Abstract
Metabolism-based therapy has been used successfully in the treatment of seizures but study of its use in other neurodegenerative disorders is growing. Data demonstrating the use of different forms of metabolism-based therapy in human trials of Alzheimer disease and Parkinson disease are discussed. Animal and in vitro studies have shed light on metabolism-based therapy’s mechanisms in these diseases, as well as ALS, aging, ischemia, trauma and mitochondrial cytopathies. Additional insights may be obtained by considering the role of metabolism-based therapy in cell disability and death (specifically apoptosis, excitotoxicity, and autophagy).
Keywords: ketogenic diet, calorie restriction, epilepsy, Alzheimer disease, Parkinson disease, neuroprotection
1. Introduction
‘When I use a word,’ Humpty Dumpty said, in a rather scornful tone,’ it means just what I choose it to mean, neither more nor less.’
“The question is,” said Alice, “whether you can make words mean so many different things.”
(Through the Looking-Glass – Chapter Six)
One common theme in medicine is that treatments used for one indication eventually are used in other diseases. Metabolism-based therapy (Table 1) was historically implemented for epilepsy but its use now has been demonstrated in a variety of neurological illnesses. Metabolism-based therapy’s effects in epilepsy may result from a variety of mechanisms. For example, the ketogenic diet, the most widely implemented form of metabolism-based therapy in neurology, may have either anticonvulsant or anti-epileptic mechanisms in epilepsy. However, most studies have not been designed to distinguish between these mechanisms. This distinction becomes more important when illnesses other than epilepsy are considered. To facilitate this distinction, and for the sake of clarity, definitions used here:
Table 1.
Metabolism-based therapies for epilepsy and other neurological disorders (selected list from human and animal studies)
| Classical ketogenic diet |
| MCT ketogenic diet |
| Ketone body supplementation |
| Polyunsaturated fat supplementation |
| Fenofibrate (peroxisome proliferator-activated receptor-alpha agonist) |
| Modified Atkins diet |
| Low Glycemic Index Treatment |
| Calorie restriction (including intermittent fasting) |
| 2-deoxy-D-glucose |
| Fructose-1,6-bisphosphate |
| Triheptanoin oil |
| Rapamycin |
Anticonvulsant: prevents the occurrence of acute seizures.
Antiepileptic therapy: suppresses or prevents epilepsy (i.e., the disease process that predisposes to recurrent unprovoked seizures).
Neuroprotectant: protects neurons from injury or degeneration.
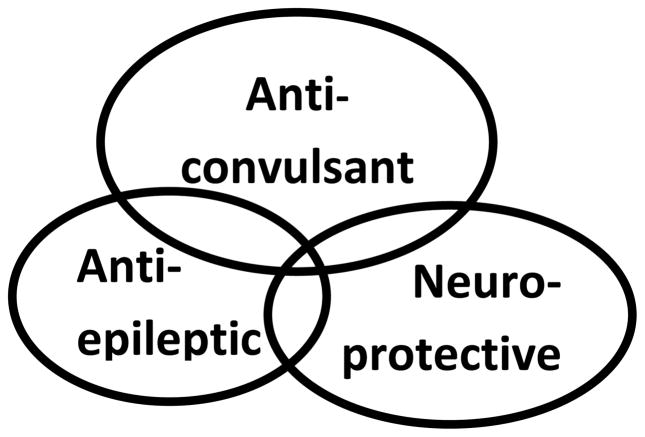
These three terms frequently commonly are used interchangeably but from a mechanistic perspective, may share only limited overlap (Fig. 1).
Figure 1.
There is some, but not complete, overlap between neuroprotection, antiepileptic agents, and anticonvulsant therapy.
1.1 Background
The ketogenic diet is the one of the oldest forms of metabolism-based anticonvulsant therapy (Hartman and Vining, 2007). However, forms of metabolism-based anticonvulsant therapy have expanded significantly, encompassing other dietary regimens, including calorie restriction (perhaps the oldest and most widely-implemented of all) (Greene et al., 2001), the modified Atkins Diet (Kossoff et al., 2003), and the Low Glycemic Index Treatment (Pfeifer and Thiele, 2005). Metabolism-based therapy also encompasses pharmacological treatments such as 2-deoxy-D-glucose (Stafstrom et al., 2009), fructose-1,6,-bisphosphate (Lian et al., 2007), fenofibrate (Porta et al., 2009), and triheptanoin oil (Willis et al., 2010). These forms of metabolism-based therapy have anticonvulsant mechanisms of action that are distinct from commercially available medicines. The longstanding belief that metabolism-based therapies shares anticonvulsant mechanisms with one another also has been challenged (Hartman et al., 2010). Thus, they will be discussed separately here to facilitate comparisons and contrasts (data on polyunsaturated fats have been discussed extensively in the literature, so they will not be considered in detail here). The perspective emphasized here will be on neuroprotection, rather than anticonvulsant and antiepileptic effects.
