Abstract
Background and Objectives
Colorectal tumors are often observed with tumor infiltrating lymphocytes, presumably as a host-immune response, and patterns may segregate by types of genomic instability. Microsatellite unstable (MSI) colorectal cancers contain a pronounced lymphocyte reaction that can pathologically identify these tumors. Colorectal tumors with elevated microsatellite alterations at selected tetranucleotide repeats (EMAST) have not been examined for lymphocyte patterns.
Methods
We evaluated a 108-person cohort with 24 adenomas and 84 colorectal cancers for MSI and EMAST. Immunohistochemical detection of CD4+ and CD8+ T cell infiltration were performed. Prognostic relevance was assessed by survival analysis.
Results
CD8+ T cell infiltration in the tumor cell nest (p=0.013) and tumor stroma (p=0.004) were more prominent in moderately- and poorly-differentiated adenocarcinoma than in adenoma and well-differentiated adenocarcinoma. CD8+ T cells in the tumor cell nest (p=0.002) and tumor stroma (p=0.009) were at higher density in tumors with ulcerating features compared with tumors with a sessile or polypoid appearance. MSI-H tumors showed a higher density of CD8+ T cell infiltrations in tumor cell nests (p=0.003) and tumor stroma (p=0.001). EMAST-positive tumors showed higher density of CD8+ T cell infiltrations than EMAST-negative tumors both in tumor cell nest (p=0.027) and in tumor stroma (p=0.003). These changes were not observed with CD4+ T lymphocytes. There was no difference in cancer patient survival based on density of CD8+ cells.
Conclusions
CD8+ T lymphocytes, but not CD4+ cells, were increased in tumor cell nests and the tumor stroma in both MSI and EMAST tumors, and showed higher infiltration in ulcerated tumors. CD8+ T lymphocyte infiltration is associated with both EMAST and MSI patterns, and increases with histological advancement.
Keywords: colorectal cancer, adenoma, T lymphocyte, microsatellite instability, elevated microsatellite alterations at selected tetranucleotide repeats
INTRODUCTION
Colorectal tumors are often associated with tumor infiltrating lymphocytes, presumably as a host immune response to malignant cells [1,2]. The immune response is purportedly initiated when cytotoxic T lymphocyte CD8+ cells or CD4+ T helper cell recruitment is triggered by “antigens” from malignant cells [2–5]. Some studies indicate the CD8+ T cells suppress cancer cell growth and micrometastasis, whereas CD4+ T cells causes degeneration of tumor cells [6]. In patients with colorectal cancer, the presence of CD8+ T cells within cancer cell nests [7] and the tumor stroma [8] are known to be associated with improved survival. Evidence is mounting that the presence of lymphocytes within a colorectal tumor confers a better prognosis for patient survival, and may be more informative than TNM staging [1].
Analysis of tumor infiltrating lymphocytes based on the type of genomic instability in the colorectal tumor is limited. One form of genomic instability is exemplified by microsatellite unstable (MSI) colorectal cancers, which has a distinct pattern for lymphocytes in and around the tumor, and can be recognized by pathologists trained to identify MSI tumors [9]. A “Crohn’s-like” pattern of lymphocytes often surrounds the MSI tumor and is so named because it resembles granuloma formation in Crohn’s disease and represents an intense lymphocyte response [2,9]. Evidence suggests that MSI tumors release neo-antigens, the product of truncated proteins produced as a result of frameshift mutation within coding microsatellites of specific genes [2–4,10]. This inflammatory process may “contain” or limit growth of the MSI tumor, and might ultimately be responsible for improved patient survival when compared to same-staged patients with non-MSI tumors. MSI is the result of a defective DNA mismatch repair (MMR) system within tumor cells [9].
A second form of MSI, EMAST, is not associated with major MMR protein defects, but is linked to heterogenous and reduced expression of the minor MMR protein hMSH3 in sporadic colorectal cancers [11–13]. EMAST can encompass some MSI tumors, but most are MSS. Recent data indicate that EMAST progresses with histological advancement from adenoma to carcinoma in the colon, is associated with lymphocyte infiltration, may be related to advanced tumor stages, and is more frequent in African American with sporadic colorectal cancers [12,13].
