Abstract
Children with Autism often show difficulties in adapting to change. Previous studies of cortisol, a neurobiologic stress hormone reflecting hypothalamic–pituitary–adrenal (HPA) axis activity, in children with autism have demonstrated variable results. This study measured cortisol levels in children with and without Autism: (1) at rest; (2) in a novel environment; and (3) in response to a blood draw stressor. A significantly higher serum cortisol response was found in the group of children with autism. Analysis showed significantly higher peak cortisol levels and prolonged duration and recovery of cortisol elevation following the blood-stick stressor in children with autism. This study suggests increased reactivity of the HPA axis to stress and novel stimuli in children with autism.
Keywords: Cortisol, Autism, Stress, HPA, Adaptability, Neurobiology
Introduction
Autism is a biologic, brain-based disorder with genetic and non-genetic etiologies. Its clinical course is influenced by neurobiological events, but these are not well understood. Children with autism are classically characterized by deficits in language and social interactions as well as displays of odd or repetitive behaviors. Though historically considered relatively rare, the Centers for Disease Control and Prevention's (CDC) Morbidity and Mortality Weekly Report currently estimates that an average of 1 in 111 children in the U.S. have an Autism Spectrum Disorder (ASD), with a gender bias of 4–5 times more males than females having ASD (Rice 2009). Despite this relatively high prevalence, our understanding of the neurodevelopmental biology and pathophysiology of these disorders remains limited. An improved understanding of neurobiological factors associated with autism may lead to more effective therapeutic interventions and additional clues as to the etiology of the disorder.
Previous studies have shown that people with autism have abnormal levels of various hormones and neuroactive substrates such as serotonin, oxytocin, vasopressin, cortisol, and dehydroepiandrosterone (DHEA) (Lam et al. 2006). These studies have made use of a variety of technologies to analyze saliva, urine, and serum samples. One of the most well studied psychoneuroendocrine systems is the hypothalamic–pituitary–adrenal (HPA) axis which is intimately involved in the stress response (Heinrichs and Koob 2004). Cortisol has been well studied in many populations as it is an important measure of the biologic reactivity to stress and both excessive and deficient cortisol responses have been associated with dysregulation of the HPA axis (Gunnar and Donzella 2002; Gillespie et al. 2009). Cortisol measures offer an excellent index of adrenocortical function during childhood that is independent of chronological age and Tanner Stages of pubertal development (Gomez et al. 1991). Any type of physical or mental stress can result in an elevation of cortisol; however, the type of situation that elicits a stress response can vary from person to person. Individuals with autism are commonly observed to react abnormally to environmental changes or novel situations that might not be stressful to others. Studies on the variation of cortisol levels in children with autism have reported conflicting findings, which is thought to be due in part to the use of different methodologies, to performing the measurements at different times of day, and to lack of adequately controlling collection conditions (Karten et al. 2005). A very interesting recently published study using a peer interaction paradigm found higher levels of cortisol in many children with autism (Corbett et al. 2010). This is of significant concern as chronic cortisol elevations can have a harmful effect on brain development (Squire 1992; Hahn et al. 1992). An association has been found between prolonged and elevated levels of cortisol and a reduced size of brain regions such as the hippocampus in individuals without autism (Karten et al. 2005). Elevated cortisol levels have been linked to other adverse associations including low security of parent–child attachments (Naber et al. 2007; Dettling et al. 2000), and perhaps the elevated level found in some studies is a reflection of poor attachment characteristics associated with autism. Other thoughts about elevated stress in children with autism could be due to the fact that these individuals have difficulty tolerating novel environments and some environmental stressors, can lead to excessive behavioral reactivity to stressful situations that could be associated with a concurrent rise in cortisol. The Childhood Autism Rating Scale reflects this characteristic by measuring adaptation to change as one of its core domains (DiLalla and Rogers 1994). In summary, the neuroendocrine stress reactivity in children with autism has not been well characterized. The aim of this study was to compare the HPA axis stress response in children with autism to normal controls in three ways, by measuring the urinary cortisol levels from first morning void and responses to a blood-stick stressor using salivary and serum cortisol levels.
