1.0. Introduction
The field of nanotechnology has emerged as an important and commercially viable technology with wide-ranging applications [1-7]. Nanotechnology uses particles of 1 billionth of a meter in size (equivalent to 7 hydrogen atoms placed side by side) [6]. Nanoparticles are typically sized between 1 and 100 nanometers. Particles of this size have high surface to volume ratios and can be used for delivering drugs, imaging agents, and penetrate areas inaccessible to larger molecules [1-7]. Apart from medicine, nanomaterials have also been used for applications as diverse as sunscreen blockers, cosmetic stabalizers and enhancers, electronics and sporting goods. While nanoparticles can perform a wide variety of functions, there has been increasing concerns and debate amongst the regulatory and scientific community regarding the fate of nanoparticles in biological systems and associated side effects these agents might have on living organisms [8-10].
Nanomedicine is growing its importance due to ability to improve therapeutic efficacy and imaging capabilities. An ideal nanoparticle based cancer therapeutic or imaging agent would: (a) rapidly detect tumor cells; (b) load one to multiple cancer drugs; (c) deliver agents rapidly to cancer cells; and, (d) be capable of measuring treatment efficacy in real time. Nanoparticles offer several advantages for cancer drug delivery compared to classical drugs [3, 6, 11, 12]. Advantages include: (a) prevention of degradation of the package such as siRNA; (b) ability to deliver higher concentrations of a drug by masking to reduce compound toxicity; (c) targeted delivery of the drug; and, (d) time dependent release of encapsulated agents [13, 14]. This review focuses on natural and synthetic nanoparticles used for drug delivery and imaging, emphasizing the toxicological aspects of each to consider prior to selection for particular applications. Methods of assessing toxicity are addressed, focusing on current limitations. Finally, major unanswered questions regarding nanotoxicity are delineated.
2.0. Nanotechnology Based Drug Delivery Systems
Nanotechnology based drugs, also know as nanodrugs, are nanosized materials made-up of natural or synthetic polymers. Nanodrugs have been developed and tested for diagnostic and therapeutic applications including detection as well as prevention or treatment of various cancer types [15]. Additional applications of nanotechnology-based drugs include use in biotechnology, healthcare, pharmaceuticals, drug delivery and skincare [2, 3, 11]. Natural and synthetic polymers such as liposomes, dextrans, poly(lactic-co-glycolic acid) (PLGA) and dendrimers have been used to prepare a wide variety of nanocarriers for delivering therapeutic and imaging agents [16-22]. Likewise carbon nanotubes, metal based (gold and silver nanoparticles / nanoshells), metal oxide based (superamagnetic nanoparticles) and semiconductor based nanoparticles such as quantum dots are also used for various clinical applications [2, 3, 23-34] (Table 1). Even though emphasis of this review is to evaluate the efficacy and safety of nanotechnology based drugs for detection and treatment of cancers, efforts also have been made to address physicochemical properties causing toxicity and methods for determining nanoparticle associated toxicity (Figure 1). The physicochemical properties of nanoparticles are due to highly reactive surfaces, chemical composition, solubility, shape, size and aggregation [8].
Table 1. Types of nanoparticle for drug delivery and biomedical applications.
Summary of various nanomaterials such as lipid, polymer, non-metal, metal, metal oxide, and semiconductor based nanoparticles being developed for drug delivery and biomedical applications.
| Nanoparticles | Applications | References |
|---|---|---|
| Lipid based NPs | ||
| Liposomes | SiRNA/DNA/asOND/drug delivery | [14, 24, 35, 36, 39, 41, 199] |
| Polymer basesd NPs | ||
| Dextran | SiRNA/DNA/asOND/drug delivery | [20, 21, 126] |
| Poly(lactic-co-glycolic acid) (PLGA) | Delivery of vaccines, peptides, proteins, micromolecules/imaging/ tissue engineering scaffolds | [12, 78, 79, 87] |
| Dendrimers | Drug delivery/imaging | [26, 199] |
| Non-metal based NPs | ||
| Carbon Nanotubes | DNA/siRNA delivery/thermal ablation | [23, 27] |
| Metal and metal oxide based NPs | ||
| Gold and Silver | Thermal ablation/imaging/antimicrobial drug delivery | [123, 200] |
| Superamagnetic | Magnetic targeting/thermal ablation/MRI Contrast agent | [140] |
| Semiconductor based NPs | ||
| Quantum Dots | Imaging, siRNA delivery, in vitro diagnostics/biosensing | [96, 145, 146, 157] |
Figure 1.
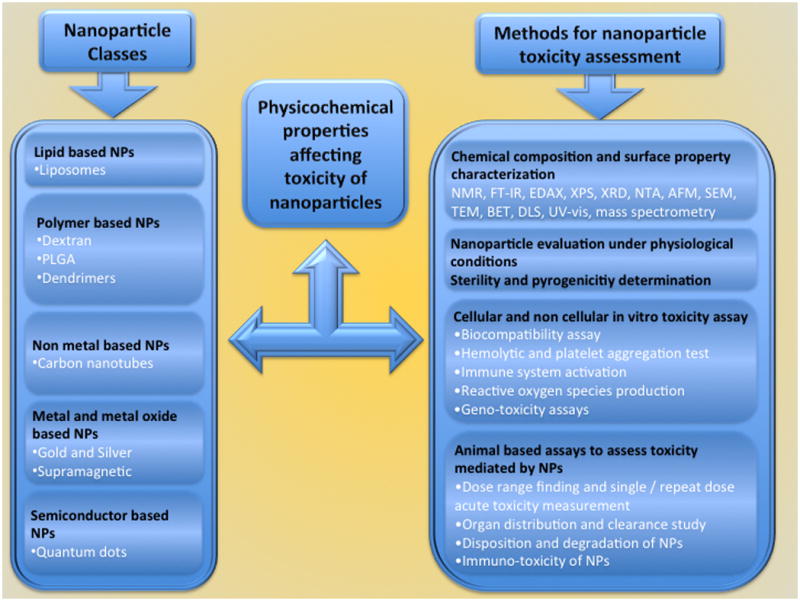
A general overview of nanoparticle classes, physicochemical properties affecting toxicity of nanoparticles and various possible methods employed for the assessment of nanoparticle based toxicity in vitro and in vivo.
2.1. Lipid based nanoparticles
2.1.1. Liposomes
Among several non-biological lipid based carriers, liposome's are widely used as delivery vehicles for carrying agents that inhibit survival of cancers of the kidney, lung, liver, prostate and skin [35]. Bangham and his co-workers in 1965 first prepared liposomes, which were called bangosomes containing entrapped solutes [14, 36]. Liposomes have several advantage over many other nanodelivery systems by being less toxic and having a high therapeutic index [37]. Liposomes are a lipid bilayer composed of amphipathic phospholipids consisting primarily of phosphotidyl cholines (PC, also called lecithins) that enclose an interior aqueous space [14, 38]. Structurally a PC molecule is made up of a head group, glycerol backbone and fatty acyl chains. The head groups of PCs are usually modified to introduce functional groups such as maleimide, which can facilitate conjugation to antibodies or other ligands, and/or polymerizable moieties to generate stable liposomes [39]. The glycerol backbone of PCs can be modified by converting carbonyl ester bonds into either ether or carbamyl esters, which help increase stability and in vivo circulation times [14]. Recent liposome preparations have included PEGylated lipids to bypass the reticulo-endothelial system and promote accumulation in tumors [40]. Based on net charge, liposomes are categorized into cationic, anionic and neutral nanoparticles [37, 39]. Clinical applications of liposomes and toxicological limitations associated with each category are discussed in the paragraphs below.
2.1.1.1. Cationic liposomes
Felgner and his co-workers were the first to prepare cationic liposomes in a formulation called Lipofectin, which was the prototype cationic liposome for delivering DNA into mammalian cells [39, 41]. Lipofectin, made by sonicating equal amounts (by weight) of a synthetic cationic lipid N-[1-(2, 3,-dioleyloxyl)propyl]-N,N,N-trimethylammonium chloride (DOT-MA) and a fusogenic lipid dioleyl phosphatidylethanolamine (DOPE), is a 50 to 200 nm liposome used to deliver DNA [39, 41]. In general, cationic liposomes consist of an amphipathic cationic lipid, with or without DOPE. The percentage of DOPE varies from 0 to 50% and functions to assist in releasing DNA by destabilizing the endosome once the liposome is taken into cells [39]. Liposomes without DOPE were found trapped in endosomes and lysosomes, resulting in to low transfection efficiencies [39, 42]. Therefore, DOPE is a critical component that influences transfection efficiency of a liposomal formulation [39]. Mechanistically, cationic liposomes deliver loaded DNA by interacting with negatively charged cells [39]. Following binding to cells, the liposome complex releases DNA into cells. However, recent studies have shown other mechanisms, such as adsorption-mediated endocytosis for cationic liposomal DNA delivery [14].
Cationic liposomes designed to express or lower protein levels of a gene of interest are generally prepared by simply combining positively charged liposomes with DNA or siRNA in order to complex the reagents leading to a structure having an overall positive charge [43]. Cationic liposomes are efficient gene or siRNA delivery vehicles yielding high transfection efficiencies and provide coupling points for conjugating to antibodies, ligands or aptamers [14]. Additional features making cationic liposomes of interest as delivery vehicles are: (a) ease of preparation and transfection procedures; (b) high percentage of nucleic complexing with liposomes; (c) lack of size limitation or packing protein requirements for encapsulating DNA or siRNA; (d) ability to transfect many cell types with high transfection efficiencies; (e) commercial availability; and, (f) lack of immunogenicity, enabling safe and repeated administration [14, 37].
2.1.1.2. Neutral nanoliposomes
One of the most important applications of neutral liposomes is for siRNA delivery [14, 39]. Neutral nanoliposomes are not toxic to normal cells such as fibroblasts or hematopoietic cells, making them potentially clinically useful [18, 44]. Liposomes developed using neutral 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) deliver siRNAs more effectively into tumor cells than cationic liposomes (DOTAP) or using naked siRNA [45]. Intravenous or intraperitoneal injections of DOPC-nanoliposomes incorporating siRNA resulted in substantial knockdown of EphA2, FAK, neuropilin-2, IL-8, Bcl-2 as well as reduction in tumor size in mice [42, 46, 47]. Systemic administration of DOPC nanoliposomes (150 μg / kg body weight, i.v.) containing siRNA targeting EphA2 in combination with paclitaxel (5 mg / kg body weight) inhibited ovarian cancer tumors more effectively compared to non-targeted siRNA or paclitaxel alone [39].
2.1.2. Clinical advantages and toxicological considerations when planning to use liposomes
Liposomes have been used as pharmacological and genetic agent carriers with unique advantages including: (a) protecting drugs or siRNA based therapeutic agents from degradation; (b) targeting to site of action through ligand peptide or antibody conjugation; and, (c) little toxicity or side effects [48]. In addition, since phospholipids used in the preparation of liposomes, such as phosphatidylcholine and phosphatidylethanolamine, also present in natural cell membranes, liposomes are the ideal candidates for preparing biocompatible and biodegradable carriers [18]. Liposomes can enhance half-life of various therapeutic agents [49]. For example, half-life of doxorubicin in blood is ∼5 minutes [50]. However, the elimination half-life is increased to 20 – 30 hours with an area under curve >60 fold when incorporated in liposome [51]. Furthermore, liposomes are known to reduce side effects of many agents by specific delivering the drug to the cancer cell; therefore, toxicity of various drugs by enhanced targeted delivery of cytotoxic drugs to solid tumors [44, 48]. Liposomal doxorubicin, an FDA approved drug has been shown to inhibit both taxane- and platinum-sensitive and resistant recurrent ovarian cancers [52, 53]. In one study it was demonstrated that Doxil, as a single-agent, reduced recurrent ovarian carcinoma with a ∼25% response rate [54]. In another phase II open label study administration of 50 mg/m2, every 4 weeks Doxil yielded partial response in 7 of 34 (20.6%) advanced refractory epithelial ovarian cancer patients [53, 55, 56].
Liposomes do have inherent problems hampering clinical utility, including: (a) low encapsulation efficiency; (b) leakage of water-soluble drugs in the presence of blood components; (c) aggregation and poor storage stability; (d) issues related to reproducibility; (e) difficulties concerning scaling-up for clinical evaluation; and, (f) toxicity [3, 57, 58]. Toxicity can occur primarily due to liposome composition, particle size or charge. Therefore, the lipid composition, formulation and charge of the liposomes should be selected to minimize potential adverse side effects. For example, cationic liposomes can interact with serum proteins, lipoproteins, and the extracellular matrix, leading to aggregation or release of agents that are loaded before reaching the target cells leading to systemic toxicity [46, 59, 60]. The chemical nature of constituents in the cationic liposomes is also a key factor that determines toxicity as they can act as surfactants and cause membrane solubilization, poration and lysis. Cationic liposomes due to the positive charge have been shown to cause liver damage, which would limit repeated dosing with agnets [60, 61]. Previous studies have shown that quaternary amines are more toxic and inhibit PKC (Protein Kinase C) activity (an indicator of transfection efficiency) compared to tertiary amines [39]. Cationic liposomes have also been shown to cause cellular influx and inflammation of lungs through reactive oxygen species induction [62]. Cationic liposomes can also lead to macrophages mediated toxicity following exposure of longer than 3 hours [63]. In conclusion the lipid chosen in a formulation are key when considering liposome use for particular applications. This can affect the toxicological aspects. For topical applications, cationic liposomes are useful but the positive charge can cause liver damage when used systemically over several weeks. Conjugating the cationic liposomes to a targeting moiety can reduce these concerns. For systemic applications a neutral formulation would be best linked to a targeting moiety to promote uptake.