Tumor lymphocyte infiltration is often described by the site within the tumor. Lymphocytes infiltrating the tumor epithelium, tumor stroma, or the tumor nest describe cells within, around, or surrounding the tumor [7,13]. Lymphocytes can also be differentiated by basic type: CD4+ T helper and CD8+ T suppressor [1]. It is the CD8+ T cells that have been suggested more closely aligned with improved prognosis for patients with colorectal cancer [7,8]. In this study, we assessed lymphocyte patterns for EMAST tumors in both adenomas and cancers. We observed a correlation of CD8+ T cells over CD4+ T cells, particularly when present in tumor nests and stroma.
MATERIALS and METHODS
Tissue samples
We evaluated a cohort of 108 patients with 24 adenomas and 84 colorectal cancers (9 well-differentiated, 69 moderately-differentiated, and 6 poorly-differentiated cell types) [12] that we characterized for MSI and EMAST. The samples were collected between 2008 and 2009 at Konkuk University Medical Center, Seoul, South Korea. The patients gave informed consent, and the study was approved by the Institutional Review Board of Konkuk University Medical Center and by the Institutional Review Board on Human Subjects Protection at University of California San Diego.
Genomic DNA extraction, MSI, and EMAST analysis
Serial 10 μm sections of tissues were cut from formalin-fixed and paraffin-embedded tissues and mounted on glass slides. Microdissection was performed to extract genomic DNA from tumor and counterpart normal tissue. Genomic DNA from microdissected tissues was isolated using Qiagen DNeasy Blood and Tissue Kit (Qiagen, USA) following the manufacturer’s instruction. Briefly, one thousand μl of xylene was added to each eppendorf tube containing microdissected tissue, and the tubes were centrifuged at 14,000 rpm for 5 min at RT. After removing the supernatant, 1000 μl of 100% ethanol was added, and all the tubes were centrifuged at 14,000 rpm for 5 min at RT. After removing the ethanol, the samples were incubated at 37°C for 15–25 minutes until the ethanol was evaporated. Tissues were incubated in lysis buffer solution containing 20 μl of proteinase K solution at 56°C overnight. On the following day, samples were treated with buffers according to the manufacturer’s instructions, and were centrifuged at 8,000 rpm for 1 min twice and 14,000 rpm for 3 min. After incubating at room temperature for 5 min, DNA was extracted by centrifuging at 8,000 rpm for 1 min.
MSI analysis was performed using five mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and three dinucleotide markers (D2S123, D17S250, and D5S346) for MSI determination and two polymorphic pentanucleotide markers (Penta C and Penta D) for sample identification. We interpreted microsatellite instability as >30% of markers mutated as MSI-high, mutation at <30% but >0% of examined loci as MSI-low, and no instability at any of the loci tested as microsatellite stable (MSS).
To determine EMAST, 5 polymorphic tetranucleotide markers (MYCL1, D9S242, D20S85, D8S321, and D20S82) were utilized [12]. Genomic DNA extracted from paired tumor and normal samples were PCR-amplified by specific primers for each EMAST marker using Platinum® PCR Supermix (Invitrogen, USA) as per the manufacturer’s protocol. The PCR products were sequenced using an ABI 3700 analyzer to identify mutation at the tetranucleotide repeats of each loci. Tumors showing frameshifts in at least 2 tetranucleotide markers were categorized as EMAST-positive tumors.
Immunohistochemical staining for T cell subtypes
Formalin-fixed, paraffin embedded sections containing colon tumor tissue were deparaffinized in xylene and rehydrated in graded alcohols to water. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 20 min. The slides were immersed in sodium citrate buffer (pH 6.0) and heated in a microwave for 10 min for antigen retrieval. After washing with phosphate-buffered saline (PBS), tissues were incubated with 1% bovine serum albumin for 30 min. Slides were then processed using a monoclonal mouse anti-human CD4 and CD8 antibody (DAKO Corp., Carpinteria, CA) in a humidity chamber overnight at 4°C. After washing with PBS, biotinylated goat anti-mouse immunoglobulin G was added for 30 minutes, followed by incubation with peroxidase-labeled streptavidin for 30 minutes at room temperature. Sections were then incubated with DAB chromogen solution for 8 min, lightly counterstained in Mayer’s hematoxylin solution and 0.08% ammonium hydroxide (10X) after rinsing with distilled water. Slides were coverslipped for microscopic analysis, and then analyzed by the methods of Naito et al [7]. The degree of CD4+ and CD8+ T cell infiltration was graded from none (no stain), mild (<5%), moderate (5–25%), and marked (>25%), respectively.