Methods
Participants
Twenty children between the ages of 3 and 10 (11 males and 9 females) meeting full DSM-IV-TR criteria for autism and 28 participants (15 males and 13 females) without a history of developmental concerns were recruited from treatment providers, community-based agencies, and health care clinics. Children with an IQ of less than 55 or children having any co-morbid medical conditions that might impact the HPA axis (e.g. endocrine disorders) were excluded. Children were excluded if they had a history of abuse or neglect. The Medical University of South Carolina Institutional Review Board (MUSC IRB) approved this study. Informed consent was obtained from each subject prior to their arrival at the Clinical and Translational Research Center (CTRC).
The Autism Diagnostic Observation Schedule (ADOS) was used to confirm the clinical diagnosis (Lord and Risi 2000). This assessment uses a highly structured scoring system where evaluations are made based on social and communicative skills, and is considered the “gold standard” for the diagnosis of autism. In addition, the lead author (ES) and study clinician (KM) independently reviewed all records available for each subject to assist in the assessment of lifetime autism criteria. A control group of 28 subjects was formed by recruiting 3–12 year old children with normal developmental history by structured interview.
We performed a Childhood Autism Rating Scale (CARS) assessment on all patients with a diagnosis of autism. The CARS consists of 14 domains assessing autism behaviors with a 15th domain assessing overall impressions of autism. Each domain is scored from 1 to 4 (total score 15–60) with the higher scores indicating more severe forms of autism. Scores below 30 classify subjects as non-autistic, between 30 and 36.5 as mildly to moderately autistic, and scores from 37 to 60 as severely autistic (Schopler et al. 1980). The Child Behavior Check List (CBCL) was completed by parents of all participants. The CBCL assesses maladaptive functioning from the perspective of the parent or caregiver. The scales derived include Total Problems, Internalizing and Externalizing Problems, and eight syndrome scales (Achenbach 1991).
Demographic information, including age, gender, race, school placement, household composition, medical insurance, socioeconomic status, religious participation, and nicotine/substance use in the home, was obtained for each study participant.
Procedures
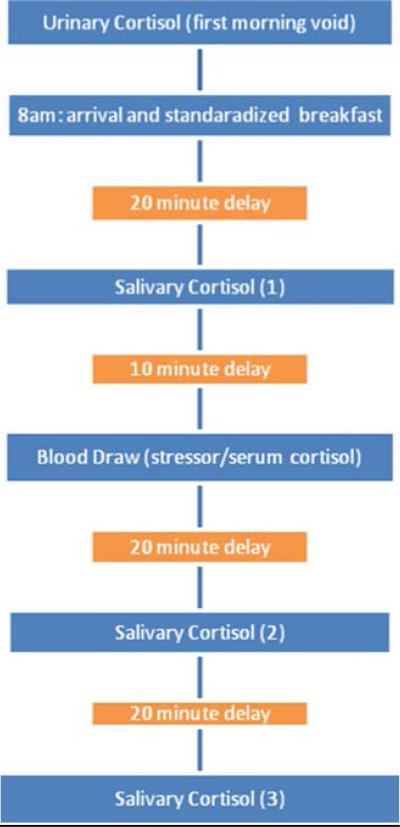
Informed consent approved by the MUSC IRB was obtained from each parent prior to participation. Because cortisol levels are known to fluctuate diurnally, efforts were made to standardize the time at which studies were performed. Each child and parent arrived at the CTRC at approximately 8 a.m. Subjects were provided with instructions and specimen collection materials prior to their visit and were asked to bring the first morning void urine sample to the visit. If a sample was unavailable at the time of arrival (e.g. parent forgot to collect the specimen), then a first morning void of the subject was collected at home on another day soon after the day of study, refrigerated, and collected by research personnel. A health professional performed a review of systems and a physical exam, including height, weight, head circumference, and vital signs, on all participants at the time of CTRC admission to ensure health and to review inclusion–exclusion data. Medications and medical conditions were reviewed. In order to minimize the potential effect of diet on cortisol, each subject was provided with a standardized, non-caffeinated breakfast of toast, jelly and apple juice to minimize any potential effect of carbohydrates on cortisol production (Gonzalez-Bono et al. 2002). Approximately 20 min after the breakfast was completed, the first salivary cortisol was collected using a standard swab from a Salimetrics kit. Ten minutes after the first salivary cortisol was collected, a blood draw was performed using a 21 gauge butterfly needle. The blood collection served both as a stressor and to collect serum for determining the baseline serum cortisol level. Salivary cortisol samples were obtained 10 min before and 20 and 40 min after the blood draw. The CTRC research setting was a novel setting to all participants as none had previously been there. A visual illustration of the methods is shown in Fig. 1 below.