An example of complications associated with liposomes is Doxil [52, 64]. Doxil is an FDA approved antineoplastic / cytotoxic anti-cancer chemotherapeutic agent made-up of liposomal doxorubicin [52, 65]. Doxil is used to treat AIDS-related Kaposi's sarcoma as well as cancers of breast, ovaries, and solid tumors [66, 67]. Doxil is known to cause irritation and induce symptoms such as flushing, shortness of breath, headache, back pain and low blood pressure [68]. Other side effects of Doxil experienced by ∼30% patients include, low blood counts, hand-foot syndrome (Palmar-plantar erythrodysethesia or PPE) including skin rash, swelling, redness, pain and peeling of skin on the palms and soles; and mouth sources [55, 56]. Side effects were prominent with four (10%) grade 4 toxicities, including severe dermatitis, leukopenia, and thrombocytopenia. Ten patients (24.4%) had Grade 3 Palmer Planter erythrodysesthesia [55, 56]. These side effects were attributed to the drug and liposome; therefore, both optimized to minimize toxicity related side effects when creating a nanoparticle for delivering a particular agent.
2.2. Polymer based nanoparticles
2.2.1. Dextrans
Dextrans are glucose polymers used for a wide variety of therapeutic applications. Dextrans are characterized by a very high content of a-1,6-glucopyranosidic linkages (95%) compared to1,3-linkages (5%) [21]. Dextrans of 40–70 kDa molecular weight have been used for delivering pharmaceutical agents as they: (a) are known to form well-defined repetitive chemical motifs; (b) soluble in water; (c) stable under acidic and basic conditions; (d) contain functional groups for derivatization; and, (e) protect drugs from degradation [21]. Furthermore, dextrans can be autoclaved and are amenable to chemical manipulations. Dextrans are made from high molecular weight, polydisperse native dextrans (molecular weight 107 to 108) by partial depolymerization using acid hydrolysis followed by fractionation [21]. Pharmacokinetic behavior of dextrans depends on physico-chemical properties mediated by the hydrophilic and lipophilic nature, molecular size, shape, flexibility and charge of the agents. For example, dextrans <70 KDa are rapidly eliminated one hour after injection, but those between 70 to 250 KDa exhibited prolonged circulation [69].
Dextrans can be conjugated to pharmaceutical agents, hormones, growth factors, enzymes and imaging molecules by irreversible or reversible methods [69]. For example, dextran-based prodrugs containing nicotinic acid, naproxen, aspirin, ketoprofen, ibuprofen, diclofenac and indomethacin were synthesized by esterification for sustained release. Other methods used for conjugating drugs to dextrans include periodate oxidation, carbamate esterification, and cyanogen bromide activation [69]. Conjugation of drugs and enzymes to dextrans improve physico-chemical properties such as solubility and stability; and help delivering drugs at the site of action [21, 69].
2.2.1.1. Clinical advantages and toxicological considerations when planning to use Dextrans
One of the major hurdles hindering development of pharmacological agents for clinic use is lack of effective delivery methods. Therefore, non-toxic drug delivery vehicles for efficient delivery of therapeutic agents are urgently needed [21]. Additionally, dextrans carrying drugs have been developed to enhance the selective action of cytotoxic drugs. Dextran conjugated of sodium phenylacetate (NaPA) inhibits human 1205 Lu melanoma tumor development more effectively than NaPA alone [70]. Similarly, a dicarboxymethyl-dextran conjugate of cisplatin had longer half-life and better colon cancer tumor inhibitory activity than cisplatin alone [71]. Enhanced efficacy of dextran-based drugs is not only due to delayed excretion by the kidneys and reduced toxicity but also due to prolonged plasma circulation time. Doxorubicin conjugated to polymeric dextrans inhibited multidrug-resistant subclone KB-V-1 cell growth more effectively by being less toxic than the free doxorubicin [72]. Furthermore, dextran conjugates synergized with other anti-cancer agents [72]. Flurbiprofe, a nonsteroidal anti-inflammatory drug causes peptic ulcers, gastrointestinal disturbances and GIT bleeding. However, conjugating flurbiprofen with dextrans reduced gastrointestinal related side effects by improving the physico-chemical properties [73]. Dextran sulphate conjugates have also been shown to reduce nephrotoxicity of gentamycin [74].
Side effects such as anaphylaxis, volume overload, pulmonary oedema, cerebral oedema, or platelet dysfunction have been reported for dextran, which can be serious [72, 75]. A rare but serious complication of dextran osmotic effect is acute renal failure [76]. Therefore, patients with history of renal insufficiency, diabetes mellitus, or vascular disorders should not be treated with dextran-based drugs. Generally, we consider these toxicological concerns minor and feel that the advantages of dextran out weigh these issues.
2.2.2. Poly(lactic-co-glycolic acid) (PLGA)
For the past three decades, various synthetic and natural biodegradable polymers have been used for the preparation of nanoparticles for a wide variety of applications. These include therapeutic delivery devices for drugs and imaging agents in biomedical and pharmaceutics, which is due to the excellent biocompatibility, permeability and controllable biodegradability of these agents [77, 78]. Polyamides, poly(amino acids), poly(alkyl-α-cyano acrylates), polyesters, poly orthoesters, polyurethanes and polyacrylamides have been routinely used to prepare device for drug loading [79, 80]. The copolymer poly(lactic-co-glycolic acid) (PLGA) is the most common biodegradable polymers approved as a compatible biomaterial in humans and has been in use since the 1970s [81]. Poly(lactic-co-glycolic acid) is a copolymer synthesized by random ring-opening copolymerization of the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. For the preparation of this copolymer, tin (II) 2-ethylhexanoate, tin (II) alkoxides or aluminum isopropoxide are usually used as catalysts [82]. PLGA undergo hydrolysis of ester linkages in the presence of water resulting in original monomers lactic acid and glycolic acid, which are byproducts of various metabolic pathways in the body [80, 83]. Degradation time of the PLGA and the release kinetics of the active agent can be modulated by varying the ratio of molecular weight and lactide /glycolide. Polymer containing a 50:50 ratio of lactic and glycolic acids is hydrolyzed much faster than those containing higher proportions of either of the two monomers [80, 84]. Since, degradation products are easily metabolized in the body via the Krebs cycle and are easily eliminated, systemic toxicity associated with PLGA based NP for drug delivery or biomaterial application is low [85].
2.2.2.1. Clinical advantages and toxicological considerations when planning to use PLGA
A wide variety of natural and synthetic biodegradable polymers such as PLGA have been investigated for drug targeting or prolonged release of agents [79, 80]. PLGA is FDA-approved elastomeric copolymers for drug delivery due to its biodegradability, biocompatibility, mechanical properties and ease of processing [86, 87]. PLGA has been investigated for drug delivery for management of variety diseases, such as arthritis, diabetes, pain, bowel disease and brain imaging, due to its biodegradability and biodistribution [86, 87]. For example, a commercially available drug delivery device using PLGA is Lupron Depot® for the treatment of advanced prostate cancer. The PLGA based nanoparticle delivery system has been shown to enhance accumulation of diagnostic and therapeutic agents due to increased permeability and retention. In animals, PLGA led to increased deposition of indocyanine green in organs and blood two to ten fold compared to free dye [88]. PLGA is commonly used in the production of a various biomedical and vascular tissue–engineering devices, like grafts, sutures, implants and prosthetic devices [80]. PLGA has also been used to mimic the biological and biomechanical properties of the native vascular tissue [89]. PLGA-based nanoparticles have been used to encapsulate paclitaxel to achieve controlled drug release to the luminal surface and inner part of ePTFE vascular grafts in order to extend the period of paclitaxel release [90]. PLGA nanoparticles have been proven to be safe. Toxicity assays have been undertaken in Balb/C mice, which showed no altertaions in the histopathlogy or tissue damage. However, bio distribution and retention studies, following oral administration of PLGA nanoparticles showed 40% particles accumulation in liver [91]. Thus, in our opinion poly(lactic-co-glycolic acid) based nanoparticles have significant potential to be used for many clinical applications. However, surface modification of the particles should be considered in order to avoid accumulation in the liver.
2.2.3. Dendrimers
Dendrimers are repeatedly branched, roughly large spherical large structures. A dendrimer is typically symmetric around a core, with a spherical three-dimensional morphology [6, 92]. Dendrimers have a core, an inner shell, and an outer shell. Each region can be synthesized having different functionality to regulate properties such as solubility, thermal stability, and attachment of compounds for a variety of different applications [92-94]. Furthermore, the synthetic processes can be controlled to establish the size and number of branches. Due to a small size of ∼10 nm, drug incorporation into dendrimers can be limiting [95, 96]. However, the exterior branches can be used for drug loading [95]. Antibody or ligand conjugation on the surface can be used to enhance targeting of these nanoparticles. Poly (amidoamine), or PAMAM, is the most well known dendrimer. Dendrimers can be used for drug and gene delivery, bacterial cell killing and as sensors [6, 92, 94]. Designing dendrimers for particular applications involves conjugating particular chemical species to the dendrimer surface [97]. This enables use for encapsulation of hydrophobic compounds and for the delivery of anticancer drugs. The physiochemical characteristics of dendrimers, including monodispersity, water solubility, encapsulation ability, and large number of functionalizable peripheral groups, can make these nanaoparticles effective drug delivery vehicles [94].
2.2.3.1. Clinical advantages and toxicological considerations when planning to use dendrimers
Dendrimers are under investigation for the delivery of DNA and anticancer drugs [93]. Usages of amino-terminated PAMAM or PPI dendrimers as non-viral gene transfer agents and enhancing the transfection of DNA by endocytosis has been published [93, 95, 98]. PAMAM dendrimers conjugated with cisplatin exhibited slower release and higher accumulation in solid tumors with minimal toxicity compared to free cisplatin [95, 98]. Likewise, silversalt conjugated PAMAM dendrimers showed antimicrobial activity against various Gram-positive bacteria [99]. Additionally, dendrimers with 4, 8, and 16 terminal ester groups converted to hydroxy-terminated molecules were able to encapsulate benzoic acid and 2,6-dibromo-4-nitrophenol. Dendrimer conjugated via an acid-labile hydrazone linkage with anticancer drug doxorubicin has been shown to reduce cytotoxicity of doxorubicin and was taken up by various cancer cell lines. In another study, anticancer drug 5-fluorouracil encapsulated into G = 4 PAMAM dendrimers with carboxymethyl PEG5000 surface chains resulted in efficient drug loading, and reduced release rate and hemolytic toxicity compared to the non-PEGylated dendrimer [100]. Recently, PAMAM dendrimers have been reported to enhance bioavailability of indomethacin in transdermal delivery applications [101]. Generations of PAMAM dendrimer/poly (styrenesulfonate) (PSS) microcapsules have been reported. These PAMAM/PSS capsules allow the selective encapsulation of a drug into the capsule core and into the dendrimer localized in the shell of the capsule, thereby providing a dual release system of either two different drugs or fast and sustained release of one drug.
Like any nanoparticles, dendrimers can cause toxicity. Dendrimers with positively charged surface groups can destabilize cell membranes and cause cell lysis. One such example is amino-terminated PAMAM dendrimers. Haemolytic effect of these dendrimers, on a solution of rat blood cells and cytotoxicity on human intestinal adenocarcinoma Caco-2 cells [102] has been reported. However, it is our opinion that the plasticity of this technology for chemical modification will enable resolution of any toxicological concerns that might arise. For example, recent studies have shown that amino-terminated PAMAM dendrimers exhibited lower toxicity than the more flexible amino-functionalized linear polymers. The degree of substitution as well as the type of amine functionality seems to be important, with primary amines being more toxic than secondary or tertiary amines [103]. To reduce or overcome cytotoxicity of cationic dendrimers surface groups such as PEG or fatty acids could be added. A partial derivatization using six lipid chains or four PEG chains on a PAMAM, have been shown to lower cytotoxicity [104]. While relatively few toxicity studies have been undertaken in animals, 10 mg/kg of PAMAM dendrimers injection to mice did not appear to cause toxic effects [105]. Hydroxy- or methoxy-terminated dendrimers did not demonstrated acute or long-term toxicity. The non-toxic properties make them promising candidates for drug delivery devices [105]. Thus, our opinion is that dendrimers will be useful clinically for a variety of applications and the plasticity of the molecules makes them amenable to chemical modification that will be useful to resolve toxicological issues.
2.3. Non-metal based nanoparticles
2.3.1. Carbon nanotubes
Carbon nanotubes are molecular-scale tubes of graphitic carbon having high mechanical strength, thermal as well as electrical conductivity and good stiffness as well as flexibility, enabling use for a variety of biomedical applications. Nanotubes have aspect ratios >100, with lengths of several mm and diameters of 0.7 to 1.5 nm for single-walled carbon nanotubes and 2 to 50 nm for multi-walled carbon nanotubes [23]. The simplest carbon nanotube is composed of graphene (a single honeycomb network of carbon atoms). The structure of a nanotube is dependent on the way graphene it is rolled in to a tube. Carbon nanotubes are insoluble in all solvents but solubility can be enhanced by chemical functionalization and by noncovalent supramolecular adsorption [106].