Statistical analysis
To study the statistical association between T cell infiltration and other variables, continuous and categorical demographic data were compared by Student t-tests and chi-square tests (Fisher’s exact test when numbers expected per cell are less than 5), respectively. A p-value of less than 0.05 was considered statistically significant.
Prognostic relevance of lymphocyte infiltration was assessed by survival analysis. The index date for survival time calculation was defined as the date for colorectal tumor. The overall survival time were calculated from the index date to the date of last information. Survival curves were determined by using the Kaplan-Meier method and were analyzed by using the log-rank test.
RESULTS
CD8+ T lymphocytes have increased infiltration in moderately- and poorly-differentiated adenocarcinomas, compared with adenoma and well-differentiated adenocarcinoma
We defined T lymphocyte patterns as cells surrounding the tumor, surrounding the epithelium, and within the epithelium as tumor margin, tumor nests, and tumor infiltration, respectively [7,13]. Patterns of CD4+ and CD8+ T cell infiltration in colorectal adenoma versus cancer are summarized in Table 1. CD8+ T cell infiltration in tumor cell nests (p=0.013) and tumor stroma (p=0.004) were more prominent in moderately- and poorly-differentiated adenocarcinomas than in adenoma or well-differentiated adenocarcinoma (Figure 1). On the other hand, CD4+ T cell infiltration in tumor cell nests (p=0.053) and tumor stroma (p=0.454) were not related to histological differentiation. In addition, other clinicopathological factors such as age, gender, size, location of the tumor, lymph node metastasis, microvascular invasion, and TNM staging were not significantly related to the density of T cell infiltration.
Table 1.
Association between T cell infiltration and histology
| Adenoma (n=24) | Well differentiated adenocarcinoma (n=9) | Moderately differentiated adenocarcinoma (n=69) | Poorly differentiated adenocarcinoma (n=6) | p-value | |
|---|---|---|---|---|---|
|
T cell infiltration in tumor cell nests
| |||||
| CD4 (none: mild: mod.: marked) | 10 : 9: 5 : 0 | 3 : 0 : 4 :2 | 40 : 12 : 12 : 5 | 3 : 2 : 0 : 1 | 0.053 |
| CD8 (mild: mod.: marked) | 13 : 11 : 0 | 2 : 6 : 1 | 18 : 41 : 10 | 1 : 2 : 3 | 0.013 |
| Ratio (CD4=CD8: CD4>CD8: CD4<CD8) | 10 : 1 : 13 | 4 : 2 : 3 | 12 : 6 : 51 | 0 : 1 : 5 | 0.045 |
|
| |||||
|
T cell infiltration in tumor stroma
| |||||
| CD4 (none: mild) | 24 : 0 | 8 : 1 | 66 : 3 | 6 : 0 | 0.454 |
| CD8 (none: mild: mod.: marked) | 14 : 10 : 0 : 0 | 3 : 6 : 0 : 0 | 26 : 32 : 10 : 1 | 0 : 2 : 4 : 0 | 0.004 |
| Ratio (CD4=CD8: CD4>CD8: CD4<CD8) | 14 : 0 : 10 | 4 : 0 : 5 | 29 : 0 : 40 | 0 : 0 : 6 | 0.077 |
Mild < 5%, Moderate 5–25%, Marked > 25% of T cell infiltration.
Figure 1. Examples of T cell infiltration in colorectal tumors.