Fig. 1.
The experimental time sequence used to assess the stress response of children with and without autism
Measures
In order to evaluate HPA response and recovery activity, we measured cortisol levels obtained in three ways. (1) Morning void urine samples were used to estimate overnight basal activity. These were performed at the Wisconsin Primate Lab. Samples were assayed in duplicate using an enzyme-linked immunoassay (EIA) method (HRP Munro) with a sensitivity of 2.8 pg/ml and direct sample preparation (Toni Ziegler, personal communication, March 2010). (2) Serum was collected using standard sampling tubes and assayed for cortisol at Nichols Institute/Quest Diagnostic Laboratories using the Bayer Advia Centaur Immunoassay System. These samples were used to establish baseline cortisol levels in the novel setting of the CTRC. (3) HPA response to the blood draw stressor was evaluated using quantitative salivary cortisol samples taken 10 min before and 20 and 40 min after the stressor (blood draw). These were collected by standard protocol utilizing salivettes and sorbettes and processed at the CTRC Core Laboratory using an expanded range, high sensitivity salivary cortisol EIA kit supplied by Salimetrics.
Statistical Analysis
Subjects were characterized by age, gender, and race using descriptive statistics. Differences in mean urinary and serum cortisol levels between children with and without autism were calculated using two independent group 2-sided t-tests with alpha = 0.05. Salivary cortisol levels at each of the three time points (before blood draw, 20 min and 40 min after blood draw) were similarly compared between groups. In addition, the area under the curve (AUC) established by the three time points was calculated for each group. The difference between groups in their respective AUC values was tested for significance again using the t-test. Breakdowns by group and by gender were evaluated using the same techniques. All calculations were made using either SPSS or SAS statistical software.
Results
Subjects
The twenty children with autism had a mean age of 83.9 months; 55% were male; 80% were Caucasian and 20% were African American or other. The 28 healthy controls had a mean age of 66.1 months; 54% were male; 71% were Caucasian and 29% were African American or other. The autism group was older (p = 0.006), but the two groups were comparable with respect to race (p = 0.737) and gender (p = 1.000).
HPA Activity
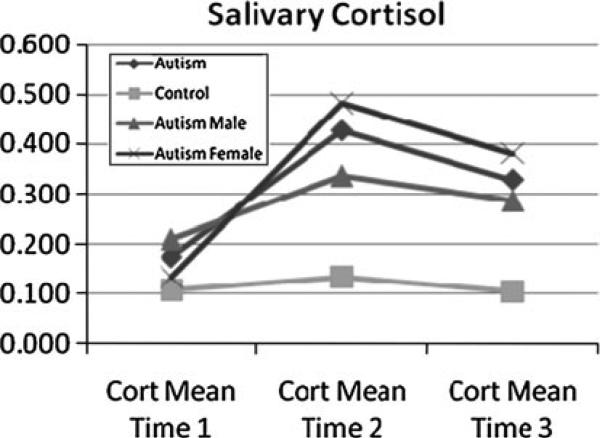
The urinary cortisol and serum cortisol levels for the two groups of children are shown in Table 1. There was no significant difference in the first morning void urinary cortisol level between the two groups (p = 0.329). However, there was a statistically significant difference in the serum cortisol obtained during the blood draw, with the autism group displaying a significantly higher serum cortisol level as compared to the control group (p = 0.014). Salivary cortisol levels in ug/dL were log transformed to ensure that the data was normally distributed. HPA responsiveness, as measured by salivary cortisol response to the stressor, is shown in Fig. 2.
