Abstract
Stem cells have potential for therapy of liver diseases, but may also be involved in the formation of liver cancer. Recently, the AASLD Henry M. and Lillian Stratton Basic Research Single Topic Conference “Stem Cells in Liver Diseases and Cancer: Discovery and Promise” brought together a diverse group of investigators to define the status of research on stem cells and cancer stem cells in the liver and identify problems and solutions on the path to clinical translation. This report summarizes the outcomes of the conference and provides an update on recent research advances. Progress in liver stem cell research includes isolation of primary liver progenitor cells (LPC), directed hepatocyte differentiation of primary LPC and pluripotent stem cells, findings of transdifferentiation, disease-specific considerations for establishing a therapeutically effective cell mass, and disease modeling in cell culture. Tumor initiating stem-like cells (TISC) that emerge during chronic liver injury share expression of signaling pathways, including those organized around TGF-β and β-catenin, and surface markers with normal LPC. Recent investigations of the role of TISC in hepatocellular carcinoma have provided insight into the transcriptional and posttranscriptional regulation of hepatocarcinogenesis. Targeted chemotherapies for TISC are in development as a means to overcome cellular resistance and mechanisms driving disease progression in liver cancer.
Keywords: Liver stem cells, liver progenitor cells, liver cancer stem cells, tumor initiating stem-like cells, liver cell therapy, embryonic stem cells, induced pluripotent stem cells
Recent advances in liver stem cell research
Liver stem cell research promises to improve the outcomes of patients with liver diseases. Advances in liver stem cell research may lead to new cell therapies and may facilitate the development of new drugs by providing faithful liver disease models.
John Gearhart, who co-directed the conference, introduced unanswered questions and technical hurdles that remain to be overcome in stem cell research. In many tissues, stem cells have yet to be specifically identified and isolated. As a consequence, the current understanding of the mechanisms that facilitate proliferation and differentiation of tissue-specific stem cells is limited, which has also hampered the generation of therapeutically effective surrogate cells from alternative cell sources such as pluripotent stem cells. To collaboratively solve these problems, stem cell researchers need to agree on a standard of phenotypical markers and functional read-outs and establish therapeutic efficacy and safety in rigorous animal models.
Research findings presented at the conference and additional advances reported in the last two years have moved the liver stem cell research field closer to realizing its potential by answering some long-standing questions, overcoming persistent technical hurdles and making unexpected discoveries.
Isolation of adult clonogenic and bipotential liver progenitor cells
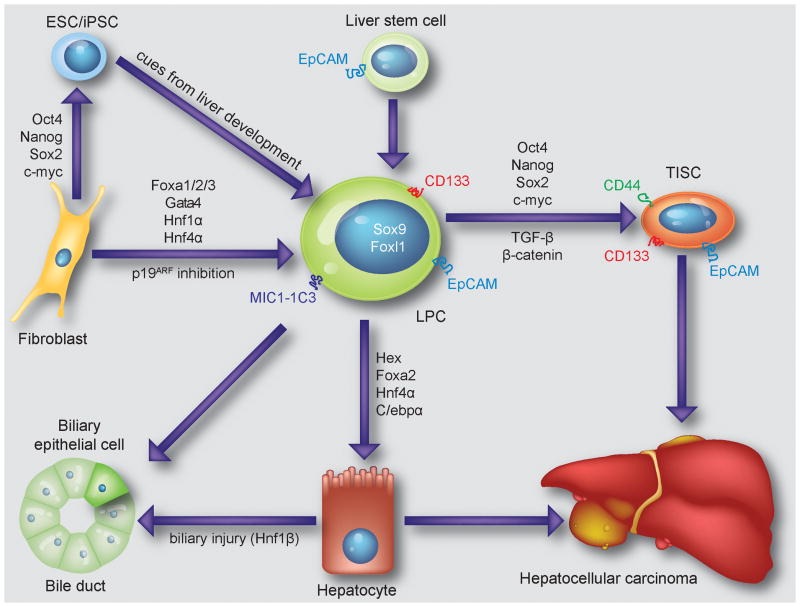
Adult LPC are believed to provide a backup system for replenishing hepatocytes and biliary epithelial cells when the regenerative capabilities of these cells are impaired such as in chronic injury states. LPC emerge and expand in periportal areas of the injured mouse, rat and human liver. Recently, the long-standing hypothesis that adult LPC reside within or derive from the epithelial lining of bile ducts has been confirmed in mice by lineage tracing of cells expressing the transcription factor Sox9 (Fig. 1).1
Figure 1.
Cartoon summarizing currently known sources of LPC and their proposed relationship to TISC and liver cancer. Recently identified cell surface markers and fate-determining transcription factors are indicated.