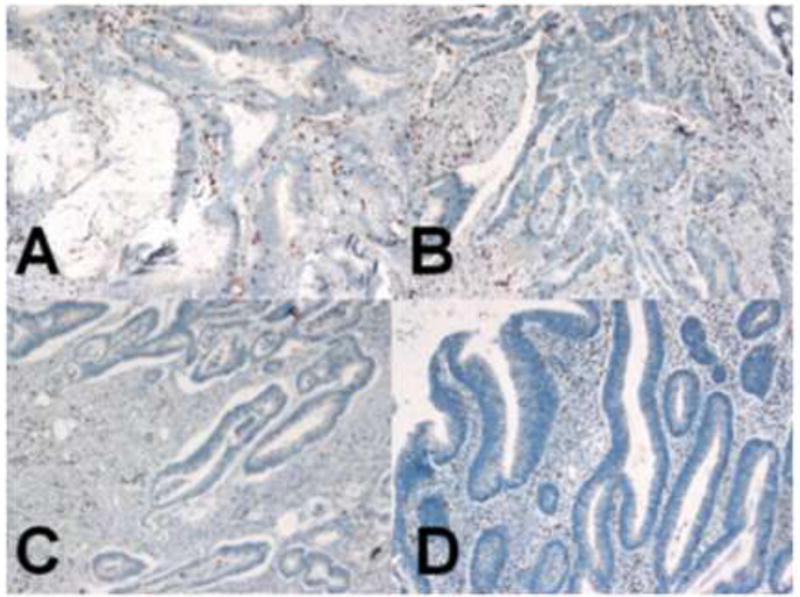
Dark brown colored nuclei indicate CD8+ T cells by anti-CD8 immunohistochemical staining (100X). (A) Poorly-differentiated adenocarcinoma and (B) moderately-differentiated adenocarcinoma showed more prominent CD9+ T cell infiltration in both tumor cell nest and tumor stroma than (C) well-differentiated adenocarcinoma and (D) adenomas.
CD8+ T cells are associated with tumor macroscopic appearance
CD8+ T cells in tumor cell nests (p=0.002) and tumor stroma (p=0.009) were at higher density in tumors with ulcerating features (Table 2) when we classified the macroscopic features into three major types: (i) protruded, elevated, or polypoid, (ii) sessile, superficial, or flat, and (iii) ulceroinfiltrative, depressed, or excavated. Tumors showing multiple macroscopic features were classified based on the predominant subtype.
Table 2.
Association between T cell infiltration and morphology
| Polypoid (n=16) | Sessile (n=25) | Ulceroinfiltrating (n=67) | p-value | |
|---|---|---|---|---|
|
T cell infiltration in tumor cell nests
| ||||
| CD4 (none: mild: mod.: marked) | 5 : 2 : 6 : 3 | 10 : 9 : 5 : 1 | 41 : 12 : 10 : 4 | 0.038 |
| CD8 (mild: mod.: marked) | 1 : 12 : 3 | 15 : 10 : 0 | 18 : 38 : 11 | 0.002 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 5 : 3 : 8 | 9 : 3 : 13 | 12 : 4 : 51 | 0.100 |
|
| ||||
|
T cell infiltration in tumor stroma
| ||||
| CD4 (none: mild) | 14 : 2 | 25 : 0 | 65: 2 | 0.104 |
| CD8 (none: mild: mod.: marked) | 3 : 11 : 1 : 1 | 14 : 11 : 0 : 0 | 26 : 28 : 13 : 0 | 0.009 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 5 : 0 : 11 | 14 : 0 : 11 | 28 : 0 : 39 | 0.266 |
Mild < 5%, Moderate 5–25%, Marked > 25% of T cell infiltration.
CD8+ T cell infiltration, and not CD4+ cells, are important in both MSI and EMAST tumors
We previously indicated that 50% of tumors from 108 patients possessing 24 adenomas and 84 colorectal cancers were EMAST-positive. [12] Additionally, by standardized MSI analysis, there were 6 MSI-H, 10 MSI-L, and 92 MSS colorectal neoplasms among the 108 tumors. All 6 MSI-H (100%) neoplasms were cancers, and all of these (100%) were also EMAST-positive, whereas 7 of 10 MSI-L neoplasms (70%; all MSI-L were cancers) were EMAST-positive. All 24 adenomas were MSS.
MSI-H tumors demonstrated a higher density of CD8+ T cell infiltration in tumor cell nests (p=0.003) and tumor stroma (p=0.001) (Table 3). To eliminate the possibility of MSI-H tumors cross-identifying EMAST tumors, we analyzed our cohort for EMAST after excluding the 6 MSI-H cases. EMAST-positive tumors showed a higher density of CD8+ T cell infiltration than EMAST-negative tumors in tumor cell nests (p=0.027) and tumor stroma (p=0.003) (Table 4). For both MSI and EMAST analyses, CD4+ T lymphocyte infiltration did not correlate with genomic subtype.