Table 1.
Comparison of mean Cortisol levels for case (children with autism) versus control children
| Biologic | Case/control | N | Mean | SE of mean | Significance |
|---|---|---|---|---|---|
| Urinary-cortisol (morning level) | Case | 20 | 315.32 | 83.20 | 0.329 |
| Control | 24 | 235.40 | 26.02 | ||
| Serum-cortisol | Case | 19 | 13.37 | 1.10 | 0.014 |
| Control | 27 | 10.09 | 0.75 |
Fig. 2.
Mean salivary cortisol levels in response to a blood draw stressor between children with Autism (further broken down by gender) and developmentally normal controls. Measurements at Time 1 were taken 10 min prior to a blood draw. Times 2 and 3 refer to measurements at 20 and 40 min post-draw, respectively. Subjects with autism display a higher peak response as well as a prolonged duration. Standard errors of the mean are as follows: Time 1 (autism 0.061, control 0.024), Time 2 (autism 0.107, control 0.018), Time 3 (autism 0.111, control 0.018)
Prior to the blood draw (time 1), the mean salivary cortisol level was not significantly different between children with autism (mean 0.175) and those without autism (mean 0.105, p = 0.242 between groups). Both groups displayed an increase in salivary cortisol levels 20 min after the blood draw, with a significantly greater peak response among children with autism (mean 0.426 for autism group versus 0.132 for those without, p = 0.014). At 40 min after the blood draw, the salivary cortisol level in the children with autism remained above their baseline level (mean 0.329), while in those without autism it dropped below their baseline level (mean 0.011, p = 0.057 between groups). The area under the curve (AUC) across the three time points was significantly different between the two groups (p = 0.013). Figure 2 also shows the change across the three time points for male and female children with autism. Females had a slightly lower level at baseline (time 1), and higher levels at times 2 (20 min post blood draw) and 3 (40 min post blood draw), but the differences were not significant at any time point (p = 0.545 at time 1, p = 0.416 at time 2, p = 0.685 at time 3). No statistically significant differences were found between the Caucasian and African-American children with autism, but only 4 children of the 20 children with autism were African American, which gave insufficient power to detect potential racial differences.
Comparisons across gender for urinary and serum cortisol levels did not reveal any statistically significant differences between females with and without autism (urinary p = 0.542, serum p = 0.106), males with and without autism (urinary p = 0.433, serum p = 0.063), or males versus females with autism (urinary p = 0.559, serum p = 0.199). In addition, tests were conducted for potential correlations (Pearson bivariate correlation) between test scores (total CARS, DSM4 total A, ABC by subscale totals) and each of the cortisol measurements: urinary cortisol, serum cortisol, and salivary cortisol. There were no significant correlations found.
In additional analyses, Pearson correlations between urine, serum, and salivary cortisol measures were assessed. For children with autism, salivary and serum cortisol measures were correlated at Time 2 (0.748, p = 0.000) and at Time 3 (0.645, p = 0.003). Salivary measures at Time 2 were correlated with salivary measures at Time 3 (0.889, p = 0.000). There was no significant correlation between age in months and any of the cortisol values (urine, serum, or salivary). For control children, salivary measures at Time 2 were also correlated with salivary measures at Time 3, but not as strongly (0.566, p = 0.002). Salivary and urine measures were correlated at Time 3 (0.407, p = 0.048).
Discussion
Behavioral symptoms of autism are known to be influenced by neurobiological factors. Many studies that have investigated stress hormone responses look at only a single time point or how the HPA axis responded to a single stressor. There have been very few studies examining how this response changes through the day or how it is affected by certain stressors, environments, and activities. Our results, derived using three methods of cortisol measurement, indicate that there is a significantly greater biologic reactivity of the HPA axis in response to a blood draw stressor in children with autism as compared to children without autism. Although there was no measurable difference in urinary morning resting levels representing basal HPA axis activity in the home environment, or differences in serum level representing exposure to a mildly novel new environment (the CTRC), the cortisol response to a blood stick stressor was significantly greater among children with autism. Specifically, these patients showed an increased magnitude of cortisol response and a prolonged duration of cortisol secretion recovery when subjected to this stressor.