2.3.1.1. Clinical advantages and toxicological considerations when planning to use carbon nanotubes
Carbon nanotubes can be used for cancer therapeutic and diagnostic applications [27, 28, 107]. Due to large surface area, carbon nanotubes can be conjugated with a variety of molecules and loaded with small molecule inhibitors and/or imaging agents for these applications [27, 106]. These agents tend to be fairly safe, dependent on what is loaded into them. Targeted carbon nanotubes conjugated to antibodies have been used to deliver drugs and plasmid DNA for inhibiting cancer [23, 27]. For example, carbon nanotubes loaded with anticancer agents doxorubicin have been used to treat cancer in animals without any toxicity issues related to free doxorubicin [108]. Liu et al., 2008 [107] demonstrated that SWCNTconjugated with paclitaxel suppressed breast tumor growth more effectively than the agent alone [107]. Intravenous injection of 10 mg/kg single-walled carbon nanotubes loaded with doxorubicin enhanced therapeutic efficacy with negligible toxicity compared to free doxorubicin treated mice [109].
Due to the fiber like shape and size of carbon nanotubes, these structures can cause cytotoxicity, inflammation and DNA damage [23, 110-116]. Single and multi-wall carbon nanotubes can induce platelet aggregation, mitochondrial dysfunction, ROS generation, changes in cell morphology in bronchial epithelial cells and keratinocytes, lipid peroxidation and oxidative stress resulting in cell death [111-113]. Studies in animals using high concentrations of nanotubes demonstrated chronic lung inflammation, including foreign-body granuloma formation and interstitial fibrosis leading to toxic effects [114, 117]. These potentially harmful effects can limit the utility of carbon nano-tubes for clinical applications. Therefore, carbon nanotubes can be used for particular applications involving prolonged drug release, but in our opinion, the route of administration should be carefully considered and limited to applications where the physical shape will not lead to problematic side- effects. These nanoparticles would not be recommended for any application involving inhalation into the lungs or applications where they might be filtered through the kidneys.
2.4. Metal and metal oxide based nanoparticles
Metallic nanoparticles contain a metal core, which is usually covered by a shell [3, 118]. The core is made-up of an inorganic metal or metal oxide, while the shell can be either an inorganic material or a metal oxide [3, 118]. Metal oxide nanoparticles can incorporate drugs as well as imaging agents and are biocompatible and stable [3, 118]. Metals present in metallic nanoparticles can interact with fluorophores to increase photo-stability, to enhance fluorescence, and reduce quenching [119, 120]. For example, silver/silica particles have been used to enhance fluorescence imaging and gold/silica nanoparticles have been used to improve optical sensing.
2.4.1. Gold or silver nanoparticles / nanoshells
Metallic colloidal gold and silver nanoparticles can be used for carrying a carrier, encapsulating imaging agents and for delivery nucleic acid based agents [3, 120-124]. Colloidal gold and silver nanoparticles are synthesized in different forms and are commercially available in various size ranges [31, 32, 120, 125, 126]. Structurally, gold nanoparticles consist of a dielectric core of silica, coated with a metallic layer of gold. The thickness of the gold influences whether the particles absorb or scatter light [124-126]. Silver nanoparticles are synthesized by various physical, chemical, and biological methods. Recent biological methods for the synthesis of metal nanoparticles that are safe and ecologically sound for the nanomaterial fabrication compared to conventional physical and chemical methods have been developed [127]. The green synthesis techniques are generally synthetic routes that utilize relatively non-toxic chemicals to synthesize nanomaterials, and include the use of non-toxic solvents such as water, biological extracts, biological systems and microwave-assisted synthesis [127, 128].
2.4.1.1. Clinical advantages and toxicological considerations when planning to use gold or silver nanoparticles / nanoshells
Gold nanoparticles can be used for photothermal ablation therapy, as sensors or photoactive agents for optical imaging, as drug carriers, and as contrast enhancers for computer tomography and X-ray absorbers for cancer therapy [31, 123]. For example, anti-EGFR antibody conjugated gold nanoshells are currently being used for the detection of EGFR expressing cells [30]. No toxicity was associated with photothermal ablation therapy in a mouse model of colon carcinoma following intravenous administration of PEG coated gold nanoshells [121, 124]. Although gold nanoparticles can deliver cancer drugs, this application has been hindered by difficulties associated with preparation of stable and nontoxic forms that can be injected into patients. Gold nanoparticles might cross a mother's placenta thereby cause deleterious toxic effects to the developing fetus [129]. Gold particles can also interact with cellular proteins that could cause free radical induced cell death or modify proteins structure leading to autoimmune related toxicity [130]. Recently, to avoid this problem, gum arabic coated nanoparticles have been tested. These nanoparticles are stable, nontoxic and can be administered orally or by injection [131].
Silver nanoparticles are being commercialized for antimicrobial activity and are being used in nanomedicine for treating wounds, burns and catheter related infections [132]. Silver nanoparticles have therapeutic potential in treating variety of diseases such as retinal neovascularization, acquired immunodeficiency syndrome and for anti cancer properties [118, 133]. For example, silver oxide nanoparticles exhibited antitumor properties following intravenous injection by decreasing tumor vessel development [134]. Silver nanoparticles can cause blood brain barrier destruction, by producing reactive oxygen species, as well as neuronal degeneration and brain edema [134]. They are also known to alter the membrane structure to damage the bacterial cell membrane by attaching to sulphur containing proteins [133]. Silver nanoparticle toxicity has recently been linked to mitochondrial targeting, reactive oxygen species production and glutathione depletion in liver cells [135]. Silver nanoparticles can release silevr ions, which can be toxic; therefore, in our opinion use of silver nanoparticles for drug delivery for humans should be limited. In our opinion gold nanoparticles are particularly effective for photoablation therapy while silver nanoparticles would best be used for controlling bacterial infections. Use outside of these fields would require technological advances to decrease the negative toxicological impacts of these agents in animals.
2.4.2. Superparamagnetic iron oxide nanoparticles (SPION)
Superparamagnetic iron oxide nanoparticles are inorganic particles of an iron oxide core coated with either silica / gold and organic materials such as phospholipids, fatty acids, polysaccharides, peptides, surfactants or polymers [136]. These particles have magnetic properties, enabling collection in a defined location or these particles can be heated in the presence of an externally applied AC magnetic field [136, 137]. Coating with peptides or antibodies enables these nanoparticles to bind to particular cells for disease treatment or imaging. This characteristic makes these nanoparticles attractive for a wide variety of applications ranging from: (a) MRI; (b) drug delivery; (c) magnetic hyperthermia; and (d) magnetically assisted cell transfection [136, 138]. Particles range in size from 50 to 160 nm. Particles coated with organic molecules are currently being evaluated in clinical trials as MRI contrast agents for detecting liver tumors and to differentiate metastatic from inflammatory lymph nodes [137].
2.4.2.1. Clinical advantages and toxicological considerations when planning to use superparamagnetic iron oxide nanoparticles
Clinical use of superparamagnetic iron oxide nanoparticles has been steadily increasing and used for gene transport, MRI, hyperthermia based treatment and for radiotherapy [138]. Superparamagnetic monocrystalline iron oxide has been used for detection of solid tumor metastases[2], detecting metastasis <2 mm in size in lymph nodes and at other sites [32]. 20 nm metal oxide nanoparticles composed of Fe2O3 or Fe3O4, have been evaluated for cancer thermal ablation therapy and MRI imaging [139]. In breast cancer, these particles have been conjugated to antibodies for targeting and imaging [140]. SPION in combination with an external magnetic field to target the nanoparticles is emerging as a potentially important area of drug delivery, which is called magnetic drug targeting [141]. This method allows delivering particles to the desired target site and maintaining them at that location during localized drug release [141-143]. This approach can have the added advantage of decreasing the toxicity of dugs causing systemic toxicity [141].
Superamagnetic nanoparticles have minimal toxicity in the human body [144]. A study comparing several metal oxide nanoparticles showed iron oxide nanoparticles to be safe and non-cytotoxic at concentrations below 100 mg/ml [144]. However, intravenous administration can lead to accumulation in a targeted organ potentially leading to iron overload, which can be toxic. High free iron levels can cause an imbalance in homeostasis, leading to DNA damage, oxidative stress and inflammation [2, 144]. Based on these reports, our opinion is that these particles can be used safely in humans provided that concentrations are maintained below 100 mg/ml and accumulation in organs is monitored to prevent iron overload. Special attention should also be given to leaching of iron (Fe+3) ions and interaction of these particles with H2O2, which could generate free radicals such as hydroxy radicals due to fenton chemistry.
2.5. Semiconductor based nanoparticles
2.5.1. Quantum dots
The general structure of a quantum dots is a core element usually containing cadmium selenide, cadmium telluride, and indium phosphide or indium arsenide either alone or in combination and a shell made of zinc sulfide [145, 146]. Quantum dots are well-studied heterogeneous groups of nanoparticles that fluoresce in different colors depending on size and constituent components [3, 147]. They are generally coated with polyethylene glycol to enable attachment to targeting molecules such as antibodies or other ligands [34]. Quantum dot absorption, distribution, metabolism, excretion and toxicity, depend on multiple factors derived from both inherent physicochemical properties and environmental conditions [147]. Size of quantum dots ranges from 2.5 to 100 nm, depending on the thickness of the coating [6].
2.5.1.1. Clinical advantages and toxicological considerations when planning to use quantum dots
Currently the main use of quantum dots is for biomedical imaging due to the broad fluorescence spectrum they are capable of emitting, which makes these nanoparticles optimal fluorophores [148]. For instance, fluorescent quantum dots can be conjugated to antibodies, receptor ligands moieties or aptemers enabling measurement of identification of cancer cells, for studying signaling events in cancer cells, for investigating peroxisomes activity and identification as well as quantification of cell membrane receptors [149-153]. Furthermore, quantum dots conjugated to targeting agents have been used for site-specific gene therapy and delivery of pharmacological agents [154, 155].
The metal core constituents of quantum dots can be toxic following removal or dissolution of the coating. Degradation of the coating can also result in severe toxicological effects. For example, quantum dots can be toxic due to the surface coating, which can be reduced by modification with N-acetylcysteine [147, 156-159]. Exposure of quantum dots surface coatings to acidic or oxidative environments, such as those present in endosomes, can cause decomposition and subsequent release of metalloid core containing Cd or Zn into the cytoplasm causing toxicity [157, 159]. Discrepancies have been reported regarding the toxicity of quantum dots in the literature, likely due to the varying physicochemical properties of individual nanoparticles. In our opinion, it will be difficult to overcome the toxicity of these particles, which will limit use in patients. However, these agents will be useful for studies characterizing cells or cellular processes occurring in clinical material, which would involve imaging or protein signaling.
3.0. Key Physicochemical Properties to Consider That can Influence Nanoparticle Toxicity
The goals for nanotechnology-based drugs are either to enhance delivery or uptake of agents into target cells or to reduce toxicity associated with pharmaceutical agents by masking. For many decades nanoparticles have been used as a strategy to reduce toxicity and side effects associated with particular drugs [3, 9]. Although, nanoparticles are intended to deliver agents into or at the vicinity of target organs, several recent findings have reported unexpected toxicities, leading to the origin of the field of nanotoxicology [3, 9]. Nanotoxicology is emerging as an important branch of nanotechnology and is the study of interactions of nanostructures with biological systems to elucidate the relationship between physical and chemical properties such as, size, shape, surface, chemistry, composition, and aggregation of nanostructured materials with induction of toxic biological responses [14]. Recently, it has been realized that nanocarrier systems can cause serious harmful effects and several studies have reported harmful effects associated with nanocarriers on organ systems [3].
Nanostructured materials are comparable to typical cellular components and proteins in size. Therefore, nanoparticles might cross the natural mechanical barriers of the human body via different routes including inhalation, intravenous, dermal, subcutaneous, oral and intraperitonial routes [8]. Nanoparticles, therefore, can induce adverse health effects. However, the fundamental cause-effect relationships are either ill defined or unexplored, hence, studies are urgently needed to demonstrate and identify various structural elements causing cyto- and organ- toxicity. Absorption of nanoparticles is also possible when the nanomaterial first interacts with proteins or cells and might affect organs such as liver, brain, spleen, blood, kidney, heart, colon, bone, etc., and cause deleterious cytotoxic effects leading to deformation and inhibition of cell growth in humans and animals [8, 160]. A number of studies have reported the potentially harmful effects of nanoparticles in in vitro and in vivo experimental models and identified key physicochemical properties influencing nanoparticle toxicity, which include particle size, composition, charge, surface area, agglomeration and dispensability (Figure 1) [8, 160, 161]. Key physicochemical properties influencing nanoparticle toxicity are discussed below and shown in Figure 1.
3.1. Particle size
Particle size is one of the critical factors that can influence the toxicity of nanoparticles [161]. Nanoparticle size varies in different dispersion mediums like deionized water and cell culture media with and without serum. For instance, 40 nm copper agglomerated 28-fold increase in size when introduced into deionized water and RPMI-1640 media, but only a nine fold increase compared to its primary size when it was put into media with serum [8, 162]. Although particles of <100 nm size possessed desirable mechanical, electrical and chemical properties that are critical for drug delivery; the smaller size can cause undesirable effects such as passing through the blood-brain barrier and triggering immune reactions as well as damaging cell membranes [8]. For example, in vitro size-dependent cytotoxicity studies using gold nanoparticles showed that 1.4 nm gold particles effectively inhibited the growth of connective tissue fibroblasts, epithelial cells, macrophages and melanoma cells, however, particles of 15 nm size are non-toxic at similar concentrations. In our opinion, for in vivo use, a size less than 100 nm and a hydrophilic surface are essential to reduce opsonization and subsequent clearance by macrophages.