LPC can be delineated from mature biliary epithelial cells, which also express Sox9, based on expression of the transcription factor Foxl1 or a combination of cell surface epitopes, including the duct cell marker MIC1-1C3 and the general stem cell marker Prominin1 (CD133) (Fig. 1).2,3 Cell isolation using these markers produces liver cell populations in which approximately 4% of the cells form colonies in culture that consist of both hepatocytes and biliary epithelial cells. Interestingly, clonogenic and bipotential adult LPC cannot only be isolated from mice with ductular reactions induced by the drug 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), but also from normal livers.3 Accumulating evidence suggests that similarly potent cells also exist in the adult human liver where they express the marker epithelial cell adhesion molecule (EpCAM) (Fig. 1).4
Improving hepatocyte differentiation of pluripotent stem cells
A detailed understanding of the signals guiding hepatic specification in development has facilitated production of cells equivalent to LPC from mouse and human embryonic stem cells (ESC) (Fig. 1).5–9 While some hepatocyte functions are lacking or underdeveloped in these ESC derivatives in culture, including expression of certain cytochrome P450 (CYP) enzymes, the cells can undergo further maturation after being transplanted into livers of mice or rats. Moreover, like primary hepatocytes, mouse ESC-derived hepatocytes can proliferate extensively after transplantation and repopulate the failing livers of fumarylacetoacetate hydrolase (FAH)-deficient mice.10 Much effort is currently focused on devising protocols that robustly produce human ESC-derived hepatocytes with similar proliferative abilities.
Despite concerns about epigenetic differences between induced pluripotent stem cells (iPSC) and ESC, evidence suggests that these pluripotent stem cell types are equally effective in giving rise to hepatocytes in culture.11–13 Moreover, liver function has been shown to be normal in chimeric mice generated by injection of wildtype mouse iPSC into FAH-deficient blastocysts.14 Because all hepatocytes are iPSC-derived in these mice, the finding establishes that mouse iPSC are in principle capable of full hepatocyte differentiation (Fig. 1). In addition, the cellular origin of human iPSC, that is, whether they are derived from hepatocytes, fibroblasts, bone marrow mesenchymal stem cells or keratinocytes, has been reported to not affect their ability for hepatic specification.15
However, advancing the differentiation of ESC or iPSC from a LPC to a mature hepatocyte stage in culture appears to require improved culture systems. Along these lines, co-culture of primary human LPC or hepatocytes with mesenchymal cells promotes or stabilizes hepatocyte differentiation, respectively.16,17 Alternatively, direct and sequential application of growth factors and matrices provided by mesenchymal liver cells can be used to more closely replicate normal liver development.16,18 Other findings presented at the conference show that prevention of epithelial-mesenchymal transition is also needed to achieve and maintain hepatocyte differentiation of human fetal liver cells in culture. These refined cell culture conditions likely have a similar effect on LPC derived from ESC or iPSC. In fact, hepatocyte differentiation and function of ESC has been shown to significantly improve on polymer matrices.19 Importantly, advanced differentiation of ESC-derived hepatocytes does not only improve their function, but may also reduce the risk of tumor formation after transplantation.20 As an alternative approach to promoting hepatocyte differentiation, forced overexpression of transcription factors such as Hex or a combination of Foxa2, Hnf4α and C/ebpα has been reported (Fig. 1).21,22
Forced and spontaneous transdifferentiation
Lineage conversion of somatic cells by forced overexpression of cell type-specific transcription factors is emerging as an alternative to reprogramming that bypasses the pluripotent state and its potential hazards. Along these lines, overexpression of the chromatin-modifying factors Foxa3 and Gata4 together with the transcription factor Hnf1α in adult mouse fibroblasts lacking the tumor suppressor p19Arf has been shown to induce conversion into cells that resemble hepatocytes.23 Similar results have been obtained by co-expressing any of the 3 Foxa genes and Hnf4α in otherwise unmodified embryonic or adult mouse fibroblasts (Fig. 1).24 Induced hepatocyte-like cells generated with these few essential factors lack certain hepatocyte functions in culture, but can repopulate livers of FAH-deficient mice and prolong their survival. If similar cells could be generated from human cells, they would have great potential for both liver research and liver cell therapy.25 An example of spontaneous lineage conversion is provided by the finding that, in states of severe biliary injury, periportal hepatocytes can activate the biliary transcription factor Hnf1β and transdifferentiate into biliary epithelial cells in rats (Fig. 1).26
Hepatocyte replacement
Successful liver repopulation of FAH-deficient mice with hepatocytes derived from mouse ESC, iPSC or by fibroblast transdifferentiation shows that the cells replicate the proliferative abilities of primary hepatocytes. However, taking advantage of post-transplant expansion to increase the efficacy of liver cell therapy has been limited to a few liver diseases that provide a growth advantage for normal hepatocytes: FAH deficiency, Wilson’s disease and progressive familial intrahepatic cholestasis. The recent finding that transplanted hepatocytes spontaneously expand and repopulate the liver in a mouse model of α1-antitrypsin deficiency extends this list to include the most common genetic liver disease.27 A strategy to achieve efficient engraftment and selective expansion of transplanted hepatocytes in other liver diseases is to combine localized liver irradiation with stimulation of hepatocyte proliferation.28,29 As reported at the conference, a clinical trial employing this strategy for therapy of metabolic liver diseases with primary human hepatocytes is currently ongoing at the University of Pittsburgh.