2. Epilepsy
2.1 Human studies: Supportive data
The ketogenic diet has been used for nearly 90 years as a treatment for seizures (Bailey et al., 2005). Numerous case series demonstrated its efficacy but its efficacy was further demonstrated in a randomized trial (Neal et al., 2008). The possibility of a more persistent beneficial effect was raised by a retrospective survey of children treated with a ketogenic diet showing that 13% of 150 children with medically intractable epilepsy (having failed to respond to an average of 6–7 medicines) were seizure free 3–6 years after initiation of the diet – and only one of the children was still consuming the diet (Hemingway et al., 2001). In this series, 29 children were not taking any anticonvulsants and an additional 28 were taking only one medicine. One interpretation is that these data support a disease-modifying or neuroprotective effect. A ketogenic diet also was successful in treating status epilepticus in two adults in status epilepticus (Wusthoff et al., 2010). A ketogenic diet stopped seizures in seven of nine patients with FIRES (fever induced refractory epileptic encephalopathy in school age children) (Nabbout et al., 2010). The diet was stopped in one of the seven patients, who then relapsed into status epilepticus and died. However, remissions have been reported after medication use as well, suggesting that a ketogenic diet simply may have been the ‘right’ anticonvulsant for these particular patients.
2.2 Human studies: Conflicting data
Not all the data for the ketogenic diet in epilepsy have been positive. A ketogenic diet was associated with resolution of epileptiform activity in medically refractory continuous spike waves of sleep (CSWS) in only one of five children after two years of treatment (one additional child had a 20% improvement in spike-wave index) (Nikanorova et al., 2009). There were no changes in IQ or neuropsychological testing in this group but improved attention and behavior was noted in 2 patients. Thus, a ketogenic diet is anticonvulsant in some, but not all, cases (the same can be said for commercially available anticonvulsants, as well). In the FIRES series, the six surviving patients all eventually developed epilepsy, providing some evidence against a ketogenic diet having long-term disease-modifying effects in all cases, even when it has provided a significant anticonvulsant effect.
2.3 Animal data
Data from animal models of epilepsy have been remarkably similar to human studies. One model of a mixed genetic-environmental epilepsy, the EL mouse, stopped having seizures after administration of a ketogenic diet (Todorova et al., 2000). However, this effect was transient, lasting only four weeks. The mechanism for decreased seizure protection was believed to be loss of ketosis and increasing blood glucose levels. Similarly, protection against electrical kindling-induced seizures has been reported but this effect was only transient (Hori et al., 1997). A ketogenic diet’s protective effects after exposure to chemoconvulsants appears to be more consistent, including spontaneous recurrent seizures after kainic acid-induced status epilepticus (Muller-Schwarze et al., 1999) and clonic convulsions after kindling with pentylenetetrazol (Hansen et al., 2009).
2.4 Summary
The ketogenic diet’s utility in patients with medically refractory epilepsy suggests its mechanism of action is distinct from medicines currently in clinical use, making it a valuable component of our armamentarium. Whether a ketogenic diet has disease-modifying properties is less clear. Data in both humans and animals suggest its anticonvulsant properties may be stronger than its ability to prevent the development of epilepsy. Other forms of metabolism-based therapy have not been studied extensively in long term studies that would shed light on anti-epileptic and disease-modifying effects in epilepsy.
3. Other Neurological Illnesses – Human and Animal Models
3.1 Alzheimer disease (AD)
A number of metabolic derangements have been associated with AD, including β-amyloid-induced toxicity, glucose dysregulation (Sims-Robinson et al., 2010), and abnormal glycogen synthase kinase activity (Baum et al., 1996). Therefore, metabolism-based therapy may have the potential to prevent progression of the pathology associated with AD.