3.2. Particle composition and charge
Nanoparticle composition and charge can effect organ accumulation and toxicity [3]. Particle surface charge is generally indicated by zeta potential. Zeta potential reflects the electrical potential of particles and is influenced by the composition of the particle and the medium in which it is dispersed. Nanoparticles with a zeta potential above (+/-) 30 mV are usually stable in suspension, as the surface charge prevents aggregation of the particles. For example, the use of cationic liposomes in in vivo models can cause dose-dependent toxicity and pulmonary inflammation [40, 58]. DOTAP, a monovalent cationic lipid constituent of cationic liposomes, and LipofectAMINE, a multivalent cationic liposome, accumulates in the vasculature and can be preferentially taken up by the liver and spleen [42]. This effect was more pronounced with the LipofectAmine compared to DOTAP [39]. Likewise, stearylamine, the first generation monoalkyl cationic lipid, caused hemagglutination and hemolysis of human erythrocytes [39]. Therefore, clinical use of this cationic lipid for delivering drugs, genes and peptides into humans is not advisable. Although cationic lipid-based delivery systems offer some promise as a potential siRNA delivery system, potential for lung and other organ related toxicities may require alternative formulations for safety [16, 60]. In our opinion, development of novel technologies preventing cationic liposomes clearance from circulation might be useful for increasing the efficacy of liposomes and lead to decreased toxicity resulting from organ or cell damage.
3.3. Particle surface area
Nanocarriers possesses large surface area due to very small particle sizes [163]. Since, nanoparticles interact with biological systems through surface area, controlling the surface properties such as composition, charge and porosity are critical factors influencing nanoparticle-associated toxicity [8, 163]. Increased surface area has the advantage of providing opportunities for antibody conjugation and material delivery but, also have inherent disadvantages making them toxic if not rationally designed. For example, the durability or stability of surface coatings inside a biological environment is a critical factor regulating the toxicological consequences associated with nanoparticles [8, 163]. While surface modifications allow nanoparticles to be stabilized and avoid agglomeration, they may shield or influence the effects of nanomaterials on biological systems. For example, PEGylation has been shown to stabilize nanoparticles such as gold nanoparticles and liposomes by reducing clearance from the circulation, thereby prolonging the chance of coming in contact with the tumor or cellular target [121]. In addition, PEGylation also protects nanoparticles from opsonization, a process by which foreign organism or particles become covered with opsonin proteins, thereby making them more visible to mononuclear phagocytic system (MPS), also known as the reticuloendothelial system (RES) [164]. Furthermore, PEG-containing copolymers found to exhibit increased circulation half-life by several orders of magnitude compared to un-PEGylated polymers [165]. Mechanistically, PEGylation creates a hydrophilic protective layer around the nanoparticles, which repel the absorption of opsonin proteins via steric forces, thereby blocking and delaying the opsonization process [165]. PEGylation can also improve efficacy by allowing nanoparticles to extravasate in pathological sites such as tumors or inflamed regions with a leaky vasculature, thereby improving the ability to directly target tumors located outside MPS-rich regions. Although PEG can decrease opsonization of nanoparticles, recent studies demonstrated that confirmation of PEG at the nanoparticle surface is a key factor influencing the opsonin repelling function [121]. For example, whereas “brush-like” and “intermediate” configurations of PEG surface reduced phagocytosis and complement activation, “mushroom-like” configuration appears to activate complement system and favor phagocytosis [166]. In our opinion, PEGylation of the surface of any nanoparticle is key to enhancing its circulation time, which would increase its probability of it being more effective.
3.4. Agglomeration and dispersability
Due to the increased surface area to volume ratio, nanoparticles tend to agglomerate via Brownian motion and van der Waal's forces [8, 167]. Prevention of agglomeration is a key factor while considering nanoparticles for clinical use since this process can alter the physicochemical properties such as size distribution, surface-to-volume ratio and surface activity as well as concentration of nanoparticles thereby effecting potential toxicity of nanoparticles due to vascular or lymphatic blockage [9]. In one study testing the risk assessment of nanoparticles composition as well as size, it has been demonstrated that aged and agglomerated copper nanoparticles (approximately 200 nm in diameter) produced greater lung inflammation than did freshly generated (11-60 nm) carbon nanoparticles. Authors of this study concluded that, although, particle composition appeared to be a more important risk factor than was the agglomeration state of the nanoparticles, further studies were required to measure the role of agglomeration fir causing serious side-effects. Several intrinsic and extrinsic factors such as nanoparticle composition, concentration, size, surface coating, dispersant characteristics (pH, presence of serum, salt and surfactant), zeta potential, sonication time and temperature can influence agglomeration [8, 167]. Sonication, detergents, lung surfactants, polyethylene glycol and serum can prevent agglomeration to decrease toxicity or deleterious side-effects [8, 168].
4.0. Approaches and Limitations for Assessing Toxicity of Nanoparticles
4.1. Methods for assessing the toxicity of nanoparticles
Nanoparticles can trigger cytotoxic, genotoxic, inflammatory and oxidative stress responses in mammalian cells, which should be considered prior to selection of a particular nanoparticle for cenrtain applications [1, 8, 110, 160, 169]. Factors triggering these toxicities are not fully understood requiring development of standardized methods for assessing toxicity. Nanoparticles possess unique physicochemical properties that can pose challenges for assessing toxicity using classical toxicity assays [8]. Therefore, more particle characterization such as size, shape, surface area, solubility, agglomeration, and elemental purity should be evaluated as a part of the toxicological assessment. These characterizations would not generally be needed for traditional pharmacological agents. Incomplete characterization could artificially influence results of many in vitro toxicity assays, causing misinterpretation of data. Furthermore, lack of standardized guidelines for nanopartical toxicological characterization could impede movement of these agents to the clinic. Recently, Dhawan et. al. [165] reported approaches and challenges encountered during nanoparticle characterization. Key elements needed to assess potential toxicity of nanomaterials include; characterization of physicochemical properties, use of cellular and non-cellular in vitro toxicity assays; and, animal based toxicological assessments (Figure 1) [170].
4.1.1. Physicochemical properties that affect nanoparticle toxicity
Various assays are available for evaluating the different physiochemical properties of nanoparticles [8, 10, 163]. Physicochemical properties that may contribute to toxic effects of nanomaterials include chemical composition, agglomeration state, particle size and size distribution, density, shape, and surface properties such as area as well as charge (Figure 1).
4.1.1.1. Chemical composition characterization
Chemical composition of nanoparticles is a key factor that can influence the physicochemical and biological properties of nanoparticles [8, 9, 163]. Chemical composition of a nanoparticle can be measured by standard methods of chemical analysis using Energy dispersive analysis of X-rays (EDAX), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD). Nuclear Magnetic Resonance (NMR), Fourier transform infrared (FT-IR) and mass spectrometry [8] (Figure 2). For an example, chemical composition can affect toxicity of liposomes that are formulated with cationic lipids and fusogenic phospholipid dioleyolphosphatidylethanolamine (DOPE). However, cationic liposomes can also have toxic effects when accumulated in vital organs, by binding to serum proteins and by disrupting cell membrane function [14, 43]. Furthermore, cationic liposomes can localize in macrophages after parenteral administration, making it necessary to examine the effect of cationic liposomes on the production of immuno-inflammatory modulators secreted by activated macrophages, and determine the effect on phagocytic macrophages as well as non-phagocytic T-lymphocytes. Cationic liposomes can down-regulate synthesis of protein kinase C (PKC)-dependent mediators nitric oxide, tumor necrosis factor-α and prostaglandin E2 by activated macrophages. Prolonged incubation of >3 h with macrophages can induce toxicity and can be determined by the lipids used in the liposomal formulation. The order is DOPE / DDAB > DOPE / DOTAP =DOPE / DC-Chol > DOPE / dimyristoyltrimethylammonium propane. Furthermore, replacement of DOPE by DPPC or the incorporation of DPPE-PEG2000 in DOPE / cationic lipids can reduced macrophages mediated toxicity and restore synthesis of PKC-dependent modulators [63]. Therefore, in our opinion, to reduce toxicity associated with nanoparticles such as cationic liposomes, the composition should be carefully considered or the liposomes should be coated with an inert material such as polyethylene glycol [3, 40]. A careful analysis of nanomaterials composition by proper characterization tools and tehniques would help researchers to get information regarding what kind of elemental species could cause toxicity, which can help using preventive methods.
Figure 2.
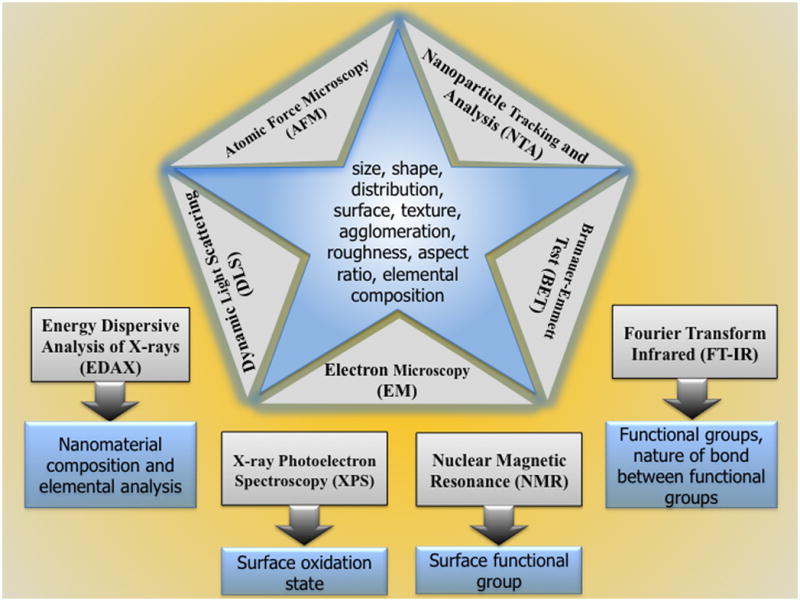
Schematics of routenely used tools and techniques for the charecaterization of nanomaterials/ nanoparticles.
4.1.1.2. Nanoparticle size, size distribution, density, shape and agglomeration state
Particle size, size distribution, density, shape and agglomeration state are key characteristics that need to be considered as triggers of toxicity while making a nanoparticle-based formulation for clinical [8, 9]. Large particles could trigger immune reactions. Furthermore, density or shape could cause a variety of toxicological problems such as blockage or organ accumulation. Although, nanoparticle characterization is often complicated by the polydispersity of samples, an approximate estimate can be obtained using Brunauer-Emmett-Teller, dynamic light scattering, transmission electron microscopy [17], scanning electron microscopy [171] atomic force microscopy or Nanoparticle Tracking Analysis approaches (Figure 2) [8]. In our opinion nanoparticle size, size distribution, density, and shape should be a routine part of the characterization to predict potential toxicological concerns.
Agglomeration of nanoparticles can occur during the synthesis or once the nanoparticles are introduced into various solutions where electrostatic interactions or chemical bonding may lead to nanoparticle aggregation [8]. Agglomeration in the vascular system could cause blockage or trigger immune recognition and elicit an immune response. Agglomeration is known to change the physicochemical properties of NPs due to the average particle size and charge, which could lead to toxicity [172, 173]. Use of the Ultrahigh-resolution system is a simple and convenient technique to characterize the extent of metal nanoparticle agglomeration in solution [174]. Other methods to determine agglomeration include nanoparticle tracking and analysis (NTA), atomic force microscopy (AFM) and electron microscopy (EM) UV-Vis, and the dynamic light scattering (DLS) technique (Figure 2) [8]. In our opinion, measurement of agglomeration should be a standard assessment that could rule out potential vascular blockage and immune related toxicities.
4.1.1.3. Surface property characterization
Surface properties of nanoparticles including surface area, charge and surface reactivity are critical regulators of nanoparticle toxicity [8, 9]. Since nanoparticles interact with cell membranes through the surface, the surface characteristics such as particle texture, porosity and charge needs to be considered as potential modulators of toxicity [8, 19, 163, 167]. Nanoparticle surface properties can be measured using Brunauer-Emmett-Teller (BET) [8]. Although BET measures size as well as surface area of nanoparticles, it does not account for size distribution, hence the need for data from additional methods for complete characterization of nanoparticle surface properties (Figure 2) [8]. Atomic force microscopy is a cost-effective method that provides details regarding nanoparticle size, morphology, surface texture and roughness. Characterization of surface reactivity can also be undertaken using the Electron Resonance Technique (Figure 2). In our opinion, nanoparticles surface properties could cause toxicity and should be part of an assessment related to potential toxicity.
4.2. Current limitations of routinely used particle characterization methods
Although several methods have been listed that could be used to characterize the physicochemical properties of nanoparticles, most have inherent drawbacks, which are detailed below.
4.2.1. Electron microscopy for particle characterization
Due to its small size, nanoparticles are irresolvable by optical microscopy, requiring more powerful methods such as electron microscopy for measuring nanoparticle size and shape [8]. Scanning electron microscopy can be used to obtain information on size distribution and shape of nanomaterials [175]. Transmission electron microscopy can provide higher resolution and therefore better information about the crystal structure and granularity of a sample [176]. However, electron microscopy is not always a good option for biological particles such as liposomes conjugated to proteins or engineered polymers like dendrimers, as these nanoparticles cannot deflect the electron beam sufficiently, thereby limiting an accurate estimate of particle size. Thus, these nanoparticles are invisible to electron microscopy and require heavy-metal staining procedures. For electron-dense nanoparticles such as metal colloids with attached PEG, targeting antibodies or drugs, electron microscopy cannot image the surface groups without the use of cryogenic methods, and as a result does not give an accurate measurement of the physiological size. A further complication is that transmission electron microscopy requires; (a) a high vacuum; (b) thin sample sections for electron-beam penetration; and, (c) sample drying, all of which can alter the physicochemical state of the nanoparticle to introduce artifacts. Therefore, in our opinion, particle characterization using electron microscopy should be used for particles without surface modifications, which can be easily visualized by processing that would not introduce artifacts.