An alternative mechanism that could be harnessed for effective liver cell replacement therapy is suggested by the ability of LPC isolated from fetal rats to spontaneously repopulate the livers of wildtype rats, in particular if the animals are aged.30 Similarly, as reported at the conference, adult LPC emerge and expand in a rat model of end-stage liver cirrhosis. However, the finding that hepatocyte function is impaired in these animals suggests that the cirrhotic liver environment may not only cause loss of hepatocyte differentiation, but may also prevent LPC maturation. Thus, liver cirrhosis may prohibit effective cell therapy with both hepatocytes and LPC.
Neo-livers
Because most chronic liver diseases are associated with cirrhosis, alternative methods of hepatocyte delivery are being developed: Colonization of lymph nodes with transplanted hepatocytes creates therapeutically effective liver tissue outside of the recipient’s liver.31 Decellularized liver matrix from cadaveric livers may provide the 3-dimensional structure needed for creating transplantable liver tissue in culture.32,33
Liver disease modeling
Patient-derived iPSC have been differentiated into hepatocytes to generate cell culture models of the liver diseases α1-antitrypsin deficiency, familial hypercholesterolemia, glycogen storage disease type 1a and Wilson’s disease.13,34 In addition, a model of maturity onset diabetes of the young type I was presented at the conference. Although iPSC-derived hepatocytes lack certain hepatocyte functions in culture, the disease phenotypes of these models are sufficiently distinctive and respond to established drugs, suggesting that they can be used to screen for new therapeutic agents for these liver diseases.
Stem cell conclusions
Recent advances in liver stem cell research can be expected to move the field forward in various ways: Even if primary LPC will not be used for transplantation directly, the newfound ability to identify and isolate adult LPC provides a system to investigate the mechanisms that guide LPC lineage choice and maturation. These analyses may reveal new regulators that, together with advanced cell culture systems, may be effective in improving hepatocyte differentiation of pluripotent stem cells or fibroblasts. Improved hepatocyte differentiation protocols would not only benefit cell therapy strategies but would also facilitate using patient-derived iPSC to screen for CYP-dependent drug hepatotoxicity or to model liver diseases that require full hepatocyte differentiation for disease manifestation.
Successful stem cell-based liver cell therapies will not only require that fully functional surrogate hepatocytes are used, but also that a therapeutically effective mass of these cells can be established in the recipient. Depending on the recipient’s liver disease, a growth advantage for transplanted hepatocytes generated from primary or pluripotent stem cells in culture can be harnessed or created to replace a sufficient number of hepatocytes. In addition, identification of the signals and pathways that afford spontaneous proliferation of adult or fetal LPC may facilitate targeted pre or post-transplant expansion of like cells derived from pluripotent stem cells. However, in liver diseases where cirrhosis acts as a barrier to conventional hepatocyte replacement therapy, alternative sites or modes for cell delivery may be required.
The conference concluded with an expert panel discussion that reviewed the challenges of translating advances in liver stem cell research into therapies for patients. As evident from recent advances, methods of efficient cell delivery and directed differentiation of pluripotent stem cells into hepatocytes have become available or appear within reach. Other tasks, such as establishing therapeutic efficacy, protection from rejection and safety of stem cell-based liver cell therapies, may require major additional efforts, including the development of large animal models of liver diseases.
Emerging concepts in the field of liver tumor initiating stemlike cells
As LPC are activated during chronic injury and cirrhosis, from which 90% of hepatocellular carcinoma (HCC) develops, LPC-derived HCC was originally proposed by Sell in 1989, five years before the sentinel work in leukemia.35,36 In the last two years an explosion of genomic profiling and molecular pathogenesis discovery has led to a more fundamental understanding of the role of tumor initiating stem-like cells (TISC) in HCC progression. TISC form the fulcrum of the hierarchic cancer model and are generally defined with four criteria: high efficiency self-renewal, differentiation along at least 2 independent lineages (i.e. hepatocyte and cholangiocyte), resistance to traditional genotoxic therapy, and capacity to establish and recapitulate the original tumor.37 Here, we review emerging concepts within the controversial field of HCC TISC, with a focus on new discoveries that continue to reiterate the redundant, overlapping, and integrated signaling pathways that are deregulated in TISC populations and HCC.