3.1.1 Ketogenic diets and ketone bodies: mixed results
A randomized, double-blind clinical placebo-controlled study of a medium chain triglyceride preparation showed a significant improvement in standardized tests of cognitive function in patients with mild to moderate AD lacking the APOε4 genotype, providing Class I evidence for the efficacy of this approach (Henderson et al., 2009). Patients with the APOε4 genotype did not benefit, however. A medium chain triglyceride diet also improved mitochondrial respiration but there was only a trend toward decreased Aβ 40 & 42 levels in aged beagles (Studzinski et al., 2008). These data provide in vivo support for prior in vitro work showing that BHB protects against Aβ 42-induced toxicity in cultured hippocampal neurons (Kashiwaya et al., 2000). Rodent trials have documented efficacy of a ketogenic diet in decreasing amounts of β–amyloid deposition, although performance in a novel object recognition test was not improved (Van Der Auwera et al., 2005).
3.1.2 Calorie restriction
Interestingly, calorie restriction also decreased β–amyloid deposition (Qin et al., 2006). The effect of calorie restriction may involve SIRT1, a sirtuin that appears to be responsible for extending lifespan in lower organisms (Qin et al., 2006). Exploratory behavior was better in a triple-transgenic murine model of AD after a 40% calorie restriction diet or an intermittent fasting paradigm (started at 3 months of age, continued until 10 or 17 months), compared to an ad lib control diet (Halagappa et al., 2007). Complications and adverse reactions to calorie restriction in rodent models also has been reported (reviewed in (Maalouf et al., 2009), indicating the need for caution when translating these studies into human patients. Differences in behavior paradigms between a ketogenic diet and forms of calorie restriction highlight the need to consider these treatments separately and provide an opportunity for further study.
3.2 Parkinson disease (PD)
3.2.1 Human data
PD is characterized by loss of dopaminergic neurons in the substantia nigra pars compacta. A ketogenic diet was associated with an improvement in Unified Parkinson’s Disease Rating Scales in five adults (Vanitallie et al., 2005). One potential explanation however, is that the change in diet led to changes in levodopa absorption (Jabre and Bejjani, 2006). One patient in the series required a decrease in her carbidopa/levodpa dose because of increased dyskinesias.
3.2.2 Animal data
Animal data for models of PD provide more direct evidence for a neuroprotective effect of ketone bodies. Treatment with β–hydroxybutyrate (BHB), one of the ketone bodies that is elevated during a ketogenic diet, was associated with decreased neuronal loss in the substantia nigra pars compacta after treatment with the mitochondrial electron transport chain complex I toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a commonly used model of PD (Tieu et al., 2003). In addition, oxygen consumption in mitochondria isolated from MPP+-treated brain tissue was increased after incubation with BHB, indicating improved mitochondrial viability. BHB protection was eliminated by treatment with 3-nitropropionic acid, a succinate dehydrogenase toxin, suggesting that BHB’s effect requires intact complex II (succinate dehydrogenase is part of complex II). These data provide in vivo support for prior in vitro work showing that BHB protects against MPP+-induced toxicity in cultured mesencephalic neurons (Kashiwaya et al., 2000). Pretreatment for six months with a 30% calorie restriction diet also led to improved movement distance and speed in a cohort of rhesus monkeys after hemiparkinsonism induced by unilateral carotid artery injection of MPTP (Maswood et al., 2004). This improvement was associated with increased glia-derived neurotrphic factor (GDNF) in the ipsilateral striatum. No changes were noted in brain-derived neurotrophic factor (BDNF). In summary, the MPTP model of PD has demonstrated the role of BHB and calorie restriction in protecting against a complex I toxin, although the clinical relevance is still somewhat unclear.
4. Neurological illnesses modeled in rodents
Some neurological illnesses have been studied in rodents but not humans. In some, human trials are underway.
4.1 Amyotrophic lateral sclerosis (ALS)
A ketogenic diet preserved motor neuron counts in a transgenic mouse model of ALS (SOD1-G93A), with a significant prolongation of time to failure in the Rotarod test (Zhao et al., 2006). Mitochondria isolated from the brains of these mice, after exposure to BHB, showed increased ATP levels and rates of ATP synthesis. They also were more resistant to a challenge with rotenone (complex I inhibitor) than malonate (a complex II inhibitor), implying that improvements in mitochondrial function require functioning complex II, similar to what was noted above (section 3.2). There is a clinical trial underway to evaluate the effectiveness of a ketogenic diet in patients with ALS (Clinicaltrials.gov identifier: NCT01035710).