A strength of this study is that there was a stressor (blood draw) and a measured HPA response as well as measures of a resting state. The significant difference in baseline salivary cortisol taken prior to the blood draw may reflect the response to the novel CTRC environment as children with autism commonly find new situations to be more stressful than would typically developing children. This is supported by the observation that although there was no significant difference in baseline levels of urinary cortisol between control participants and children with autism, a statistically significant difference in serum cortisol levels was observed. It is clearly accepted by anyone working with children that have Autism that their behavioral responses can be extreme. One study speculated that the cortisol awakening response (CAR) of the HPA axis could be a factor in how youth with high-functioning autism react to new situations. This suggests that the biologic conditions produced by stress hormones are associated with the behavioral response of these individuals. Non-significant findings between the experimental group of young children and control group led to the hypothesis that in some children with autism the CAR variations in the HPA may develop during adolescence or later in life (Zinke et al. 2010).
Our findings are similar to those of Richdale and Prior (1992) who demonstrated hypersecretion of cortisol in children with autism who were integrated into a regular school system, suggesting a hyper-responsiveness to the environmental stress of the school environment. This has been demonstrated by Corbett, et al. as they found that a peer interaction paradigm situation triggered an HPA response in many children with autism (2010).
Elevated cortisol levels have been linked to other adverse associations including low security of parent–child attachments (Naber et al. 2007; Dettling et al. 2000), and perhaps this elevated level is a reflection of poor attachment characteristics associated with autism. However, much variability is noted in the responses of children and variable responses were seen in some of our 20 children.
One of the largest studies conducted in humans with severe Autism Spectrum Disorder (ASD) (N = 48) found higher levels of plasma adrenocorticotropic hormone (ACTH), which was suggested to be a better marker for acute stress while cortisol, whose levels were not significantly different as compared to the control group, is a better marker for basal functioning. The highest ACTH levels were found in the subjects with most severe ASD. However in our study, higher elevations of cortisol were not associated with greater ASD impairment as measured with the CARS.
Several other studies have found dysregulation in the HPA response in subjects with ASD (Richdale and Prior 1992; Nir et al. 1995; Yamazaki et al. 1975). Lopata et al. (2008) found significantly increased salivary cortisol levels in response to conditions of peer familiarity in children with high-functioning ASD. Unfortunately, many studies examining HPA responsiveness in children with autism are weakened by lack of appropriate controls, invasive sampling techniques, mixed diagnoses, and very small samples (Maher et al. 1975; Tordjman et al. 1997; Curin et al. 2003; Corbett et al. 2006).
Although data has been inconsistent, some studies show alterations in the normal circadian patterns of cortisol in children with autism (Richdale and Prior 1992; Yamazaki et al. 1975; Tordjman et al. 1997; Aihara and Hashimoto 1989; Hill et al. 1977; Hoshino et al. 1987). The results seem to be more apparent in populations with greater cognitive impairment; however Brosnan et al. (2009) showed an increased cortisol awakening response in adolescent males with Asperger Syndrome. It has also been postulated that children with autism show poor negative feedback regulation of the HPA axis. Hoshino et al. (1987) demonstrated that some children with autism had abnormal diurnal rhythm and/or failure to suppress cortisol in response to dexamethasone, which would normally be expected to cause a strong negative feedback response. The results suggest that in children with autism the negative feedback mechanism regulating the HPA axis may be less efficient, resulting in prolonged cortisol increase following activation by a stress response.
When studying the HPA axis, it is important to take into account the diurnal variability of the axis which has been shown to be altered in several studies. Corbett et al. (2009) found increased variation in circadian rhythms as well as statistically significant cortisol elevations from exposure to a nonsocial stimulus (placement in a mock MRI machine) among ASD subjects. Another study by Corbett et al. showed decreased salivary cortisol levels in the morning with increased levels in the evening. Autistic subjects also showed increased circadian variability within and between subjects. Some have suggested that ann abnormal circadian pattern may be due to dysfunction of the pineal gland (Bourgeron 2010).