4.2.2. Dynamic light scattering
The dynamic light scattering technique is widely used for measuring particle size in solution [177]. However, since dynamic light scattering measures the equivalent-sphere hydrodynamic diameter, it does not provide information regarding the particle shape [177]. Furthermore, dynamic light scattering cannot be used to measure particle size if the sample absorbs at the wavelength of the laser used by the size-measuring instrument [8]. Intensity of scattered light from small spherical particles is proportional to particle diameter to the 6th power; therefore, larger particles scatter light much more efficiently than smaller particles [162]. Small traces of agglomerates or dust in a sample can skew size measurements. Furthermore, dynamic light scattering, unless conducted in-line with a separation/fractionation method, cannot separate similar sized populations, such as monomers and dimers of the same species [8]. Therefore, in our opinion, other particle characterization methods, such as transmission electron microscopy should be using in conjunction with dynamic light scattering for unambiguous characterization of nanoparticles size and shape.
4.2.3. Nanoparticle evaluation under physiological conditions
The most challenging area of nanoparticle characterization is evaluation under physiological conditions that resemble or mimic the state present in animals [163]. Many properties of nanoparticles are environmental and condition dependent. The size distribution at physiological pH and ionic strength might differ from the distribution in water or in a dry state [163]. Plasma proteins are known to bind nanoparticles in the blood and the protein-bound size would be a more relevant determinant of disposition and clearance than the unbound free-particles [9]. Changes in size due to agglomeration could trigger immune reactions or cause agglomeration leading to toxicological effects. Size measurements using dynamic light scattering could be valuable for this type of characterization since it can provide a measure of the hydrodynamic size of a nanoparticle in biological fluids, such as blood plasma [177]. Characterization by TEM and FTIR along with DLS would give further detailed information about the change and shape of NPs under physiological conditions (Figure 2). In our opinion, nanoparticle evaluation under physiological conditions should be a routine requirement for characterization and should be considered when assessing possible toxicological concerns.
4.2.4. Sterility and pyrogenicity determination of nanoparticles
Nanoparticles need to be evaluated for pyrogenicity and endotoxin contamination since these factors will induce toxic effects. Dependent on constituent components involved in the assembly of the nanoparticles, the end product could contain bacterial endotoxins [178]. Due to the induction of inflammatory signaling mediators such as cytokines, chemokines and prostaglandins, endotoxins can cause fever, organ damage and fibril reactions. The FDA has recommended two types of pyrogen characterization tests. A limulus amoebocyte lysate assay also known as bacterial endotoxin test, uses blood from the Horseshoe crab (Limulus polyphenus) and is a sensitive method to measure bacterial endotoxins [178]. This test can detect picogram quantities of bacterial endotoxin. The second FDA approved test for measuring endotoxins is the rabbit pyrogen test [170], which is an in vivo test capable of identifying various pyrogenic substances. This test measures the rise in temperature of rabbits following intravenous injection of the test solution. The limulus amoebocyte lysate test has been widely used in preclinical pharmaceutical development. However, a limitation of the test is that most nanoparticles interfere with the assay [168]. Alternate pyrogenicity tests have been proposed but might also be subject to nanoparticle-interference issues [168]. In our opinion, sterility and pyrogenicity of nanoparticles are fundamental requirements and should be performed routinely to prevent toxicity related concerns.
4.3. In vitro cell culture models for toxicity assessment of nanoparticles
In vitro toxicity of nanoparticles in cultured cells is essential to fully understand the mechanisms of action of nanoparticle therapeutics in biological systems. Unfortunately, nanoparticle-based agents are often poorly suited to conventional in vitro pharmacological assays as nanoparticles adsorb proteins during assays. Nanoparticles such as gold colloids scatter light and can invalidate colorimetric assays that rely on absorbance measurements [125]. Furthermore, some nanoparticles have very large molar-extinction coefficients with emission wavelengths causing false positive results in colorimetric assays using plate readers. Therefore, in our opinion, the absorption spectra of a nanoparticle in solution should be routinely performed and used to determine which tests can be undertaken without concerns regarding overlap for colorimetric assays that rely on absorbance measurements. Despite these concerns, the following tests are recommended for the initial in vitro toxicity testing (Figure 1).
4.3.1. Biocompatibility assays
Cultured cell-based assays such as MTT, sulforhodamine B, bromodeoxy uridine incorporation and cell cycle FACS analysis that measure cell viability should be used to determine the biocompatibility of nanomaterials on living cells [179]. These cell based assays measures loss of membrane integrity, metabolic activity, monolayer adherence and monitor progression through the cell cycle. Membrane integrity assays, such as the trypan blue exclusion assay and lactate dehydrogenase (LDH)-leakage assay [180], can be of particular importance for certain nanoparticles such as cationic liposomes that can disrupt cell membranes integrity under certain circumstances [181]. Tetrazolium dye reduction, ATP and 3H-thymidine incorporation assays can be used to measure cell viability and proliferation rates by monitoring cellular metabolic activity [168]. Nanoparticles that are antioxidants, such as certain functionalized fullerenes, might interfere with these assays by enhancing tetrazolium-dye reduction causing an overestimation of cell viability. Progression through the cell cycle can also be monitored by DNA staining and flow cytometry and cell-cycle effects have been demonstrated for a variety of nanoparticle samples, including carbon nanotubes [182]. In our opinion, toxicity assessment of nanoparticles on cultured cells is easy and inexpensive and should be used as a first assessment of toxicological effect on a living system.
4.3.2. Hemolytic and platelet aggregation tests
Nanoparticels can interfere with normal blood cell function, therefore, hemolytic and platelet aggregation tests should be evaluated especially when particles are injected intravenously [168]. Since intravenous administration could cause hemolysis or platelet aggregation leading to a variety of pathological conditions, including anemia and jaundice, it is important to determine whether nanoparticles lead to these effects thereby contributing toxicity. Platelet aggregation test determines the anticoagulant or thrombogenic properties of nanoparticles. In our opinion most nanoparticles would at some point come in contact with blood cells, therefore, basic hemolytic and platelet aggregation tests should be used to measure effects on these cell types. These tests are inexpensive and fairly easy to execute.
4.3.3. Immune system activation assays
Nanoparticles interaction with the immune system can be a major source of toxicological effects. This effect can be analyzed in cell-based assays on cells present in organs from the immune system [168]. Though standard immunological culture based tests exist, the preclinical immunotoxicity testing of nanoparticles has been hampered due to the absorbance and emission spectra of the nanoparticles, which in turn interferes with the colorimetric methods creating false positive or false negative effects in enzyme assays. The plaque forming cell assay is a test to measure nanoparticle related toxicity in vitro [168]. Although the plaque forming cell assay has been shown to predict immune toxicity, the main hurdle is that nanoparticles are usually taken up by phagocytic cell of the reticuloendothelial system, which triggers immune responses [168]. Tests for macrophage function and reticuloendothelial uptake can be useful for characterizing immune system modulation by the nanoparticle. In our opinion, a major source of nanoparticles related toxicity is immune mediated; therefore, preclinical immunotoxicity testing of nanoparticles is essential when preparing particles for testing in animal models or in humans.
4.3.4. Reactive oxygen species and oxidative stress detection assays
Reactive oxygen species (ROS) and/or free radicals have shown to be produced by a variety of nanoparticles, which can lead to toxicological effects [183, 184]. A number of assays are available to measure cellular ROS production. The electroparamagnetic resonance technique has been widely used to assess nanoparticle induced ROS generation. This technique uses spin probes or traps in combination with specific reagents enabling identification and quantification of the free radical species [185]. Another method for the detection of ROS production is the fluorescent dye 2,7-dichlorofluorescin (DCFH) assays [186]. In this assay, the dye is delivered to the cells with the diacetate group intact, which renders the molecule relatively lipophilic, enabling access to cells, which can be measured by fluorimetric or by flow-cytometric techniques. The Cytochrome C assay is another assay used to measure intracellular ROS production by cells [187].
A number of markers are available to detect oxidative stress by measuring lipid peroxidation using the thiobarbituric acid reactive substances assay and the Trolox equivalent antioxidant capacity assay. Glutathione levels can also be measured as an indicator of oxidative stress, using the total Glutathione (GSSG/GSH) assay kit. Another sensitive marker of oxidative stress induced by nanoparticles is to measure mRNA expression changes of oxidative stress-dependent genes [188]. In our opinion, nanoparticles can trigger reactive oxygen species production and oxidative stress leading to toxicity, therefore, test should be undertaken to measure both to limit these problems during clinical development.
4.3.5. Genotoxicity assays
Currently, cell culture based genotoxicity assessment strategies for nanoparticles are limited. Various established genotoxicity assays are available to screen for potential chemical carcinogens, however, only a few assays such as the salmonella reverse mutation assay, the micronucleus test, and the alkaline comet assay have been used for the nanoparticles [189]. The salmonella reverse mutation assay, also known as the Ames test, is most widely used in vitro assay. However due to differences in membrane structure and composition of bacteria compared to mammalian cell membranes, and to what extent bacterial mutagenicity tests identify the true genotoxic potency of nanoparticles remains a concern. Therefore, mutagenicity assays in mammalian cells could provide better and more relevant information compared to the bacterial assay [190]. Micronucleus and alkaline comet assay have also been used to evaluate clastogenic (chromosome breaking) effects of nanoparticles in mammalian cells [191]. The main advantage of the micronucleus assay is that it detects chromosomal and genomic mutations but can only be used on dividing cells [190]. Although the comet assay is used to measure chromosomal and genomic mutations in live and dead cells, the disadvantage of this assay is that it does not measure fixed mutations, which can be overcome using the micronucleus assay. Assessing genotoxicity mediated by nanoparticles, in our opinion, is an underdeveloped area and will limit clinical evaluation of nanoparticles. Our recommendation is that the micronucleus and comet assay be used to measure the effect of nanoparticles of DNA integrity.
4.4. Animal based assays to assess toxicity mediated by nanoparticles
Characterization of nanoparticle-based agents in an animal system is an essential part of assessing nanotoxicology [192]. These animal toxicity assessment tests can be time consuming and costly but provide useful and important information regarding formulation-associated toxicity. In addition, animal studies are important since several studies have shown that in vitro results alone do not always translate into in vivo results. Therefore, in our opinion it is essential to evaluate the bio-distribution and toxicity of nanoparticle in animal systems. The FDA guidelines need to be considered regarding drug formulations for preclinical studies when planning these studies (United States, Food and Drug Administration CBER/CDER Guidance for industry: Developing medical imaging drug and biological products part 1: conducting safety assessments, June 2004). Some of the standard assay for screening for toxicity in animals measure; (a) dose range finding and single / repeat dose acute toxicity of nanoparticles; (b) organ distribution and clearance of nanoparticles; (c) disposition and degradation of nanoparticle constituents and toxicity associated with released products; and, finally (d) immunotoxicity mediated by nanoparticles.
4.4.1. Dose range finding and single / repeat dose acute toxicity measurements
Dose-range finding toxicity studies can be measured by intravenous administration of nanoparticles in male or female rats or mice [193]. Single or repeat-dose toxicity tests are common procedures used to establish dose-range finding toxicity. Experimentally, animals are usually monitored for up to 14-days, however, in some instances (such as for immunotoxicity assessment) it might require longer periods, which can be extended up to 28 days. In our opinion, parameters such as mortality, body weight, organ weight, clinical chemistry, hematology, gross pathology and histology of control versus treatment groups should be compared as assessments of nanoparticle-associated toxicity[193].
4.4.2. Organ distribution and clearance study
Determination of absorption, distribution, metabolism and excretion (ADME) rates of nanoparticles is essential for evaluating potential toxic effects [161]. However, many difficulties are associated with these studies as measuring individual constituents of a multicomponent nanoparticle is labor intensive and challenging [193]. In our opinion this is an important area that will need significant development and standardization.
4.4.3. Disposition and degradation of nanoparticles
Constituents of nanoparticles undergo degradation when administered in animals. Therefore, determination of disposition and pharmacokinetic (PK) properties of nanoparticles is essential [161, 193]. Disposition and PK can be evaluated by administering nanoparticles to mice or rats followed by detecting the constituents or constituent degradation products in tissues harvested at various time points. Experimentally, these studies use radiolabels as well as scintillation counting, inductively coupled plasma mass spectroscopy (ICP-MS), electron microscopy in conjunction with energy dispersive spectroscopy (EM-EDX) or high performance liquid chromatography (HPLC) to detect and quantify nanoparticles or constituents in tissues [8, 193]. In our opinion nanoparticles degradation in biological systems and accumulation of the constituent components could be a source of toxicity. Therefore, determining the nature of the breakdown products and sites where they accumulate can be key aspects when assessing potential nanoparticles toxicity.
4.4.4. Immunotoxicity of nanoparticles
Since nanoparticles can trigger immune responses, it is important to determine whether the nanoparticles or constituent parts trigger immunotoxicity-related reactions [130, 194]. However, relatively few methods exist to accurately assess immunotoxicity of nanoparticles. The lymph node proliferation assay (LNPA) can be used to estimate nanoparticle-associated toxicity, but it has inherent disadvantages [161, 193]. For example, nanoparticles can travel through the lymphatic system when injected subcutaneously and might give inaccurate results, hence requiring alternate routes of administration. The latest FDA guidelines for immunotoxicity assessment of human pharmaceuticals, ICH S8, need to be considered while assessing the immune related toxicity of nanoparticles [193]. FDA recommends examining immune cell function in those cases in which the preliminary study indicated potential immunotoxicity through changes in gross pathology hematology, clinical chemistry, immune organ weights or histological evaluation. In our opinion, assessing immunotoxicity of nanoparticles in animal models is one of the most important tests that should be undertaken related to nanoparticles mediated toxicity. Immune reactions can cause death and should be taken seriously, therefore, it is highly recommended to undertake these tests and if observed, to use approaches to minimize factors causing these effects.