4.2 Aging
4.2.1 Ketogenic diets
In late-adult rats, medium chain triglyceride diet (10 and 20% MCT) exposure for 8 weeks was associated with an increased density of synapses and synaptic mitochondria in neurons in the outer molecular layer of the dentate gyrus (an ‘anti-aging’ effect), but in contrast, these parameters were decreased in neurons from the stratum molecular of the CA1 region (an ‘accelerated aging’ effect) (Balietti et al., 2008). After exposure to the 20% MCT diet, these rats showed an increase in numerical density of succinate dehydrogenase-positive mitochondria in Purkinje cells from the cerebellar vermis (an area that shows age-related degeneration) (Balietti et al., 2010). Conflicting findings between neurons in various regions suggest that metabolism-based therapy has different effects that may be specific for a given cell type. These data provide further support (but do not prove) the hypothesis that ketone bodies exert their effects through an effect on complex II (see section 4.1). Using a different model, a ketogenic diet was associated with improvements in an object recognition test and T-maze in aged rats, although the specific mechanism of neurodegeneration in that model is unknown (Xu et al., 2010).
4.2.2 Calorie restriction
A 30% calorie restriction for 20 years leads to a decrease in age-related neurodegeneration in rhesus monkeys, evidenced by increased volumes of gray matter in subcortical structures (including the putamen and cingulate gyrus), although changes were not noted in frontal and temporal cortex (the latter two areas show volume loss in aging humans) (Colman et al., 2009).
4.3 Post-ischemia models
4.3.1 Brain Ischemia
A number of metabolic pathways are activated after prolonged ischemia (Semenza, 2007). Adolescent rats pretreated for 25 days with a ketogenic diet showed less degeneration of neurons in the CA1 field of the hippocampus, Purkinje cells in the cerebellum, and thalamic reticular nucleus after global cerebral ischemia induced by cardiac arrest after cardiac vessel occlusion (in contrast, levetiracetam did not protect these neurons in this model) (Tai et al., 2008). Protection against focal ischemia (measured by infarct volume) after middle cerebral artery occlusion in young adult rats was noted after treatment with either a ketogenic diet (pretreatment for 3 weeks) or intracerebroventricular BHB infusion (pretreatment for 4 days) (Puchowicz et al., 2008). Elevated levels of the anti-apoptosis protein Bcl-2 were noted in rats treated with either regimen, compared to controls. Acetoacetate pretreatment and BHB post-treatment (but not pretreatment) protect against post-stroke ischemia in rats (Massieu et al., 2001, Suzuki et al., 2002). In a different model, calorie restriction and intermittent fasting decreased the volume of infracted tissue, as well (Marie et al., 1990). Further study of these treatments after (rather than before) induction of strokes would increase the translational utility of this approach.
4.3.2 Heart ischemia
A ketogenic diet also may provide benefit in heart muscle after ischemia. Hearts from adult rats on a ketogenic diet for 19 weeks were subjected to an isolated heart perfusion model that was designed to mimic effects of global ischemia (Al-Zaid et al., 2007). Compared to a normal diet, tissue from ketogenic diet-treated rats showed an increase in reperfusion recovery in coronary flow, a persistence of functional recovery, and an increased number of mitochondria.
4.4 Mitochondrial cytopathies
Mitochondria have long been suspected to the primary organelle where ketogenic diets exerts their effects, given their role in metabolism and neuroprotection (Bough and Rho, 2007). The use of ketogenic diets in patients with mitochondrial disorders has been evolving. Initial concerns about deficits in fatty acid metabolism have been replaced by the realization that some patients actually may benefit from a ketogenic diet (Kang et al., 2007). In the laboratory, mice that accumulate mitochondrial DNA mutations with age ( ‘Deletor’ mice) showed decreased numbers of ragged red fibers and decreased muscle mitochondrial structural distortion after treatment with a ketogenic diet (Ahola-Erkkila et al., 2010). These data provide in vivo support for a similar finding in cultured cells (Santra et al., 2004).
4.5 Traumatic brain injury
Use of a ketogenic diet in traumatic brain injury is covered elsewhere in this supplement.
5. Potential mechanisms of neuroprotection
5.1 Overview: cell death and disability
Ultimately, neurprotection is responsible for preservation of cell viability and function. Much of the current work on neuroprotection uses models and paradigms that focus on cell death. Cell death actually represents a spectrum of various mechanisms and morphological subtypes, including necrosis, apoptosis, and autophagy. Growing evidence has demonstrated significant mechanistic overlap between them. Processes that contribute to cell protection via antioxidant effects (e.g., glutathione) are discussed elsewhere in this supplement.