Deregulation of TGF-β signaling as a molecular pathway to TISC-based malignancy
Transforming growth factor-β (TGF-β) signals through intermediary SMAD proteins, which activate differentiation programs and inhibit cell cycle progression during early carcinogenesis.38 In TISC-driven hepatocarcinogenesis, the loss of intermediary regulators, such as β2-Spectrin, results in malignant transformation of liver stem and progenitor cells to TISC, via loss of differentiation and growth arrest signals (Fig. 1).39 Within liver TISC populations, increased expression of ESC transcription factors Oct4 and Nanog, driven by loss of TGF-β differentiation signals, propagates self-renewal characteristics. Small molecule promoters of TGF-β signaling, which may restore growth arrest and differentiation signals in TISC, have been proposed as a TISC targeting strategy. In related work, targeting of the fifth subunit of the COP9 signalosome (CSN5), which is functionally interconnected with TGF-β signaling, resulted in decreased tumor growth of human HCC cell lines in vivo.40
As chronic hepatitis C virus (HCV) infection is the primary cause of HCC in the United States, murine models of HCV-induced HCC are highly relevant. In HCV core+ or NS5A+ transgenic mice, up-regulation of TLR4 expression during HCV-induced chronic injury was associated with impaired TGF-β signaling, up-regulated Nanog expression, and increased malignant potential, a process that is exacerbated by high fat diet.41,42 TLR4 activation occurred predominantly in Nanog-dependent CD133+CD49f+ TISC. Targeting Nanog directly in these TISC results in decreased tumor initiation, by down-regulating cellular growth regulators. TLR4-initiated and Nanog-dependent activation of yes-associated protein 1 (YAP1), a regulator of Hippo signaling, results in inhibition of TGF-β through suppression of nuclear translocation of SMAD3. Silencing Yap1 results in suppression of Nanog transcription, restoration of TGF-β/SMAD3 signaling, and sensitization of TISC to rapamycin and sorafenib.
Deregulation of β-catenin signaling in liver TISC
Canonical β-catenin signaling through activation of TCF/LEF promoters is a general mechanism of stem cell function, resulting in stem cell proliferation, survival, and inhibition of differentiation. Mutations in β-catenin and related complex proteins often result in β-catenin activation without Wnt initiation.43 Although β-catenin mutations are well characterized in HCC, mutations that protect β-catenin from degradation are not by themselves sufficient to induce HCC in murine models.43,44β-catenin activation is also found in normal LPC proliferating in response to chronic liver injury. Conversely, in liver-specific β-catenin knockout mice, LPC proliferation is reduced in response to DDC diet.45 Further illustrating the importance of β-catenin for LPC proliferation, rare LPC with normal β-catenin expression eventually emerge in this model and repopulate the liver.46
Genomic profiling in support of TISC-based HCC
Genomic profiling has emerged as a powerful tool for understanding of comprehensive regulatory pathways in cancer biology, and recent work proposes that HCC can be subdivided within established differentiation stages based on profiling analysis.47 Based on transcriptome profiles, HCC with a progenitor (EpCAM+) phenotype demonstrates TISC traits such as self-renewal, bipotency, tumor-sphere formation, and increased tumor initiation compared to EpCAM− HCC.48 Recent work also demonstrates that HCC expressing a cytokeratin-19 signature is TISC-derived and carries a poor prognosis.49 In addition, integrative profiling provides insight into molecular mechanisms favoring tumor metastasis.48,50,51 Within TISC-based tumors, genomic profiling confirms activation of key oncogenic signals from MAPK, PI3K, and β-catenin pathways compared to mature hepatocyte-based HCC. These findings are supported by work demonstrating that TISC, identified by “side-population” analysis, exhibit strong tumor initiation ability, chemotherapy resistance, and express high levels of the pluripotency-associated transcription factors Nanog, Oct4, c-Myc, and Sox2. This TISC signature is enriched using a 3-day treatment with the DNA methyltransferase inhibitor zebularine followed by isolation of the side-population. During this enrichment process, methyltransferase inhibitors induce differentiation in all but the most resistant TISC.37
Chromatin accessibility, epigenetic regulation, and transcriptional regulation in TISC
Polycomb factors, such as Enhancer of zeste homolog 2 (EZH2), act as epigenetic chromatin modifiers and transcriptional repressors, and are important in stem cell self-renewal programs.52 In HCC, EZH2 suppresses Wnt antagonists, resulting in functional β-catenin activation.53
MicroRNAs are non-coding regulators of gene expression, and microRNA-mediated control of proliferation in liver stem cells and hepatocytes during liver regeneration and control of differentiation in TISC during carcinogenesis have been proposed.54–56 Specifically within HCC, molecular alterations manifesting as small changes across multiple genes, can be explained by changes in microRNA expression.56 MicroRNA expression profiling of HCC identified microRNA-181 as up-regulated in EpCAM+ TISC.57β-catenin drives microRNA-181, which targets the hepatocyte differentiation promoting genes CDX2 and GATA6. In addition, microRNA-122, the most abundant microRNA in hepatocytes, has been identified as an inhibitor of AFP expression and aggressive features in HCC,58 providing another link between TISC-based HCC and poor prognosis.