Table 3.
Association between T cell infiltration and MSI
| MSS (n=92) | MSI-L (n=10) | MSI-H (n=6) | p-value | |
|---|---|---|---|---|
|
T cell infiltration in tumor cell nests
| ||||
| CD4 (none: mild: mod.: marked) | 45 : 21 : 19 : 7 | 7 : 1 : 1 : 1 | 4 : 1 : 1 : 0 | 0.822 |
| CD8 (mild: mod.: marked) | 34 : 50 : 8 | 0 : 7 : 3 | 0 : 3 : 3 | 0.003 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 25 : 9 : 58 | 1 : 1: 8 | 0 : 0 : 6 | 0.323 |
|
| ||||
|
T cell infiltration in tumor stroma
| ||||
| CD4 (none: mild) | 89 : 3 | 9 : 1 | 6 : 0 | 0.498 |
| CD8 (none: mild: mod.: marked) | 40 : 41 : 11 : 0 | 1 : 7 : 2 : 0 | 2 : 2 : 1 : 1 | 0.001 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 43 : 0 : 49 | 2 : 0 : 8 | 2 : 0 : 4 | 0.236 |
Mild < 5%, Moderate 5–25%, Marked > 25% of T cell infiltration.
Table 4.
Association between T cell infiltration and EMAST
| EMAST-positive (n=48) | EMAST-negative (n=54) | p-value | |
|---|---|---|---|
|
T cell infiltration in tumor cell nests
| |||
| CD4 (none: mild: mod.: marked) | 25 : 10 : 9 : 4 | 27 : 12 : 11 : 4 | 0.988 |
| CD8 (mild: mod.: marked) | 13 : 28 : 7 | 21 : 29 : 4 | 0.027 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 10 : 5 : 33 | 16 : 5 : 33 | 0.373 |
|
| |||
|
T cell infiltration in tumor stroma
| |||
| CD4 (none: mild) | 46 : 2 | 52 : 2 | 0.878 |
| CD8 (none: mild: mod.: marked) | 16 : 24 : 8 | 25 : 24 : 5 | 0.003 |
| Ratio (CD4=CD8 : CD4>CD8 : CD4<CD8) | 18 : 0 : 30 | 27 : 0 : 27 | 0.390 |
Mild < 5%, Moderate 5–25%, Marked > 25% of T cell infiltration.
Prognostic value of CD4+ and CD8+ T cell infiltration in colorectal tumors
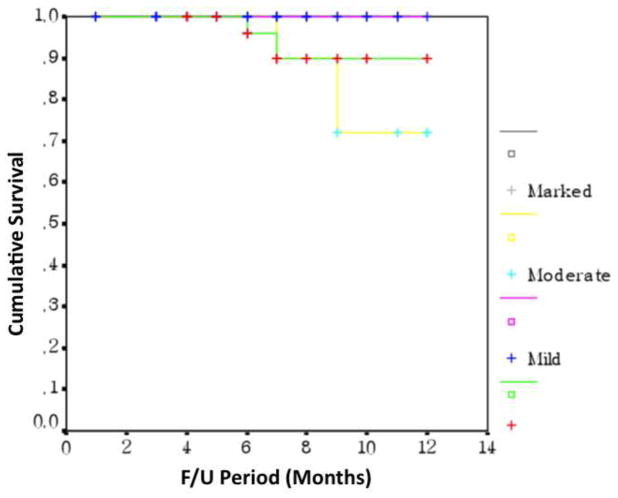
Of 84 cancer patients, 4 patients died during the follow up period (Table 5). There was no statistically significant difference in survival based on the degree of T cell infiltration (Figure 2).
Table 5.