5.2 Apoptosis
Apoptosis is a programmed form of cell death that can be triggered by a number of extracelluar and intracellular stimuli that activate a proteolytic cascade (involving caspases) (Gorman, 2008). In contrast to some forms of cell death that result from energy failure, apoptosis requires ATP to proceed. A balance of pro-death factors (e.g., Bad, Bim, Bax, and Bak) and anti-death factors (e.g., Bcl-2 and Bcl-xL) maintains the balance of cell viability and the need for orderly tissue remodeling (which plays key roles in organ development and maturation). In terms of neurological disorders, blocking apoptosis (e.g., a Bax/Bak conditional double knockout) increases neuron survival in neurological disorders, such as a murine model of ALS (Reyes et al., 2010). A number of apoptosis proteins (including Bad, Bim, and Bax) have roles in cell death after status epilepticus (Niquet and Wasterlain, 2004). The role of these proteins in less severe forms of cellular disability (i.e., intermittent recurrent seizures, rather than status epilepticus) is unknown.
The importance of ‘apoptosis proteins’ in maintaining normal cell function and viability has been increasingly recognized (Cheng et al., 2006). For example, Bcl-xL has a role in cell metabolism and is necessary for synaptic activity (Li et al., 2008), while Bad plays a role in glycolysis (Danial et al., 2003). This raises the possibility that these proteins play roles in both normal cellular activity as well as cell death (Cheng et al., 2006). Thus, studies only examining the role of these proteins in neuronal death could be extended by studying their role during normal cell function as well as periods of cellular disability (i.e., before a death stimulus).
A ketogenic diet appears to decrease morphological changes associated with apoptosis after kainic acid exposure (Noh et al., 2003). One potential neuroprotective effect of a ketogenic diet may be to prevent dissociation of the pro-apoptosis protein Bad from its binding partner 14-3-3 (Noh et al., 2006). Dissociation of Bad from 14-3-3 is one way to activate the apoptosis cascade.
5.3 Excitotoxicity
Excititoxicity results from overexposure of cells to the excitatory neurotransmitter glutamate and has mechanistic features of both necrosis and apoptosis (Ankarcrona et al., 1995). Reactive oxygen and nitrogen species play an important role in this type of cell death and are discussed elsewhere in this supplement. Ligands such as NMDA, AMPA, and kainic acid bind different types of glutamate receptors and activate similar cascades (Lau and Tymianski, 2010). Studies examining the effect of a ketogenic diet on kainic acid-induced cell death have shown different results in different strains of mice (Noh et al., 2003, Samala et al., 2008). Hippocampal slices exposed to NMDA show less cell death after incubation with BHB compared to control media but interestingly, BHB did not improve induced epileptiform-like firing in these preparations (Samoilova et al., 2010). This finding stands in some contrast to other recent data showing that another ketone body produced during a ketogenic diet, acetoacetate, alters chloride transport in vesicular glutamate transporters, which would be predicted to alter glutamate trafficking (Juge et al., 2010). The difference in specific ketone body (i.e., BHB not suppressing epileptiform activity but acetoacetate altering glutamate trafficking) is consistent with animal studies showing that acute treatment with acetoacetate, but not BHB, is anticonvulsant in mice (Rho et al., 2002). Neocortical neurons exposed to BHB and acetoacetate produced less reactive oxygen species after exposure to glutamate (Maalouf et al., 2007). Both intermittent fasting (i.e., every-other day fasting) and the glycolysis inhibitor 2-deoxy-D-glucose pretreatment protect against kainic acid-induced CA3 neuronal death in adult rats (Bruce-Keller et al., 1999, Lee et al., 1999). BDNF may play a neuroprotective role in the intermittent fasting paradigm (contrasted with the lack of BDNF changes in the MPTP model, noted in section 3.2) (Duan et al., 2001). Together, these data suggest a role for ketone bodies, limited caloric intake, and decreased glucose utilization in protection against excitotoxicity, although the effects of BHB and acetoacetate may differ. Growth factors, such as BDNF and GDNF may play different roles in metabolism-based therapies, depending on the paradigm studied.