TISC surface markers and alternative strategies of identification
According to the hierarchical model of tumor formation and maintenance, tumor eradication requires TISC-targeted therapy, which requires target identification. Several surface markers, many of which are used as liver stem and progenitor cell markers, have been utilized to identify liver TISC in human and murine models.
CD133 is an epithelial membrane protein expressed on the apical cell surface. CD133 expression has been identified within several human HCC cell lines, and recent work demonstrates a mechanistic link between TGF-β signaling and epigenetic modification as a regulator of CD133 expression within Huh7 cells, with increasing CD133 expression correlating with tumor initiation (Fig. 1).59 A prospective isolation of single CD133+ TISC from liver-specific Pten-deficient animals demonstrates robust tumor initiation in immune-deficient and syngenic immune-competent hosts.60 Follow-up work demonstrates that the chronic inflammatory state of the model, not the oncogenic signals resulting from Pten loss, is the primary driver of TISC formation.61
EpCAM is present in the developing liver and biliary system, and absent in mature hepatocytes.4,62 EpCAM thus serves as a potential link between LPC and TISC populations (Fig. 1). In HCC, EpCAM expression correlates with in vivo tumor initiation as well as patient survival.48,57
CD44 expression is well characterized within breast TISC populations (CD44highCD24low).63 In HCC, CD44 expression correlates with tumor initiation, metastatic potential, and chemotherapy resistance (Fig. 1).64,65 In vitro, the inhibition of CD44 expression results in reduction of TISC characteristics.66
As TISC are proposed to be rare populations, one concern is the variability of CD133 and other marker expression, which ranges from less than 1% in MHCC97-H cells to 60% in Huh7 cells.37,65 This discrepancy suggests that isolating TISC based on co-expression of multiple markers will be more effective than use of a single marker. A mechanism for deregulated signaling resulting in specific TISC surface marker expression is exemplified as β-catenin deregulation promoting EpCAM expression.48 External factors, such as matrix stiffness, have also been implicated in promoting TISC; although increasing matrix stiffness was associated with chemotherapy resistance, decreasing matrix stiffness was associated with other TISC characteristics, such as CD133 and CD44 expression.67
Alternative methods for TISC isolation include functional assays such as side-population, in which the exclusion of Hoechst dye identifies TISC,68,69 and aldehyde dehydrogenase activity.70 A functional assay may be superior to cell surface markers for TISC isolation, as functional assays isolate cells based on the ability to detoxify, a key TISC characteristic.37
TISC-based therapies
In terms of novel TISC-based therapies for HCC, there is a synergistic action between the HDAC inhibitor vorinostat and the PARP inhibitor ABT1888 in HCC cell lines.71 The use of HDAC inhibitors is supported by the epigenetic modifications that enable maintenance of the dedifferentiated state within TISC populations.37 This combination provided a significant increase in DNA damage and apoptosis in TISC populations, which are resistant to standard genotoxic therapies. In terms of targeting signaling pathways in TISC, ON123300, an ATP mimetic kinase inhibitor,72,73 inhibits tumor cell proliferation and induces apoptosis, primarily through inhibition of CDK4 and ARK5, without causing broader hepatotoxicity. This ATP kinase inhibitor may provide alternative TISC targeting.
Novel chemoprevention strategies aimed at targeting TISC through inhibition of the MAP Kinase pathway and/or vitamin D supplementation have been proposed.74–76 Key insights include the marked vitamin D deficiency observed in cirrhotic patients who develop HCC. In the setting of cirrhosis, vitamin D supplementation may act as a chemoprevention strategy by restoring TGF-β signaling as vitamin D up-regulates β2- spectrin in cirrhotic patients. Within alcoholic liver disease, a sensitization of liver macrophages to portal endotoxin, which activates TLR4 on macrophages, results in the production of inflammatory cytokines and activation of p38 MAPK, both contributing to activation of the NF-kB signaling cascade.42,77 Liver cirrhosis results from increased sensitivity of hepatic stellate cells to TGF-β, leading to increased proliferation and production of extracellular matrix via activation of p38 MAPK signaling.