Four colorectal cancer patients who died during the follow up period
| Staging at the time of diagnosis | Pathology | Endoscopic finding | MSI status | EMAST | Follow up period to death | CD8+ T cells in cancer stroma | CD8+ T cell in cancer cell nest | CD4+ T cells in cancer stroma | CD4+ T cell in cancer cell nest |
|---|---|---|---|---|---|---|---|---|---|
| T3N0M0 | MD | Ulcerating | MSI-L | Neg. | 13 mo. | None | Mod. | None | None |
| T3N2M0 | MD | Ulcerating | MSS | Pos. | 9 mo. | Mod. | Mod. | None | None |
| T3N2M0 | MD | Ulcerating | MSS | Neg. | 7 mo. | Mod. | Marked | None | Mod. |
| T3N1M0 | MD | Ulcerating | MSS | Neg. | 14 mo. | None | Mod. | None | Mild |
MD, moderately-differentiated adenocarcinoma; MSI, microsatellite instability; EMAST, elevated microsatellite alterations at selected tetranucleotide repeats.
Figure 2. Kaplan-Meier survival curves for CD8+ T cell lymphocytes in colorectal cancer.
The density of infiltration of CD8+ T lymphocytes in tumor stroma showed no difference in patient survival (p=0.982, log-rank test).
DISCUSSION
In this study, we examined patterns of CD4+ and CD8+ T cell infiltration along the adenoma-carcinoma continuum in the colon, linkage to specific clinicopathological variables, and MSI and EMAST genomic patterns. CD8+ T cell infiltration, but not CD4+ T cell infiltration, in tumor cell nests and tumor stroma were correlated with histology, morphology, and MSI and EMAST genetic patterns. To the best of our knowledge, this is the first evaluation of the type of T cell infiltration with EMAST in colorectal cancers.
MSI is a well-defined pattern of genomic instability seen in up to 20% of sporadic colorectal cancers, and caused by loss of DNA mismatch repair function through hypermethylaton of the mismatch repair gene hMLH1 [9]. A well-defined pattern of T cells, termed a “Crohn’s-like reaction”, is recognized by pathologists and chronically surrounds MSI-H tumors [9]. Presumably triggered by truncated proteins as a result of frameshifts within genes, neo-antigens trigger T cell infiltration that results in containment of the tumor [2,3,10]. This response could be responsible for the observed improved progress among patients with MSI-H tumors.
EMAST, recently described for colorectal cancer, is present in ~60% of colon and 33% of rectal tumors, and appears to be associated with inflammation [11–13]. EMAST may be caused by reduced somatic nuclear expression of the minor mismatch repair protein hMSH3 [11–13]. Its association with T lymphocytes has not been previously described. It is unclear if inflammation triggers EMAST, or vice versa. Unlike patients with MSI-H tumors, patients with EMAST tumors are more likely to be advanced stage [13]. Thus, an understanding of any difference in patterns between MSI and EMAST tumors becomes more paramount.
Different T cell subsets within colorectal cancers have been described [5]. In our study, CD8+ T cell infiltration was related to specific clinicopathological factors, whereas CD4+ T cell infiltration was not. A recent study showed that chemotherapy-induced elimination of myeloid derived suppressor cells caused stimulation of tumor-infiltrating CD8+ T cells, but not CD4+ T cells, produced detectable levels of IFN-γ, and promoted T cell-dependent antitumor responses [14]. Immunization with guanylyl cyclase C-expressing viral vectors opposed nascent tumor growth of pulmonary metastasis in mouse models, reflecting systemic lineage-specific tolerance characterized by CD8+ T cell antibody responses (and not CD4+) [15]. A study examining patients with esophageal squamous cell carcinoma for CD4+ and CD8+ T cells showed that patients with abundant CD8+ T cells in either the cancer cell nest or the mesenchymal stroma had a better prognosis, while CD4+ cells were not predictive [16]. Although it is not fully clear why CD8+ T cells are closely related to the observed clinicopathological factors more than CD4+ T cells, these studies suggest that the tumor immune response depends more on CD8+ T cell infiltration, and corroborates our findings in the present study.
It is interesting that ulcerated tumors showed prominent CD8+ T cell infiltration compared with tumors that have a sessile or polypoid appearance. We have shown previously that EMAST was associated with ulcerated cancers and may be a consequence of increased local inflammation [12,13]. Colorectal tumors develop an increasing level of inflammatory cells as they progress from adenoma to cancer, and would be highest in cancers in which mucosa is breached, as in ulcerated cancers [12]. We assume that some T cells promote progression of colorectal cancer as part of chronic inflammation, whereas other T cell responses lead to tumor regression. Of significance, patients with inflammatory bowel disease have increased risk of developing colorectal cancer, reflecting tumor promotion by chronic inflammation [17]. However, it is still unclear if the presence of tumor infiltrating CD8+ T lymphocytes represents the result of an inflammatory response that facilitates either tumor progression or a protective host response against cancer.