5.4 Autophagy
5.4.1 Autophagy in neurological disorders
Autophagy (‘self-eating’) is an energy-requiring programmed form of protein and organelle catabolism. Autophagy provides macromolecules for use in times of stress (in part, a recycling system) and is important in cell death, survival under stress, and cell ‘quality control’ (Yang and Klionsky, 2010). Blocking autophagy leads to loss of cortical and cerebellar neurons and accumulation of intraneuronal inclusion bodies containing ubiquitinated proteins (Komatsu et al., 2006). Abnormalities in autophagy also have been demonstrated in neurological disorders that involve accumulation of abnormal proteins, such as AD (Jaeger and Wyss-Coray, 2010).
Lafora disease, one form of progressive myoclonus epilepsy, involves accumulation of cytoplasmic polyglucosan inclusions but it is not clear whether they are the cause or the effect of the disorder (Knecht et al., 2010). Mutations in Laforin, one of the proteins affected in this disease, are associated with decreased autophagy, raising the question of whether correcting this process would improve outcomes (Aguado et al., 2010). A trial in patients with Lafora disease showed a ketogenic diet did not halt progression of illness but four of the five patients had mutations in malin (the other protein mutated in Lafora disease) and patients were studied after the progressive nature of their illness had been established (Cardinali et al., 2006). Utility of a ketogenic diet in patients earlier in their course or in those with laforin mutations is unknown.
5.4.2 Autophagy and mTOR
One link between autophagy and metabolism is the mTOR (mammalian target of rapamycin) pathway (Laplante and Sabatini, 2009). mTOR is sensitive to changes in glucose and protein levels and has an effect on protein synthesis, lipid metabolism, and lipid synthesis (Laplante and Sabatini, 2009). Decreases in mTOR activity lead to increased autophagy. Laforin’s activity in the autophagy pathway (noted above) is mediated via mTOR (Aguado et al., 2010). Further demonstrating the importance of mTOR in neurological disease, patients with tuberous sclerosis complex (TSC) have mutations in the mTOR pathway and treatment with the mTOR inhibitor rapamycin decreases seizure frequency in patients with TSC (Orlova and Crino, 2010, Krueger et al., 2010). Independent of its effect in TSC, rapamycin also decreases spontaneuous recurrent seizures after kainic acid-induced status epilepticus, suggesting an additional role for mTOR inhibition in decreasing epileptogenesis (Zeng et al., 2009). Rapamycin improves spatial memory and decreases Aβ 42 levels in a transgenic mouse model of AD (PDAPP), with a concomitant increase in neuronal autophagy (Spilman et al., 2010). Rapamycin also decreases the extent of brain damage in a neonatal rat hypoxia-ischemia model, with a concomitant increase in autophagy (Carloni et al., 2010). Together, these findings suggest a neuroprotective role for the nutrient signal-integrating mTOR pathway.
6. Common themes in different neuroprotection models
In reviewing the literature, some common themes emerge regarding the neuroprotective effects of metabolism-based therapies. Because of the convergence of metabolic processes and neuroprotection, much work has focused on mitochondria. Mitochondrial oxygen consumption and increased ATP synthesis have been noted after BHB exposure and these effects appear to be mediated via a complex II-dependent mechanism in some models described here. Although this points to ketone bodies enhancing mitochondrial function in neurons, there are data showing cell-specific changes in synaptic mitochondrial density (with both increases and decreases) after medium chain triglyceride diets in aged rodents, indicating the effect of ketone bodies may be restricted to a subset of neurons. Other measures of mitochondrial parameters have shown changes after exposure to a ketogenic diet, as well. Studies of a ketogenic diet in SSADH-deficient mice showed an increase in mitochondrial area (a surrogate measure of mitochondrial biomass) in the somata of pyramidal cells from CA1 hippocampal neurons (Nylen et al., 2009). Nonepileptic rats on a ketogenic diet had increased numbers of transcripts for many mitochondrial proteins and increased mitochondrial fragmentation in dentate gyrus neurons (Bough et al., 2006). How these parameters translate into a neuroprotective effect is unclear. Increased ATP levels may make a compromised neuron more ‘resistant’ to an electrical insult but an alternative explanation is that adenosine (a product of ATP metabolism) may, by itself, exert neuroprotective effects (Masino et al., 2009). Neuroprotective effects of a ketogenic diet and BHB at the mitochondrial level also may be mediated by protective factors such as Bcl-2 and other apoptosis-related proteins, or by relieving oxidative stress (discussed elsewhere in this supplement).