5.0. Strategies to Overcome Nanoparticle Mediated Toxicity
5.1. Masking the nanoparticles to overcome toxicity related issues
Surface protection or masking is a key factor that can moderate the toxicity of nanoparticles. Surface protection is routinely used for reducing the toxicological effects caused by nanoparticles [35]. Methods such as: (a) surface coating of nanoparticles with biocompatible hydrophilic polymers/surfactants; or (b) formulation of nanoparticles with biodegradable copolymers with hydrophilic polyethylene glycol (PEG), polyethylene oxide, polyoxamer, poloxamine and polysorbate 80 (Tween 80) have been used for masking the nanoparticle surfaces. For example, coupling high molecular weight polymers such as PEG to the liposome surface can coat cationic liposome [40]. PEG then forms a physical barrier around the liposome perimeter. Each molecule of the PEG thus coated around liposome can bind to ∼136 water molecules thereby influencing the extent of hydration of the liposomal formulation [39].
Mechanistically, PEGylation creates a hydrophilic protective layer around the nanoparticles, which repel the absorption of opsonin proteins via steric forces, thereby blocking and delaying the opsonization process [14, 40]. Opsonization is a process by which a foreign organism or particle becomes covered with opsonin proteins, thereby making it more visible to mononuclear phagocytic system, also known as the reticuloendothelial system [165]. Opsonins are serum components that aid in the process of phagocytic recognition. For example, complement proteins C3, C4, and C5 and immunoglobulins binds to nanoparticles thereby enhancing recognition by macrophages of liver [165]. The duration of the opsonization process varies from a matter of seconds to many days. Once the particles are opsonized, phagocytosis that is the engulfing and eventual destruction or removal of foreign materials from the bloodstream usually occurs. Together these two processes form the main clearance mechanism for the removal of undesirable components from the blood. Furthermore, PEGylation can also improve the efficacy by allowing nanoparticles to extravasate into pathological sites such as tumors or inflamed regions with a leaky vasculature, thereby improve the ability to directly target tumors located outside MPS-rich regions [67, 165].
Recent studies confirmed that PEG at the nanoparticle surface is a key factor influencing the opsonin repelling function. Whereas “brush-like” and “intermediate” configurations of PEG surface reduced phagocytosis and complement activation, the “mushroom-like” configuration activated the complement system promoting phagocytosis [195]. PEG molecules also helped reduce the circulation clearance in an ultrasound assisted topical delivery of 4.75:4.75:0.5 molar ratio DOTAP, 1,2-dioleoyl-sn-glycero-3phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (DSPE-PEG2000) nanoliposomes loaded with siRNA targeting Akt3 and V600EB-Raf [196]. Although PEG molecules reduce circulation clearance, the amount of PEG used in the liposome preparation can influence the structural integrity of the liposome. For example, >15 mol% PEG can cause unfavorable structural changes in the liposomes [39]. Therefore a typical PEGylated liposome formulation usually contains 5 to 10 mol% PEG.
PEGylation can also be used to mask gold nanoparticles, carbon nanotubes, dendrimers and other nanoparticles [29, 64]. For example, conjugation of prostate membrane antigen (PSMA) specific antibody to the amino groups of PEGylated QDs (QD605) have been used to detect prostate cancer cells expressing PSMA [65]. Diagnostic and therapeutic agents of these types can be covalently attached, encapsulated, or absorbed on to nanoparticles that are masked by PEGylation. In our opinion, masking the surface of nanoparticles is a key strategy for reducing toxicity and enhancing the circulation time of nanoparticles.
5.2. Adding targeting moieties to nanoparticles
Targeting of nanoparticles using surface bound targeting moieties is an approach for reducing toxicity [197]. Targeted nanoparticles have improved efficacy at much lower concentrations compared to untargeted agents thereby reducing toxicity [11, 12]. For example, antibody or peptide targeted immunoliposomes can improve drug efficacy and lower toxicity [38, 64]. Targeted delivery of a mixture of siRNA against MDM2, c-myc and VEGF (1:1:1) loaded in to nanoparticles inhibited melanoma metastasis by 70 - 80% compared to free siRNA or non-targeted nanoparticles in experimental mouse models [198]. Mechanistically, targeted liposomes function by “active targeting” to recognize targeted cells to enhance therapeutic efficacy [198]. Active targeting of liposomes involves specific interaction of antibodies or peptides linked to the nanoparticle with receptors and uptake of encapsulated drugs by receptor-mediated endocytosis. Factors interfering with targeting might therefore influence drug delivery and liposomal efficacy [11]. Differences in efficacy of targeted liposomes could be due to changes in liposome accumulation, internalization, degradation or intracellular fate of the drug.
Selection of an appropriate ligand for targeting is a critical factor influencing the success of immunoliposomes or other immune-targeted nanoparticles. The selected ligand should be: (a) produced in large quantities requiring minimal purification steps; (b) stable; and, (c) be conjugated easily to the nanoparticles without affecting properties of either nanoparticles or ligand [14]. Among various ligands tested, antibodies are stable, easy to produce using hybridoma technology and can be highly selective. However, antibodies' Fc-receptor can cause accumulation of immunoliposomes in the liver and spleen, which has the potential to enhance immunogenicity [14]. To circumvent problems associated with Fc-receptors, recent emphasis has been on creating fragments of antibodies possessing only F(ab')2, Fab', and scFv. scFv fragments seem to be more effective and less cytotoxic, but are not stable [14]. In our opinion, targeting of nanoparticles to particular cells is a clear advantage of this technology, which could enable lower concentrations of the nanoparticle to be used resulting in fewer toxicological side effects. This approach should be used if a targeting moiety can be identified that could be used for targeted delivery into particular tumors or tumor cells.
6. Expert Opinion
6.1. What Does The Future Hold For Nanotechnology Based Drugs
6.1.1 Key findings and major weaknesses in nanotechnology related research
Use of nanotechnology for biomedical applications is growing rapidly with both beneficial and harmful effects on human health and the environment. Tremendous efforts have been devoted to enhance efficacy of drugs by increasing circulation in blood, enhancing intracellular penetration and aiding targeting moieties to specific cell or tumor types. Some of these nanoparticles have been evaluated clinically, while many others are still under preclinical evaluation. In our opinion, nanomedicine still has several limitations preventing its full clinical potential. For example, lack of reproducibility with respect to particle characterization and agent loading, methods to scale-up from laboratory preparations to bulk clinical grade formulations, appropriate assays to measure toxic contaminating substances and procedures to alleviate nanoparticles associated toxicities. These are areas that need attention in order to realize the full translational potential of nanomedicine in cancer, which is to develop non-toxic delivery vehicles and imaging agents for the treatment of cancer.
6.1.2. Challenges to overcome in order to achieve the full potential of nanotechnology
Strategies to evaluate and accurately measure nanoparticles based toxicity and efficacy in cell culture as well as in animal systems remains a challenge to this field. Specifically, these challenges are: a) preparation strategies for protein and polysaccharide based nanostructures need improvement for obtaining evenly dispersed and stable nanoparticles; b) cytotoxicity, biodegradability, biocompatibility and immune response of nanoparticles need to be evaluated for clinical drug delivery; c) development and production of the nanoparticles are still at the laboratory scale, therefore, biologists and chemical engineers need to collaborate for the large-scale production of these nanoparticles and; d) combined efforts of bioengineers and toxicologist through chemical modification and nanomaterials sciences in designing nanoparticulate structures will improve the pharmacokinetics and biodistribution of the drug delivery system.
6.1.3. The future of nanomedicine
Nanotechnology will be used in the future to resolve a variety of issues related to cancer drug delivery and for the imaging of cnacer cells. Targeted drug therapies or “smart drugs” would be a boon in the field of cancer drug delivery and development. Sucessful agents would have fewer side effects and would be more effective at lower concentrations than traditional therapies. It is predicted that in the future, an increasing number of these types of agents will receive FDA approval for clinical evaluation. Moreover, efforts from the National Cancer Institute in establishing the Alliance for Nanotechnology in Cancer will enhance research capabilities and expand multi-disciplinary team research in this field to advance prevention, diagnostic, and/or treatment efforts.
6.1.4. Particular area of interest in nanotechnology
An interesting area of nanotechnology involves theranostics, which is the development of agents that could serve as both a therapeutic and imaging agents. This area could revolutionize medicine and form the basis for new, more effective treatments. In the future, nanotechnology will also aid the formation of molecular systems that may be strikingly similar to living systems. These tools could form the basis for regeneration or replacement of body parts lost due to infection, accident, or disease. Thus, the future for nanomedice remains bright and will become even more significant as issues such as nanoparticles toxicity are resolved.
Article highlights.
Unique, multifunctional nanoparticles for targeting, imaging and diagnosis would be beneficial for the detection and treatment of various diseases.
New experimental methods and guidelines to aid in the design of safe nanomedicines are required.
Nanotechnology has significant potential for advancing therapeutic efficacy and imaging in cancer; however, these agents can be toxic.
Toxicity needs to be considered when selecting nanoparticles for a particular application.
Methods for assessing nanoparticles toxicity need to be improved and standardized across all nanotechnology platforms in order to speed the application of nanoparticles use in humans.
Acknowledgments
Declaration of Interest
GP Robertson has recieved financial support from the National Institutes of Health (NIH CA-127892-01A, NIH CA-136667-02, NIH CA-138634-02) as well as support from the Foreman Foundation for Melanoma Research. A Sharma has recieved support from the National Institutes of Health (NIH CA-142060-02, the Bassumian Trust, PA and the Tobacco Settlement Fund.
Footnotes
S Madhunapantula has declares that he has no conflict of interest and has recieved no payment in the preperation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to reders.