Unanswered questions in the field of liver TISC
New work reinforces that the TGF-β and β-catenin pathways are central to the process of TISC transformation and maintenance. Transcriptome profiling confirms poor prognosis of TISC-based HCC. At the conference, several issues were identified as areas of focus for future work. One unresolved issue is whether liver TISC have reduced rates of proliferation compared to the bulk tumor population. A quiescent state is proposed for TISC, but strong evidence is lacking. A second outstanding issue is the origin of TISC. Although the hierarchic cancer model proposes that TISC are derived from stem cells, they may also originate from hepatocyte dedifferentiation, through loss of β2-Spectrin or up-regulation of β-catenin and resultant up-regulation of Oct-4 and Nanog.
A third issue is the need for agreement on the phenotype of TISC. For example, in the field of hematopoietic malignancy, CD34+CD38− is a standard immune-phenotype for TISC, while CD44high/+CD24low/− is used to identify TISC in breast cancer. As reviewed above, surface markers such as EpCAM, CD133, CD49f, CD44, and others, have all been proposed for identifying TISC, as have functional traits such as efflux of Hoechst 33342 associated with the side-population.
A final unanswered question is the effect of TISC targeted therapy on the LPC population of the regenerating liver. As LPC and TISC share many common pathways for proliferation and maintenance of stemness, targeting TGF-β or β-catenin may reduce the effectiveness of LPC to regenerate the liver during cirrhosis.
TISC conclusions
Although liver researchers proposed the concept of progenitor cell-derived cancer several years before leukemia researchers, the existence of liver TISC is still debated. Recent advances provide additional support for the liver TISC concept and, importantly, may aid therapeutic decision making by allowing risk assessment. Targeted anti-TISC therapies hold significant promise, but a better understanding of the origin and phenotype of these cells appears necessary.
Summary.
Significant progress has been made in liver stem cell and liver cancer stem cell research in the last two years. Although normal and cancerous liver stem cells or LPC naturally share many characteristics and markers, on most accounts, the two fields have been moving forward independently. The dual focus of the conference provided an opportunity to gather insight or update one’s knowledge of the other field. In addition, the conference identified commonalities that may serve as a basis for future advances. For example, new markers of normal LPC may be useful for further fractionation of heterogeneous TISC populations. Conversely, signaling pathways and transcription factors regulating TISC characteristics may also play a role in non-cancerous liver regeneration. In addition, new cell culture or in vivo cell delivery systems will likely benefit research on both normal and cancerous liver stem cells or LPC. The hope is that increased exchange and collaboration between the two fields will accelerate the development of therapies for patients with liver disease and liver cancer.
Acknowledgments
Support: CBR acknowledges funding from an American Cancer Society Research Scholar Award (MGO-116519) and the National Institutes of Health (R03DK088013). HW acknowledges funding from a California Institute for Regenerative Medicine New Faculty Award (RN2-00950) and the National Institutes of Health (R01DK080852 and P30DK026743). The American Association for the Study of Liver Disease Henry M. and Lillian Stratton Basic Research Single Topic Conference “Stem Cells in Liver Diseases and Cancer: Discovery and Promise” was supported from National Institute of Health funding RO1CA042857 (LM), RO1CA106614 (LM), and PO1CA130821 (LM).
The American Association for the Study of Liver Disease (AASLD) Henry M. and Lillian Stratton Basic Research Single Topic Conference “Stem Cells in Liver Diseases and Cancer: Discovery and Promise” was held in Atlanta, GA, March 19–20, 2011. The meeting provided an overview and update on ongoing research efforts seeking to obtain a detailed understanding of stem cell biology in liver disease and cancer for the development of new therapies. This review is dedicated to Nelson Fausto, a leader in the field.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- CSC
cancer stem cells
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- ESC
embryonic stem cells
- EpCAM
epithelial cell adhesion molecule
- FAH
fumarylacetoacetate hydrolase
- HCV
Hepatitis C virus
- HCC
hepatocellular carcinoma
- iPSC
induced pluripotent stem cells
- LPC
liver progenitor cells
- TGF-β
transforming growth factor-β
- TISC
tumor initiating stem-like cells
- TLR-4
toll-like receptor-4
- YAP1
yes-associated protein 1
Literature cited
- 1.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, Wells RG, Grompe M, Greenbaum LE, Kaestner KH. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–92. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH, Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN, Theise ND. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;53:964–73. doi: 10.1002/hep.24122. [DOI] [PubMed] [Google Scholar]