As additional studies are conducted, we it's important that we continue to look at multiple ways that the HPA axis is functioning, times of day as well as the specific stressor tested. Stressors related to novel experiences and social interactions, not considered by others to be stressful, may be especially stressful for those with autism. The interaction between stress and the multi layered variability of the HPA axis activity is known to be complex. Using both a Trier-style psychosocial stressor test and a control test with no intended stressor, Jansen et al. (2003) demonstrated higher cortisol levels in children with autism after the control test and lower levels after the psychosocial test when compared to normal children. Although cortisol levels did not increase for children with autism during the stressor, their heart rate significantly increased indicating that the test was perceived as stressful. When trying to replicate these results with adults, Jansen et al. (2006) found increased levels of oxytocin but no difference in cortisol levels between Autism and controls when given the psychosocial stressor task. In both studies, however, the researchers found a decreased heart rate in those with Autism in response to the psychosocial stressor and hypothesized that this is due to a physiological dysfunction in the stress response (Jansen et al. 2003, 2006).
Our study provides evidence for dysregulation of the HPA stress response in individuals with autism. In this study, no correlation was found between the severity of autism symptoms, as measured by the CARS, and measures of HPA response. Although we attempted to control many variables that potentially confound studies of the HPA axis, our study is still limited by the difficulty of regulating induced stress in a population known for their abnormal and widely varied perception of and response to stress. However, there are several strengths of our study that include the controlled environment of the CTRC, a high carbohydrate breakfast and the use of a same time of day standardized protocol and procedures. Prepubertal participants were chosen, and we had an almost equal number of males and females in both groups. We used standard measures of the severity of autistic features. There is no way to comprehensively evaluate which environmental stressors are perceived as most stressful in this population i.e. novel environment, social interactions, meeting new people, anticipation of blood draw, etc. Future research that aims to evaluate the role of different stressors and the biologic mechanisms of other systems might provide insight into the association of increased cortisol response and behavior. This was a small pilot study to demonstrate the feasibility of conducting biologic studies with prepubertal children with autism in our CTRC and to begin exploring the associations of behavior and biology in these children. Further research looking at larger groups of test subjects in more controlled scenarios over extended periods of time and monitoring multiple stress and resilience systems at the same time may be required if we are to someday understand the scope of HPA dysregulation and its effects in individuals with autism.
Acknowledgments
This research project was supported by Award Number UL1RR029882 from the National Center for Research Resources, as well as Award Number P50 DA016511 from the Office of Research on Women's Health at the National Institutes of Health.
Control participants were from grant K23 NIH/NIMH K23MH064111: Neurodevelopmental Biology of Neglected Children (PI: Eve G. Spratt) and additional resources were obtained from grant NIH/NIDA K24DA00435: Midcareer Investigator Award in Patient-Oriented Research (PI: Kathleen T. Brady).
The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Many thanks to Quest Diagnostics for performing serum cortisol measurements. Thanks to Drs. Lindsay DeVane and Megan Gunnar, as well as research assistants Loriann Uerebroth, Samantha Friedenberg, Lauren English, and Doreen Condon, for assistance and advice with this project.