- •1.Medina C, Santos-Martinez MJ, Radomski A, et al. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–8. doi: 10.1038/sj.bjp.0707130. This review article highlightscurrent status of nanoparticle, pharmacological developments and nanoparticle-basedtoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang MF, Lei L, Guo SR, et al. Recent progress in nanotechnology for cancer therapy. Chin J Cancer. 2010;29:775–80. doi: 10.5732/cjc.010.10075. [DOI] [PubMed] [Google Scholar]
- 3.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133–49. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leucuta SE. Nanotechnology for Delivery of Drugs and Biomedical Applications. Curr Clin Pharmacol. 2010 doi: 10.2174/157488410793352003. [DOI] [PubMed] [Google Scholar]
- 5.Jain KK. Role of nanobiotechnology in the development of personalized medicine. Nanomedicine (Lond) 2009;4:249–52. doi: 10.2217/nnm.09.12. [DOI] [PubMed] [Google Scholar]
- 6.Bharali DJ, Khalil M, Gurbuz M, et al. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomedicine. 2009;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka Y, Yoshikawa T, Tsutsumi Y. Nano-safety science for assuring the safety of nanomaterials. Nippon Eiseigaku Zasshi. 2010;65:487–92. doi: 10.1265/jjh.65.487. [DOI] [PubMed] [Google Scholar]
- ••8.Dhawan A, Sharma V. Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. This review providesa critical information on thechallenges faced in the field of nanotechnology andmethods employed in nanotoxicology. [DOI] [PubMed] [Google Scholar]
- ••9.Oberdoster G. Safety assessment for nanotechnology and nanomedicine: concept of nanotoxicology. J ournal of INTERNAL MEDICINE. 2009;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. This article discusses in detail physico- chemical characterization of nanoparticles and key aspects of nanotoxicology. [DOI] [PubMed] [Google Scholar]
- 10.Oberdorster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••11.Alexis F, Pridgen EM, Langer R, et al. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010:55–86. doi: 10.1007/978-3-642-00477-3_2. This book chapter discusses advances in nanoparticle technology platforms development enphasizing on preclinical and clinical of nanocarrier drug delivery systems and their impact for cancer therapy. [DOI] [PubMed] [Google Scholar]
- 12.Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–20. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youns M, Hoheisel JD, Efferth T. Therapeutic and Diagnostic Applications of Nanoparticles. Curr Drug Targets. 2010 doi: 10.2174/138945011794815257. [DOI] [PubMed] [Google Scholar]
- 14.Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo H, Li J, Chen X. Antitumor effect of N-succinyl-chitosan nanoparticles on K562 cells. Biomed Pharmacother. 2010;64:521–6. doi: 10.1016/j.biopha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Hobel S, Aigner A. Nonviral delivery platform for therapeutic RNAi: pegylated siRNA/cationic liposome complexes for targeting of the proto-oncogene bcl-2. Future Oncol. 2009;5:13–7. doi: 10.2217/14796694.5.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Mikhaylova M, Stasinopoulos I, Kato Y, et al. Imaging of cationic multifunctional liposome-mediated delivery of COX-2 siRNA. Cancer Gene Ther. 2009;16:217–26. doi: 10.1038/cgt.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podesta JE, Kostarelos K. Chapter 17 - Engineering cationic liposome siRNA complexes for in vitro and in vivo delivery. Methods Enzymol. 2009;464:343–54. doi: 10.1016/S0076-6879(09)64017-9. [DOI] [PubMed] [Google Scholar]
- 19.Aumelas A, Serrero A, Durand A, et al. Nanoparticles of hydrophobically modified dextrans as potential drug carrier systems. Colloids Surf B Biointerfaces. 2007;59:74–80. doi: 10.1016/j.colsurfb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull (Tokyo) 2010;58:1423–30. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- 21.Bisht S, Maitra A. Dextran-doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:415–25. doi: 10.1002/wnan.43. [DOI] [PubMed] [Google Scholar]
- 22.Simi CK, Emilia Abraham T. Hydrophobic grafted and cross-linked starch nanoparticles for drug delivery. Bioprocess Biosyst Eng. 2007;30:173–80. doi: 10.1007/s00449-007-0112-5. [DOI] [PubMed] [Google Scholar]
- 23.Shen C, Brozena AH, Wang Y. Double-walled carbon nanotubes: Challenges and opportunities. Nanoscale. 2010 doi: 10.1039/c0nr00620c. [DOI] [PubMed] [Google Scholar]
- 24.Tran MA, Smith CD, Kester M, et al. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14:3571–81. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 25.Cloninger M. Dendrimers and protein cages as nanoparticles in drug delivery. Drug Discov Today. 2004;9:111–2. doi: 10.1016/S1359-6446(03)02954-4. [DOI] [PubMed] [Google Scholar]
- 26.Caminade AM, Majoral JP. Dendrimers and nanotubes: a fruitful association. Chem Soc Rev. 2010;39:2034–47. doi: 10.1039/b926408f. [DOI] [PubMed] [Google Scholar]
- 27.Thakare VS, Das M, Jain AK, et al. Carbon nanotubes in cancer theragnosis. Nanomedicine (Lond) 2010;5:1277–301. doi: 10.2217/nnm.10.95. [DOI] [PubMed] [Google Scholar]
- 28.Huang N, Wang H, Zhao J, et al. Single-wall carbon nanotubes assisted photothermal cancer therapy: animal study with a murine model of squamous cell carcinoma. Lasers Surg Med. 2010;42:638–48. doi: 10.1002/lsm.20968. [DOI] [PubMed] [Google Scholar]
- 29.Kah JC, Wong KY, Neoh KG, et al. Critical parameters in the pegylation of gold nanoshells for biomedical applications: an in vitro macrophage study. J Drug Target. 2009;17:181–93. doi: 10.1080/10611860802582442. [DOI] [PubMed] [Google Scholar]
- 30.Melancon MP, Lu W, Yang Z, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther. 2008;7:1730–9. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauck TS, Chan WC. Gold nanoshells in cancer imaging and therapy: towards clinical application. Nanomedicine (Lond) 2007;2:735–8. doi: 10.2217/17435889.2.5.735. [DOI] [PubMed] [Google Scholar]
- 32.Ji X, Shao R, Elliott AM, et al. Bifunctional Gold Nanoshells with a Superparamagnetic Iron Oxide-Silica Core Suitable for Both MR Imaging and Photothermal Therapy. J Phys Chem C Nanomater Interfaces. 2007;111:6245. doi: 10.1021/jp0702245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C, Rait A, Pirollo KF, et al. Nanoimmunoliposome delivery of superparamagnetic iron oxide markedly enhances targeting and uptake in human cancer cells in vitro and in vivo. Nanomedicine. 2008;4:318–29. doi: 10.1016/j.nano.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhyner MN, Smith AM, Gao X, et al. Quantum dots and multifunctional nanoparticles: new contrast agents for tumor imaging. Nanomedicine (Lond) 2006;1:209–17. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 35.Tran MA, Watts RJ, Robertson GP. Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigment Cell Melanoma Res. 2009;22:388–99. doi: 10.1111/j.1755-148X.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen TM. Liposomes. Opportunities in drug delivery Drugs. 1997;54 4:8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 37.Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv 2011. 2011:326497. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park YS. Tumor-directed targeting of liposomes. Biosci Rep. 2002;22:267–81. doi: 10.1023/a:1020190606757. [DOI] [PubMed] [Google Scholar]
- 39.Farhood H, G X, Son K, Yang YY, Lazo JS, Huang L. Gene Therapy for Neoplastic Diseases. Annals of the New York Academy of Sciences; 1994. Cationic Liposomes for Direct Gene Transfer in Therapy of Cancer and Other Diseases†; pp. 23–35. [DOI] [PubMed] [Google Scholar]
- 40.Dadashzadeh S, Mirahmadi N, Babaei MH, et al. Peritoneal retention of liposomes: Effects of lipid composition, PEG coating and liposome charge. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landen CN, Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 43.Miller CR, Bondurant B, McLean SD, et al. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–83. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 44.Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approches for the delivery of siRNA in cancer. Journal of INTERNAL MEDICINE. 2009;267:44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 45.Gewirtz AM. RNA targeted therapeutics for hematologic malignancies. Blood Cells Mol Dis. 2007;38:117–9. doi: 10.1016/j.bcmd.2006.10.162. [DOI] [PubMed] [Google Scholar]
- 46.Spagnou S, Miller AD, Keller M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry. 2004;43:13348–56. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- 47.Akar U, Chaves-Reyez A, Barria M, et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4:669–79. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 48.Huwyler J, Drewe J, Krahenbuhl S. Tumor targeting using liposomal antineoplastic drugs. Int J Nanomedicine. 2008;3:21–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Yu D, Peng P, Dharap SS, et al. Antitumor activity of poly(ethylene glycol)-camptothecin conjugate: the inhibition of tumor growth in vivo. J Control Release. 2005;110:90–102. doi: 10.1016/j.jconrel.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 50.Johansen PB. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemotherapy and Pharmacology. 1981;5:267–70. doi: 10.1007/BF00434396. [DOI] [PubMed] [Google Scholar]
- 51.Lindner LH, Eichhorn ME, Eibl H, et al. Novel temperature-sensitive liposomes with prolonged circulation time. Clin Cancer Res. 2004;10:2168–78. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 52.Alberts DS, Garcia DJ. Safety aspects of pegylated liposomal doxorubicin in patients with cancer. Drugs. 1997;54 4:30–5. doi: 10.2165/00003495-199700544-00007. [DOI] [PubMed] [Google Scholar]
- 53.Alberts DS, Liu PY, Wilczynski SP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200) Gynecol Oncol. 2008;108:90–4. doi: 10.1016/j.ygyno.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holloway RW, Finkler NJ, Nye LP, Bigsby GE, Ortiz BH. Doxil and gemcitabine combination therapy for recurrent ovarian cancer: Results of a phase II trial. Journal of Clinical Oncology; 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition); 2004. p. 5090. [Google Scholar]
- 55.Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15:987–93. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 56.Muggia FM. Clinical efficacy and prospects for use of pegylated liposomal doxorubicin in the treatment of ovarian and breast cancers. Drugs. 1997;54 4:22–9. doi: 10.2165/00003495-199700544-00006. [DOI] [PubMed] [Google Scholar]
- 57.Barenholz Y. Liposome application: problems and prospects. Current Opinion in Colloid & Interface Science. 2001;6:66–77. [Google Scholar]
- 58.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, et al. Nanoparticles and the immune system. Endocrinology. 2010;151:458–65. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dokka S, Toledo D, Shi X, et al. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–5. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 60.Lv H, Zhang S, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–9. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–82. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Dokka S, T D, Shi X, Castranova V, Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000 May;:521–25. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 63.Filion Ma, P NC. Major limitations in the use of cationic liposomes for DNA delivery International Journal of Pharmaceutics. 1998;162:159–70. [Google Scholar]
- 64.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 65.Larocque j, bharali dj, mousa sa. Cancer detection and treatment: the role of nanomedicine. Molecular Biotechnology. 2003;42:358–66. doi: 10.1007/s12033-009-9161-0. [DOI] [PubMed] [Google Scholar]
- 66.Berry G, Billingham M, Alderman E, et al. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi's sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol. 1998;9:711–6. doi: 10.1023/a:1008216430806. [DOI] [PubMed] [Google Scholar]
- 67.Perez AT, Domenech GH, Frankel C, et al. Pegylated liposomal doxorubicin (Doxil) for metastatic breast cancer: the Cancer Research Network, Inc., experience. Cancer Invest. 2002;20 2:22–9. doi: 10.1081/cnv-120014883. [DOI] [PubMed] [Google Scholar]
- 68.Goram AL, Richmond PL. Pegylated Liposomal Doxorubicin: Tolerability and Toxicity. Pharmacotherapy. 2001;21:751–63. doi: 10.1592/phco.21.7.751.34572. [DOI] [PubMed] [Google Scholar]
- 69.Dhaneshwar SS, K M, Gairola N, Kadam SS. Dextran: A promising macromolecular drug carrier. Indian Journal of Pharmaceitical Sciences. 2006;68:705–14. [Google Scholar]
- 70.Gervelas C, Avramoglou T, Crepin M, et al. Growth inhibition of human melanoma tumor cells by the combination of sodium phenylacetate (NaPA) and substituted dextrans and one NaPA-dextran conjugate. Anticancer Drugs. 2002;13:37–45. doi: 10.1097/00001813-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Ichinose K, Tomiyama N, Nakashima M, et al. Antitumor activity of dextran derivatives immobilizing platinum complex (II) Anticancer Drugs. 2000;11:33–8. doi: 10.1097/00001813-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Lam W, Leung CH, Chan HL, et al. Toxicity and DNA binding of dextran-doxorubicin conjugates in multidrug-resistant KB-V1 cells: optimization of dextran size. Anticancer Drugs. 2000;11:377–84. doi: 10.1097/00001813-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Shrivastava SK, Jain DK, Trivedi P. Dextrans--potential polymeric drug carriers for suprofen. Pharmazie. 2003;58:804–6. [PubMed] [Google Scholar]
- 74.Porter KA, Blackburn GL, Bistrian BR. Safety of iron dextran in total parenteral nutrition: a case report. J Am Coll Nutr. 1988;7:107–10. doi: 10.1080/07315724.1988.10720226. [DOI] [PubMed] [Google Scholar]
- 75.Dhaneshwar S, Kandpal M, Gairola N, Kadam SS. Dextran: A promising macromolecular drug carrier. Indian Journal of Pharmaceitical Sciences. 2006;68:705–14. [Google Scholar]
- 76.Darrell B, Phillip O, Buncke J, Harry J. Dextran-Induced Acute Renal Failure after Microvascular Muscle Transplantation. Plastic & Reconstructive Surgery. 2001 December;108:2057–60. doi: 10.1097/00006534-200112000-00034. [DOI] [PubMed] [Google Scholar]
- 77.Hawker CJ, Wooley KL. The convergence of synthetic organic and polymer chemistries. Science. 2005;309:1200–5. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 78.Jain R, Shah NH, Malick AW, et al. Controlled drug delivery by biodegradable poly(ester) devices: different preparative approaches. Drug Dev Ind Pharm. 1998;24:703–27. doi: 10.3109/03639049809082719. [DOI] [PubMed] [Google Scholar]
- 79.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 80.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 81.Wickline SA, Neubauer AM, Winter PM, et al. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–80. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 82.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17:247–89. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 83.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 84.Kitchell JP, Wise DL. Poly(lactic/glycolic acid) biodegradable drug-polymer matrix systems. Methods Enzymol. 1985;112:436–48. doi: 10.1016/s0076-6879(85)12034-3. [DOI] [PubMed] [Google Scholar]
- 85.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 86.Musumeci T, Ventura CA, Giannone I, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325:172–9. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 87.Musumeci T, Vicari L, Ventura CA, et al. Lyoprotected nanosphere formulations for paclitaxel controlled delivery. J Nanosci Nanotechnol. 2006;6:3118–25. doi: 10.1166/jnn.2006.452. [DOI] [PubMed] [Google Scholar]
- 88.Saxena V, Sadoqi M, Shao J. Polymeric nanoparticulate delivery system for Indocyanine green: biodistribution in healthy mice. Int J Pharm. 2006;308:200–4. doi: 10.1016/j.ijpharm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Vorp DA, Maul T, Nieponice A. Molecular aspects of vascular tissue engineering. Front Biosci. 2005;10:768–89. doi: 10.2741/1571. [DOI] [PubMed] [Google Scholar]
- 90.Lim HJ, Nam HY, Lee BH, et al. A novel technique for loading of paclitaxel-PLGA nanoparticles onto ePTFE vascular grafts. Biotechnol Prog. 2007;23:693–7. doi: 10.1021/bp060338i. [DOI] [PubMed] [Google Scholar]
- 91.Semete B, Booysen L, Lemmer Y, et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 6:662–71. doi: 10.1016/j.nano.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 92.Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40:173–90. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 93.Medina SH, El-Sayed ME. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 2009;109:3141–57. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 94.Nanjwade BK, Bechra HM, Derkar GK, et al. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38:185–96. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 95.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 96.Surendiran A, Sandhiya S, Pradhan SC, et al. Novel applications of nanotechnology in medicine. Indian J Med Res. 2009;130:689–701. [PubMed] [Google Scholar]