Other potential neuroprotective mechanisms of metabolism-based therapy may include growth factors (e.g., BDNF or GDNF) or sirtuins, as discussed previously. There is a handful of preclinical studies pointing to the beneficial effects of mTOR inhibition in neurodegenerative disorders. Finally, the ketone body acetoacetate may modulate glutamate trafficking, providing a potential common link between some forms of metabolism-based therapy and many types of neurodegenerative disorders.
7. Conclusions
Metabolism-based therapy encompasses a number of different specific interventions (Table 1). Metabolism-based therapies have been used in epilepsy and their application is being actively investigated in a number of other neurodegenerative disorders (Table 2). As some of these interventions involve lifestyle changes and in order to optimize the population of patients that might benefit most from them, there has been a significant effort to unravel their neuroprotective mechanisms. In order to characterize specific effects of metabolism-based therapies, future studies would benefit from designs that distinguish between anticonvulsant, antiepileptic, and neuroprotective mechanisms.
Table 2.
Studies of metabolism-based therapies in neurodegenerative diseases
| Human | Animal &/or in vitro | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | AD | PD | AD | PD | ALS | Aging | Post-ischemia | Mitochondrial Cytopathy |
| ‘Classical’ ketogenic diet | X | X | X | X | X | X | ||
| MCT | X | X | ||||||
| Ketone bodies | X | X | X | X | X | |||
| CR | X | X | ||||||
Abbreviations: AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; CR, calorie restriction; MCT, medium chain triglyceride; PD, Parkinson disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Aguado C, Sarkar S, Korolchuk VI, Criado O, Vernia S, Boya P, Sanz P, De Cordoba SR, Knecht E, Rubinsztein DC. Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum Mol Genet. 2010;19:2867–76. doi: 10.1093/hmg/ddq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola-Erkkila S, Carroll CJ, Peltola-Mjosund K, Tulkki V, Mattila I, Seppanen-Laakso T, Oresic M, Tyynismaa H, Suomalainen A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet. 2010;19:1974–84. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- Al-Zaid NS, Dashti HM, Mathew TC, Juggi JS. Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischaemia. Acta Cardiol. 2007;62:381–9. doi: 10.2143/AC.62.4.2022282. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–73. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Bailey EE, Pfeifer HH, Thiele EA. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005;6:4–8. doi: 10.1016/j.yebeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, Bertoni-Freddari C, Aicardi G, Lattanzio F, Fattoretti P. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron. 2010;41:143–8. doi: 10.1016/j.micron.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Balietti M, Giorgetti B, Fattoretti P, Grossi Y, Di Stefano G, Casoli T, Platano D, Solazzi M, Orlando F, Aicardi G, Bertoni-Freddari C. Ketogenic diets cause opposing changes in synaptic morphology in CA1 hippocampus and dentate gyrus of late-adult rats. Rejuvenation Res. 2008;11:631–40. doi: 10.1089/rej.2007.0650. [DOI] [PubMed] [Google Scholar]
- Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol Chem Neuropathol. 1996;29:253–61. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, Mcfall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Cardinali S, Canafoglia L, Bertoli S, Franceschetti S, Lanzi G, Tagliabue A, Veggiotti P. A pilot study of a ketogenic diet in patients with Lafora body disease. Epilepsy Res. 2006;69:129–34. doi: 10.1016/j.eplepsyres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–77. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Cheng WC, Berman SB, Ivanovska I, Jonas EA, Lee SJ, Chen Y, Kaczmarek LK, Pineda F, Hardwick JM. Mitochondrial factors with dual roles in death and survival. Oncogene. 2006;25:4697–705. doi: 10.1038/sj.onc.1209596. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–6. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–26. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12:2263–80. doi: 10.1111/j.1582-4934.2008.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, Mcgowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–8. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–20. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Nielsen AH, Knudsen KE, Artmann A, Petersen G, Kristiansen U, Hansen SH, Hansen HS. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem Int. 2009;54:199–204. doi: 10.1016/j.neuint.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108:898–905. doi: 10.1542/peds.108.4.898. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–8. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Jabre MG, Bejjani BP. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2006;66:617. doi: 10.1212/01.wnl.0000216108.57529.b1. author reply 617. [DOI] [PubMed] [Google Scholar]