References
- Achenbach TM. Manual for the child behavior checklist/4-18. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Aihara R, Hashimoto T. Neuroendocrinologic studies on autism. No To Hattatsu. 1989;21:154–162. [PubMed] [Google Scholar]
- Bourgeron T. Cold Spring harbor symposia on quantitative biology. Vol. 72. Cold Spring Harbor Laboratory Press; 2010. The possible interplay of synaptic and clock genes in autism spectrum disorders. [DOI] [PubMed] [Google Scholar]
- Brosnan M, Turner-Cobb J, Munro-Naan Z, Jessop D. Absence of a normal cortisol awakening response (CAR) in adolescent males with Asperger syndrome (AS). Psychoneuroendocrinology. 2009;34(7):1095–1100. doi: 10.1016/j.psyneuen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31:59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research. 2009;2(1):39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism. 2010;1(13) doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curin JM, Terzic J, Petkovic ZB, Zekan L, Terzic IM, Susnjara IM. Lower cortisol and higher ACTH levels in individuals with autism. Journal of Autism and Developmental Disorders. 2003;33:443–448. doi: 10.1023/a:1025019030121. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in child care. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- DiLalla DL, Rogers SJ. Domains of the childhood autism rating scale: Revelance for diagnosis and treatment. Journal of Autism and Developmental Disorders. 1994;24:115–128. doi: 10.1007/BF02172092. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: Genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MT, Malozowski S, Winterer J, Vamvakopoulos NC, Chrousos GP. Urinary free cortisol values in normal children and adolescents. The Journal of Pediatrics. 1991;118:256–258. doi: 10.1016/s0022-3476(05)80496-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Hormones and Behavior. 2002;41:328–333. doi: 10.1006/hbeh.2002.1766. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Hahn WE, Van Ness J, Chaudhar N. Overview of the molecular genetics of mouse brain. Molecular Genetic Neuroscience. 1992:332. [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. Journal of Pharmacology and Experimental Therapeutics. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hill SD, Wagner EA, Shedlarski JG, Sears SP. Diurnal cortisol and temperature variation of normal and autistic children. Developmental Psychobiology. 1977;10:579–583. doi: 10.1002/dev.420100612. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoyama F, Watanabe M, Murata S, Kaneko M, Kumashiro H. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Japanese Journal of Psychiatry and Neurology. 1987;41:227–235. doi: 10.1111/j.1440-1819.1987.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, van der Gaag R-J. Differentiation between Autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28:582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Wiegant VM, Westenberg HGM, Lahuis B, van Engeland H. Autonimic and neuroendocrine responses to a psychosocial stressor in adults with Autistic Spectrum Disorder. Journal of Autism and Developmental Disorders. 2006;36:891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Olariu A, Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends in Neuroscience. 2005;28:171–172. doi: 10.1016/j.tins.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: A review of the literature. Research in Developmental Disabilities. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lopata C, Volker MA, Putnam SK, Thomeer ML, Nida RE. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning Autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(10):1866–1877. doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S. A standard measure of social and communication deficits associated with the spectrum of Autism. The Autism Diagnostic Observation Schedule- Generic. 2000;30:205–223. [PubMed] [Google Scholar]
- Maher KR, Harper JF, Macleay A, King MG. Peculiarities in the endocrine response to insulin stress in early infantile Autism. Journal of Nervous and Mental Disease. 1975;161:180–184. doi: 10.1097/00005053-197509000-00005. [DOI] [PubMed] [Google Scholar]
- Naber FB, Swinkels SH, Buitelaar JK, Bakermans-Kranenburg MJ, van IJzendoorn MH, Dietz C, et al. Attachment in toddlers with autism and other developmental disorders. Journal of Autism and Developmental Disorders. 2007;37(6):1123–1138. doi: 10.1007/s10803-006-0255-2. [DOI] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders. 1995;25:641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Rice C. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network. 2009 CDC: MMWR Surveillance Summaries, 58, 1–20. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5810a1.htm.
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with Autism. Journal of Autism and Developmental Disorders. 1992;22:433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R, DeVellis R. Toward objective classification of childhood autism: Childhood autism rating scale (CARS). Journal of Autism and Developmental Disorders. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol. Journal of Child Psychology and Psychiatry. 1997;38:705–715. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Saito Y, Okada F, Fujieda T, Yamashita I. An application of neuroendocrinological studies in autistic children and Heller's syndrome. Journal of Autism and Childhood Schizophrenia. 1975;5:323–332. doi: 10.1007/BF01540679. [DOI] [PubMed] [Google Scholar]
- Zinke K, Fries E, Kliegel M, Kirschbaum C, Dettenborn L. Children with high-functioning autism show a normal cortisol awakening response (CAR). Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.03.009. Epub April 19 2010. [DOI] [PubMed] [Google Scholar]