Due to the short follow up period of our subjects (less than 2 years, as subjects were enrolled from 2008), we could not find any significant association between patient survival and CD8+T cell infiltration. Previous studies have shown that CD8+ T cells in cancer cell nests predict better survival [1,7,18]. It has been suggested that the number of CD8+ T cells within the primary tumor might be a good indicator of the presence of a systemic immunosurveillance mechanism, and that the improved survival associated with infiltration of lymphocytes may be the result of an effective suppression of tumor local extension or micrometastasis to distant organs. Longer follow up will verify whether patients with EMAST-positive tumors that show marked CD8+ T cell infiltration in tumor cell nests and/or tumor stroma have better survival.
In summary, we found that increased CD8+ T cell infiltration, but not CD4+ T cell infiltration, in tumor cell nests and tumor stroma are associated with moderately or poorly-differentiated colorectal cancers, ulcerated tumors, MSI-positive tumors, and EMAST-positive tumors. A long term follow up study is needed to know the prognostic value of CD8+ T cell infiltration in these tumors.
Acknowledgments
This study was supported by Konkuk University, the US Public Health Service (DK067287), the UCSD Digestive Diseases Research Development Center (DK080506), and the SDSU/UCSD Comprehensive Cancer Center Partnership (CA132379 and CA132384).
Footnotes
Roles of the authors:
Sun-Young Lee - Collected samples as a colonoscopist at Konkuk University, conducted experiments & study design at UCSD, and wrote manuscript
Katsuya Miyai - Analyzed the samples as a pathologist at UCSD
Hye Seung Han – Analyzed samples as a pathologist at Konkuk University
Dae-Yong Hwang and Moo Kyung Seong – Collected samples as colorectal surgeons at Konkuk University
Heekyung Chung, Barbara H. Jung, Bikash Devaraj, and Kathie L. McGuire – Helped in conducting experiments at UCSD
John M. Carethers – Study design, wrote and edited manuscript, and provided funding
References
- 1.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Speetjens FM, Kuppen PJ, Morreau H, van der Burg SH. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;135:711–2. doi: 10.1053/j.gastro.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Linnebacher M, Gebert J, Rudy W, et al. Frameshift peptide-derived T-cell epitopes: A source of novel tumor-specific antigens. Int J Cancer. 2001;93:6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 4.Williams DS, Bird MJ, Jorissen RN, et al. Nonsense mediated decay resistant mutations are a source of expressed mutant proteins in colon cancer cell lines with microsatellite instability. PLoS One. 2010;5:e16012. doi: 10.1371/journal.pone.0016012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroentrol. 2006;12:7233–7238. doi: 10.3748/wjg.v12.i45.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 8.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–99. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuschenbach M, Kloor M, Morak M, Wentzensen N, Germann A, Garbe Y, Tariverdian M, Findeisen P, Neumaier M, Holinski-Feder E, von Knebel Doeberitz M. Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Fam Cancer. 2010;9:173–9. doi: 10.1007/s10689-009-9307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haugen AC, Goel A, Yamada K, et al. Genetic instability caused by loss of MutS homologue 3 in human colorectal cancer. Cancer Res. 2008;68:8465–8472. doi: 10.1158/0008-5472.CAN-08-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SY, Chung H, Deveraj B, et al. Microsatellite alterations at selected tetranucleotide repeats are associated with morphologies of colorectal neoplasias. Gastroenterology. 2010;139:1519–1525. doi: 10.1053/j.gastro.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaraj B, Lee A, Cabrera BL, Miyai K, Luo L, Ramamoorthy S, Keku T, Sandler RS, McGuire KL, Carethers JM. Relationship of EMAST and microsatellite instability among patients with rectal cancer. J Gastrointest Surg. 2010;14:1521–8. doi: 10.1007/s11605-010-1340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 15.Snook AE, Li P, Stafford BJ, et al. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009;69:3537–3544. doi: 10.1158/0008-5472.CAN-08-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 17.Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61–73. doi: 10.5009/gnl.2008.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschoolmeester V, Baay M, Van Marck E, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]