- Jaeger PA, Wyss-Coray T. Beclin 1 complex in autophagy and Alzheimer disease. Arch Neurol. 2010;67:1181–4. doi: 10.1001/archneurol.2010.258. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–8. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:5440–4. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht E, Aguado C, Sarkar S, Korolchuk VI, Criado-Garcia O, Vernia S, Boya P, Sanz P, Rodriguez De Cordoba S, Rubinsztein DC. Impaired autophagy in Lafora disease. Autophagy. 2010;6:991–3. doi: 10.4161/auto.6.7.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Krauss GL, Mcgrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–91. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–42. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, Mcnay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–74. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XY, Khan FA, Stringer JL. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27:12007–11. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–64. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Bralet AM, Gueldry S, Bralet J. Fasting prior to transient cerebral ischemia reduces delayed neuronal necrosis. Metab Brain Dis. 1990;5:65–75. doi: 10.1007/BF01001047. [DOI] [PubMed] [Google Scholar]
- Masino SA, Kawamura M, Wasser CA, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7:257–68. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massieu L, Del Rio P, Montiel T. Neurotoxicity of glutamate uptake inhibition in vivo: correlation with succinate dehydrogenase activity and prevention by energy substrates. Neuroscience. 2001;106:669–77. doi: 10.1016/s0306-4522(01)00323-2. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:18171–6. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999;10:1517–22. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V, Aberastury M, Silva W, Dulac O. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–7. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Nikanorova M, Miranda MJ, Atkins M, Sahlholdt L. Ketogenic diet in the treatment of refractory continuous spikes and waves during slow sleep. Epilepsia. 2009;50:1127–31. doi: 10.1111/j.1528-1167.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Niquet J, Wasterlain CG. Bim, Bad, and Bax: a deadly combination in epileptic seizures. J Clin Invest. 2004;113:960–2. doi: 10.1172/JCI21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Kim YH, Han JY, Park CH, Kang AK, Shin HS, Kang SS, Cho GJ, Choi WS. Ketogenic diet protects the hippocampus from kainic acid toxicity by inhibiting the dissociation of bad from 14-3-3. J Neurosci Res. 2006;84:1829–36. doi: 10.1002/jnr.21057. [DOI] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–28. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead OC., 3rd The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(−/−) mice. Biochim Biophys Acta. 2009;1790:208–12. doi: 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65:1810–2. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- Porta N, Vallee L, Lecointe C, Bouchaert E, Staels B, Bordet R, Auvin S. Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia. 2009;50:943–8. doi: 10.1111/j.1528-1167.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, Lamanna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–16. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, Macgrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–54. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Reyes NA, Fisher JK, Austgen K, Vandenberg S, Huang EJ, Oakes SA. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J Clin Invest. 2010;120:3673–9. doi: 10.1172/JCI42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Anderson GD, Donevan SD, White HS. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43:358–61. doi: 10.1046/j.1528-1157.2002.47901.x. [DOI] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–27. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Samoilova M, Weisspapir M, Abdelmalik P, Velumian AA, Carlen PL. Chronic in vitro ketosis is neuroprotective but not anti-convulsant. J Neurochem. 2010;113:826–35. doi: 10.1111/j.1471-4159.2010.06645.x. [DOI] [PubMed] [Google Scholar]
- Santra S, Gilkerson RW, Davidson M, Schon EA. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol. 2004;56:662–9. doi: 10.1002/ana.20240. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6:551–9. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–47. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, Mackay WA, Beckett TL, Henderson ST, Murphy MP, Sullivan PG, Burnham WM. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-beta precursor protein (APP) levels in the aged dog. Brain Res. 2008;1226:209–17. doi: 10.1016/j.brainres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Beta-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn J Pharmacol. 2002;89:36–43. doi: 10.1254/jjp.89.36. [DOI] [PubMed] [Google Scholar]
- Tai KK, Nguyen N, Pham L, Truong DD. Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm. 2008;115:1011–7. doi: 10.1007/s00702-008-0050-7. [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–40. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Van Der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab (Lond) 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–30. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- Willis S, Stoll J, Sweetman L, Borges K. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis. 2010;40:565–72. doi: 10.1016/j.nbd.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51:1083–5. doi: 10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- Xu K, Sun X, Eroku BO, Tsipis CP, Puchowicz MA, Lamanna JC. Diet-induced ketosis improves cognitive performance in aged rats. Adv Exp Med Biol. 2010;662:71–5. doi: 10.1007/978-1-4419-1241-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, Macgrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]