- 5.Kubo A, Kim YH, Irion S, Kasuda S, Takeuchi M, Ohashi K, Iwano M, Dohi Y, Saito Y, Snodgrass R, Keller G. The homeobox gene Hex regulates hepatocyte differentiation from embryonic stem cell-derived endoderm. Hepatology. 2010;51:633–41. doi: 10.1002/hep.23293. [DOI] [PubMed] [Google Scholar]
- 6.Han S, Dziedzic N, Gadue P, Keller GM, Gouon-Evans V. An Endothelial Cell Niche Induces Hepatic Specification Through Dual Repression of Wnt and Notch Signaling. Stem Cells. 2010;29:217–228. doi: 10.1002/stem.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, Weber A, Vallier L. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–65. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 8.Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, Tolstikov V, Zern MA. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28:674–86. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- 9.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–9. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Liu P, Liu C, Xiang D, Deng L, Li W, Wangensteen K, Song J, Ma Y, Hui L, Wei L, Li L, Ding X, Hu Y, He Z, Wang X. Hepatoblast-like progenitor cells derived from embryonic stem cells can repopulate livers of mice. Gastroenterology. 2010;139:2158–2169. e8. doi: 10.1053/j.gastro.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, Wang G, Daley GQ, Lee JH, Church GM, Forbes SJ, Iredale JP, Wilmut I. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–35. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J, Semple R, Weber A, Lomas DA, Vallier L. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–36. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espejel S, Roll GR, McLaughlin KJ, Lee AY, Zhang JY, Laird DJ, Okita K, Yamanaka S, Willenbring H. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120–6. doi: 10.1172/JCI43267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Yao HL, Cui CB, Wauthier E, Barbier C, Costello MJ, Moss N, Yamauchi M, Sricholpech M, Gerber D, Loboa EG, Reid LM. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology. 2010;52:1443–54. doi: 10.1002/hep.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ploss A, Khetani SR, Jones CT, Syder AJ, Trehan K, Gaysinskaya VA, Mu K, Ritola K, Rice CM, Bhatia SN. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci U S A. 2010;107:3141–5. doi: 10.1073/pnas.0915130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C, Gerber DA, Alvaro D, Reid LM. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 19.Hay DC, Pernagallo S, Diaz-Mochon JJ, Medine CN, Greenhough S, Hannoun Z, Schrader J, Black JR, Fletcher J, Dalgetty D, Thompson AI, Newsome PN, Forbes SJ, Ross JA, Bradley M, Iredale JP. Unbiased screening of polymer libraries to define novel substrates for functional hepatocytes with inducible drug metabolism. Stem Cell Res. 2011;6:92–102. doi: 10.1016/j.scr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Payne CM, Samuel K, Pryde A, King J, Brownstein D, Schrader J, Medine CN, Forbes SJ, Iredale JP, Newsome PN, Hay DC. Persistence of functional hepatocyte-like cells in immune-compromised mice. Liver Int. 2011;31:254–62. doi: 10.1111/j.1478-3231.2010.02414.x. [DOI] [PubMed] [Google Scholar]
- 21.Inamura M, Kawabata K, Takayama K, Tashiro K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H, Kiyokawa N, Umezawa A, Hayakawa T, Furue MK, Mizuguchi H. Efficient generation of hepatoblasts from human ES cells and iPS cells by transient overexpression of homeobox gene HEX. Mol Ther. 2011;19:400–7. doi: 10.1038/mt.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacob R, Rudrich U, Rothe M, Kirsch S, Maasoumy B, Narain N, Verfaillie CM, Sancho-Bru P, Iken M, Popescu I, Schambach A, Manns MP, Bock M. Induction of a mature hepatocyte phenotype in adult liver derived progenitor cells by ectopic expression of transcription factors. Stem Cell Res. 2011;6:251–61. doi: 10.1016/j.scr.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–9. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 24.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–3. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 25.Willenbring H. A simple code for installing hepatocyte function. Cell Stem Cell. 2011;9:89–91. doi: 10.1016/j.stem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Limaye PB, Bowen WC, Orr A, Apte UM, Michalopoulos GK. Expression of hepatocytic- and biliary-specific transcription factors in regenerating bile ducts during hepatocyte-to-biliary epithelial cell transdifferentiation. Comp Hepatol. 2010;9:9. doi: 10.1186/1476-5926-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J, Yannam GR, Roy-Chowdhury N, Hidvegi T, Basma H, Rennard SI, Wong RJ, Avsar Y, Guha C, Perlmutter DH, Fox IJ, Roy-Chowdhury J. Spontaneous hepatic repopulation in transgenic mice expressing mutant human alpha1-antitrypsin by wild-type donor hepatocytes. J Clin Invest. 2011;121:1930–4. doi: 10.1172/JCI45260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, Yannam GR, Enke C, Solberg TD, Adelson AB, Platt JL, Fox IJ, Roy-Chowdhury J, Guha C. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–67. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenig S, Yuan Q, Krause P, Christiansen H, Rave-Fraenk M, Kafert-Kasting S, Kriegbaum H, Schneider A, Ott M, Meyburg J. Regional transient portal ischemia and irradiation as preparative regimen for hepatocyte transplantation. Cell Transplant. 2011;20:303–11. doi: 10.3727/096368910X520074. [DOI] [PubMed] [Google Scholar]