- 97.Chandrasekar D, Sistla R, Ahmad FJ, et al. The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials. 2007;28:504–12. doi: 10.1016/j.biomaterials.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 98.Svenson S. Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm. 2009;71:445–62. doi: 10.1016/j.ejpb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 99.Balogh L, Swanson DR, Tomalia DA, et al. Dendrime-Silver Complexes and Nanocomposites as Antimicrobial Agents. Nano Letters. 2000;1:18–21. [Google Scholar]
- 100.Bhadra D, Bhadra S, Jain S, et al. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–24. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 101.Chauhan AS, Sridevi S, Chalasani KB, et al. Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin. J Control Release. 2003;90:335–43. doi: 10.1016/s0168-3659(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 102.Jevprasesphant R, Penny J, Jalal R, et al. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–6. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 103.Fischer D, Li Y, Ahlemeyer B, et al. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 104.El-Sayed M, Ginski M, Rhodes C, et al. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Release. 2002;81:355–65. doi: 10.1016/s0168-3659(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 105.Bourne N, Stanberry LR, Kern ER, et al. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob Agents Chemother. 2000;44:2471–4. doi: 10.1128/aac.44.9.2471-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Z, Tabakman S, Welsher K, et al. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–60. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaudhuri P, Soni S, Sengupta S. Single-walled carbon nanotube-conjugated chemotherapy exhibits increased therapeutic index in melanoma. Nanotechnology. 2010;21:025102. doi: 10.1088/0957-4484/21/2/025102. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z, Fan AC, Rakhra K, et al. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew Chem Int Ed Engl. 2009;48:7668–72. doi: 10.1002/anie.200902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh S, Nalwa HS. Nanotechnology and health safety--toxicity and risk assessments of nanostructured materials on human health. J Nanosci Nanotechnol. 2007;7:3048–70. doi: 10.1166/jnn.2007.922. [DOI] [PubMed] [Google Scholar]
- 111.Radomski A, Jurasz P, Alonso-Escolano D, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–93. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 113.Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–26. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 114.Warheit DB, Laurence BR, Reed KL, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–25. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 115.Ravichandran P, Periyakaruppan A, Sadanandan B, et al. Induction of apoptosis in rat lung epithelial cells by multiwalled carbon nanotubes. J Biochem Mol Toxicol. 2009;23:333–44. doi: 10.1002/jbt.20296. [DOI] [PubMed] [Google Scholar]
- 116.Reddy AR, Reddy YN, Krishna DR, et al. Pulmonary toxicity assessment of multiwalled carbon nanotubes in rats following intratracheal instillation. Environ Toxicol. 2010 doi: 10.1002/tox.20632. [DOI] [PubMed] [Google Scholar]
- 117.Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–31. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60:1289–306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Geddes CD, Parfenov A, Lakowicz JR. Photodeposition of silver can result in metal-enhanced fluorescence. Appl Spectrosc. 2003;57:526–31. doi: 10.1366/000370203321666542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 121.Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–7. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 122.Kawano T, Yamagata M, Takahashi H, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J Control Release. 2006;111:382–9. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 123.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 124.O'Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 125.Murphy CJ, Gole AM, Stone JW, et al. Gold nanoparticles in biology: beyond toxicity to cellular i maging. Acc Chem Res. 2008;41:1721–30. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 126.Zwiorek K, Kloeckner J, Wagner E, et al. Gelatin nanoparticles as a new and simple gene delivery system. J Pharm Pharm Sci. 2005;7:22–8. [PubMed] [Google Scholar]
- 127.Zamiri R, Azmi BZ, Sadrolhosseini AR, et al. Preparation of silver nanoparticles in virgin coconut oil using laser ablation. Int J Nanomedicine. 2011;6:71–5. doi: 10.2147/IJN.S14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmad N, Sharma S, Singh VN, et al. Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A Novel Approach Towards Weed Utilization. Biotechnol Res Int 2011. 2011:454090. doi: 10.4061/2011/454090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keelan JA. Nanotoxicology: nanoparticles versus the placenta. Nat Nanotechnol. 2011;6:263–4. doi: 10.1038/nnano.2011.65. [DOI] [PubMed] [Google Scholar]
- 130.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34:J234–46. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L, Yu F, Cole AJ, et al. Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging. AAPS J. 2009;11:693–9. doi: 10.1208/s12248-009-9151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shahverdi AR, Fakhimi A, Shahverdi HR, et al. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–71. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 133.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 134.Rutberg FG, Dubina MV, Kolikov VA, et al. Effect of silver oxide nanoparticles on tumor growth in vivo. Dokl Biochem Biophys. 2008;421:191–3. doi: 10.1134/s1607672908040078. [DOI] [PubMed] [Google Scholar]
- 135.Hussain SM, Hess KL, Gearhart JM, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19:975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 136.Neuberger T, Schöpfa B, Hofmannb H, Hofmannc M, Rechenberg VN. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. Journal of Magnetism and Magnetic Materials. 2005;293:483–96. [Google Scholar]
- 137.Hofmann-Amtenbrink M, Rechenberg VON B, Hofmann H. Superparamagnetic nanoparticles for biomedical applications. 2009:121–49. [Google Scholar]
- 138.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 139.Saboktakin MR, M A, Ramazanov MA. Synthesis and characterization of superparamagnetic nanoparticles coated with carboxymethyl starch (CMS) for magnetic resonance imaging technique. J Carbohydr Polym. 2009;78(2):292–95. [Google Scholar]
- 140.Meng J, F J, Galiana G, et al. LHR- Functionalized superparamagnetic iron oxide nanoparticles for breast cancer targeting and contrast enhancement in MRI. [J] Mat Sci Eng C-BioS Science. 2009;29:1467–79. [Google Scholar]
- 141.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11 2:S81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 142.Rudge S, Peterson C, Vessely C, et al. Adsorption and desorption of chemotherapeutic drugs from a magnetically targeted carrier (MTC) J Control Release. 2001;74:335–40. doi: 10.1016/s0168-3659(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 143.Ström V, Hultenby K, Gröttner C, Teller J, Xu B, Holgersson J. A novel and rapid method for quantification of magnetic nanoparticle cell interactions using a desktop susceptometer. Nanotechnology. 2004;15:457–66. [Google Scholar]
- 144.Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A. 2010 doi: 10.1002/jbm.a.32972. [DOI] [PubMed] [Google Scholar]
- 145.Smith AM, Dave S, Nie S, et al. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn. 2006;6:231–44. doi: 10.1586/14737159.6.2.231. [DOI] [PubMed] [Google Scholar]
- 146.Tang M, Xing T, Zeng J, et al. Unmodified CdSe quantum dots induce elevation of cytoplasmic calcium levels and impairment of functional properties of sodium channels in rat primary cultured hippocampal neurons. Environ Health Perspect. 2008;116:915–22. doi: 10.1289/ehp.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–72. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chan WC, Maxwell DJ, Gao X, et al. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. 2002;13:40–6. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 149.Colton HM, Falls JG, Ni H, et al. Visualization and quantitation of peroxisomes using fluorescent nanocrystals: treatment of rats and monkeys with fibrates and detection in the liver. Toxicol Sci. 2004;80:183–92. doi: 10.1093/toxsci/kfh144. [DOI] [PubMed] [Google Scholar]
- 150.Dubertret B, Skourides P, Norris DJ, et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 151.Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 152.Lidke DS, Nagy P, Heintzmann R, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- 153.Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 154.Rudge SR, Kurtz TL, Vessely CR, et al. Preparation, characterization, and performance of magnetic iron-carbon composite microparticles for chemotherapy. Biomaterials. 2000;21:1411–20. doi: 10.1016/s0142-9612(00)00006-5. [DOI] [PubMed] [Google Scholar]
- 155.Scherer F, Anton M, Schillinger U, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–9. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 156.Choi AO, Cho SJ, Desbarats J, et al. Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J Nanobiotechnology. 2007;5:1. doi: 10.1186/1477-3155-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoshino A, Fujioka K, Oku T, et al. Quantum dots targeted to the assigned organelle in living cells. Microbiol Immunol. 2004;48:985–94. doi: 10.1111/j.1348-0421.2004.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 158.Shiohara A, Hoshino A, Hanaki K, et al. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004;48:669–75. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 159.Hoshino A, Hanaki K, Suzuki K, et al. Applications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse body. Biochem Biophys Res Commun. 2004;314:46–53. doi: 10.1016/j.bbrc.2003.11.185. [DOI] [PubMed] [Google Scholar]
- 160.El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: an overview. J Toxicol 2009. 2009:754810. doi: 10.1155/2009/754810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hall JB, Dobrovolskaia MA, Patri AK, et al. Characterization of nanoparticles for therapeutics. Nanomedicine (Lond) 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 162.Murdock RC, Braydich-Stolle L, Schrand AM, et al. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008;101:239–53. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- 163.Powers KW, Brown SC, Krishna VB, et al. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation Toxicol Sci. 2006;90:296–303. doi: 10.1093/toxsci/kfj099. [DOI] [PubMed] [Google Scholar]
- 164.Barreto JA, O'Malley W, Kubeil M, et al. Nanomaterials: Applications in Cancer Imaging and Therapy. Advanced Materials. 2011;23:H18–H40. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 165.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 166.Manocha B, Margaritis A. Production and Characterization of Œ≥-Polyglutamic Acid Nanoparticles for Controlled Anticancer Drug Release. Critical Reviews in Biotechnology. 2008;28:83–99. doi: 10.1080/07388550802107483. [DOI] [PubMed] [Google Scholar]
- 167.Borm P, Klaessig FC, Landry TD, et al. Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 168.Hall JB, D MA, Patriak, McNeil SE. Characterization of nanoparticles for therapeutics. Nanomedicine (Lond) 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 169.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 170.United States Pharmacopeia (United States Pharmacopeial Convention IURPT [Google Scholar]
- 171.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–60. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dhawan A, Sharma V, Parmar D. Nanomaterials: A challenge for toxicologists. Nanotoxicology. 2008;3:1–9. [Google Scholar]
- 173.Gosens I, Post JA, de la Fonteyne LJ, et al. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol. 2010;7:37. doi: 10.1186/1743-8977-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Skebo JE, Grabinski CM, Schrand AM, et al. Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. Int J Toxicol. 2007;26:135–41. doi: 10.1080/10915810701226248. [DOI] [PubMed] [Google Scholar]
- 175.Hatziantoniou S, Deli G, Nikas Y, et al. Scanning electron microscopy study on nanoemulsions and solid lipid nanoparticles containing high amounts of ceramides. Micron. 2007;38:819–23. doi: 10.1016/j.micron.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 176.P Somani, U M. Importance of transmission electron microscopy for carbon nanomaterials research. Modern research and educational topics in microscopy. 2007:634–42. [Google Scholar]
- 177.Berne B, P R. Dynamic light scattering: with applications to chemistry, biology and physics. NY, USA: Dover Publications; 2000. [Google Scholar]
- 178.United States Pharmacopeia (United States Pharmacopeial Convention IBET [Google Scholar]
- 179.Mickuviene I, Kirveliene V, Juodka B. Experimental survey of non-clonogenic viability assays for adherent cells in vitro. Toxicol In Vitro. 2004;18:639–48. doi: 10.1016/j.tiv.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 180.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journ Immuno Method. 1998;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 181.Hong S, Bielinska AU, Mecke A, et al. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug Chem. 2004;15:774–82. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 182.Tuschl H, Schwab CE. Flow cytometric methods used as screening tests for basal toxicity of chemicals. Toxicol In Vitro. 2004;18:483–91. doi: 10.1016/j.tiv.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 183.Stone V, Shaw J, Brown DM, et al. The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol In Vitro. 1998;12:649–59. doi: 10.1016/s0887-2333(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 184.Foucaud L, Wilson MR, Brown DM, et al. Measurement of reactive species production by nanoparticles prepared in biologically relevant media. Toxicol Lett. 2007;174:1–9. doi: 10.1016/j.toxlet.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 185.Schins RP, Duffin R, Hohr D, et al. Surface modification of quartz inhibits toxicity, particle uptake, and oxidative DNA damage in human lung epithelial cells. Chem Res Toxicol. 2002;15:1166–73. doi: 10.1021/tx025558u. [DOI] [PubMed] [Google Scholar]
- 186.Wilson MR, Lightbody JH, Donaldson K, et al. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 2002;184:172–9. doi: 10.1006/taap.2002.9501. [DOI] [PubMed] [Google Scholar]
- ••187.Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39:613–26. doi: 10.1080/10408440903120975. This is an up-to-date article on nonanoparticle toxicity testing emphasizing on various assay methods toassess the cytotoxity and biological reactivity of nanoparticles. [DOI] [PubMed] [Google Scholar]
- 188.Xiao GG, Wang M, Li N, et al. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–90. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 189.Landsiedel R, Kapp MD, Schulz M, et al. Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations--many questions, some answers. Mutat Res. 2009;681:241–58. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 190.Jacobsen NR, Pojana G, White P, et al. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells. Environ Mol Mutagen. 2008;49:476–87. doi: 10.1002/em.20406. [DOI] [PubMed] [Google Scholar]
- 191.Mroz RM, Schins RP, Li H, et al. Nanoparticle-driven DNA damage mimics irradiation-related carcinogenesis pathways. Eur Respir J. 2008;31:241–51. doi: 10.1183/09031936.00006707. [DOI] [PubMed] [Google Scholar]
- 192.Grainger DW. Nanotoxicity assessment: all small talk? Adv Drug Deliv Rev. 2009;61:419–21. doi: 10.1016/j.addr.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Home - Nanotechnology Characterization Lab. 2011 [cited 2011 11]; Available from: http://ncl.cancer.gov/
- 194.Jang J, Lim DH, Choi IH. The impact of nanomaterials in immune system. Immune Netw. 2010;10:85–91. doi: 10.4110/in.2010.10.3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mohanraj V, C Y. Nanoparticles – A Review. Tropical Journal of Pharmaceutical Research. 2006:561–73. [Google Scholar]
- 196.Tran MA, Gowda R, Sharma A, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Emerich DF, Thanos CG. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol Eng. 2006;23:171–84. doi: 10.1016/j.bioeng.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 198.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–6. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gardikis K, Hatziantoniou S, Bucos M, et al. New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy. J Pharm Sci. 2010;99:3561–71. doi: 10.1002/jps.22121. [DOI] [PubMed] [Google Scholar]
- 200.Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]


