sectional design that it does not allow for causal inferences to be drawn regarding the relationship between muscle mass and physical function. It remains possible that declines in functional performance among the LF group occurred prior to the onset of significant muscle atrophy. Secondly, the small sample size in this study makes it susceptible to type II error. Finally, although the participants were all sedentary, subtle differences in daily physical activity could contribute to differences in functional outcomes and were not accessed.
In conclusion, our data suggest that age-related differences in lower extremity tissue composition conflict between the femoral and tibiofibular regions. Furthermore, muscle volume of the femoral region appears to be more strongly associated with physical function in older adults than do tibiofibular muscle volume and adipose tissue compartments. These findings are relevant to both researchers and clinicians interested in preventing or treating functional decline among older adults. Future prospective studies are needed to determine optimal methods for maintaining muscle mass and physical function of older adults, particularly those who are already functionally-limited. Additionally, these results may have important implications in the development of a widely-accepted clinical definition of sarcopenia.
Highlights.
Femoral muscle mass differed across age groups and functional statuses.
Femoral muscle volume was associated with physical function of older adults.
Tibiofibular muscle volume was not significantly associated with function.
Advanced age was associated with elevated intermuscular adipose tissue (IMAT).
IMAT did not differ between high- and low-functioning older adults.
Acknowledgments
This study was funded by the University of Florida (UF) Claude D. Pepper Older Americans Independence Center (OAIC). The OAIC is supported by a grant from the National Institutes of Health/National Institute on Aging (1P30AG028740). TB was partially supported by an award from the University of Florida Clinical and Translational Science Institute (1KL2RR029888).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults--united states, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. The surgeon general’s call to action to improve the health and wellness of persons with disabilities. 2005. [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Misic MM, Rosengren KS, Woods JA, Evans EM. Muscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adults. Gerontology. 2007;53:260–266. doi: 10.1159/000101826. [DOI] [PubMed] [Google Scholar]
- 7.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12:493–498. doi: 10.1007/BF02982711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther. 2007;87:1334–1347. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, Sewall A, Goodpaster B, Satterfield S, Cummings SR, Harris TB. Health, Aging and Body Composition Study, Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: A randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann RA, Hagy J. Biomechanics of walking, running, and sprinting. Am J Sports Med. 1980;8:345–350. doi: 10.1177/036354658000800510. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans NS, Liu K, Criqui MH, Ferrucci L, Guralnik JM, Tian L, Liao Y, McDermott MM. Associations of calf skeletal muscle characteristics and peripheral nerve function with self-perceived physical functioning and walking ability in persons with peripheral artery disease. Vasc Med. 2011;16:3–11. doi: 10.1177/1358863X10395656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner JD, McCully KK, Landsberg LA, Sands LP, Tycenski P, Hofmann MT, Wetterholt KL, Shaw CE. Physical determinants of independence in mature women. Arch Phys Med Rehabil. 1995;76:373–380. doi: 10.1016/s0003-9993(95)80664-4. [DOI] [PubMed] [Google Scholar]
- 18.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 19.Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, Newman AB. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: A randomized clinical trial. J Obes. 2011;(2011):516576. doi: 10.1155/2011/516576. Epub 2010 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, Pepe G, Tarantini F, Berni A, Marchionni N, Di Bari M. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16:390–395. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, Guralnik JM. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: Analysis from the In CHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64:223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity non uniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 25.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estrada M, Kleppinger A, Judge JO, Walsh SJ, Kuchel GA. Functional impact of relative versus absolute sarcopenia in healthy older women. J Am Geriatr Soc. 2007;55:1712–1719. doi: 10.1111/j.1532-5415.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 27.Lebrun CE, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13:474–481. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- 28.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB. Health, Aging and Body Composition Study, Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 29.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l’OSteoporose) study. Am J Clin Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 30.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 32.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, Fritsche A, Schick F. Intermuscular adipose tissue (IMAT): Association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29:1340–1345. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 34.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, Fantin F, Bosello O, Cominacini L, Harris TB, Zamboni M. Adipose tissue infiltration in skeletal muscle of healthy elderly men: Relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295–299. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, Wheeler VW, Evans RW, Zmuda JM. Adipose tissue infiltration in skeletal muscle: Age patterns and association with diabetes among men of african ancestry. Am J Clin Nutr. 2008;87:1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]