- 30.Menthena A, Koehler CI, Sandhu JS, Yovchev MI, Hurston E, Shafritz DA, Oertel M. Activin A, p15INK4b signaling, and cell competition promote stem/progenitor cell repopulation of livers in aging rats. Gastroenterology. 2011;140:1009–20. doi: 10.1053/j.gastro.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2011;140:656–666. e2. doi: 10.1053/j.gastro.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Chen S, Li W, Guo X, Zhao P, Xu J, Chen Y, Pan Q, Liu X, Zychlinski D, Lu H, Tortorella MD, Schambach A, Wang Y, Pei D, Esteban MA. Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr223. [DOI] [PubMed] [Google Scholar]
- 35.Sell S, Dunsford HA. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–63. [PMC free article] [PubMed] [Google Scholar]
- 36.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 37.Marquardt JU, Factor VM, Thorgeirsson SS. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol. 2010;53:568–77. doi: 10.1016/j.jhep.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fausto N, Mead JE, Gruppuso PA, Braun L. TGF-beta in liver development, regeneration, and carcinogenesis. Ann N Y Acad Sci. 1990;593:231–42. doi: 10.1111/j.1749-6632.1990.tb16115.x. [DOI] [PubMed] [Google Scholar]
- 39.Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, Zouhairi ME, Mendelson J, Shetty K, Kallakury B, Berry DL, Shin KH, Mishra B, Reddy EP, Kim SS, Mishra L. Transforming growth factor-beta adaptor, beta2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology. 2011;53:1676–84. doi: 10.1002/hep.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YH, Judge AD, Seo D, Kitade M, Gomez-Quiroz LE, Ishikawa T, Andersen JB, Kim BK, Marquardt JU, Raggi C, Avital I, Conner EA, Maclachlan I, Factor VM, Thorgeirsson SS. Molecular targeting of CSN5 in human hepatocellular carcinoma: a mechanism of therapeutic response. Oncogene. 2011 doi: 10.1038/onc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machida K, Tsukamoto H, Liu JC, Han YP, Govindarajan S, Lai MM, Akira S, Ou JH. c-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repair. Hepatology. 2010;52:480–92. doi: 10.1002/hep.23697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, Seki E, Deshaies R, Miyake K, Lai MM. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–53. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr, Dar MJ, Khillan J, Dai C, Monga SP. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–13. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, Thorgeirsson SS, Wiltrout RH. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–27. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–22. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XF, Tan X, Zeng G, Misse A, Singh S, Kim Y, Klaunig JE, Monga SP. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–65. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo HG, Park ES, Thorgeirsson SS, Kim YJ. Exploring genomic profiles of hepatocellular carcinoma. Mol Carcinog. 2011;50:235–43. doi: 10.1002/mc.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, Thorgeirsson SS. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2010;51:1401–9. doi: 10.1002/hep.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oishi N, Wang XW. Novel therapeutic Strategies for Targeting Liver Cancer Stem Cells. Int J Biol Sci. 2011;7:517–35. doi: 10.7150/ijbs.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, Wang XW. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–12. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, Woodward WA, Hsu JM, Hortobagyi GN, Hung MC. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell. 2011;19:86–100. doi: 10.1016/j.ccr.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H, Ching AK, Cheung KF, Wong HK, Tong JH, Jin H, Choy KW, Yu J, To KF, Wong N, Huang TH, Sung JJ. EZH2-Mediated Concordant Repression of Wnt Antagonists Promotes {beta}-Catenin-Dependent Hepatocarcinogenesis. Cancer Res. 2011;71:4028–4039. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]
- 54.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2010 doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 55.Song G, Sharma AD, Roll GR, Ng R, Lee AY, Blelloch RH, Frandsen NM, Willenbring H. MicroRNAs control hepatocyte proliferation during liver regeneration. Hepatology. 2010;51:1735–43. doi: 10.1002/hep.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011;121:141–58. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 57.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–80. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima K, Takata A, Vadnais C, Otsuka M, Yoshikawa T, Akanuma M, Kondo Y, Kang YJ, Kishikawa T, Kato N, Xie Z, Zhang WJ, Yoshida H, Omata M, Nepveu A, Koike K. MicroRNA122 is a key regulator of alpha-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma. Nat Commun. 2011;2:338. doi: 10.1038/ncomms1345. [DOI] [PubMed] [Google Scholar]
- 59.You H, Ding W, Rountree CB. Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta. Hepatology. 2010;51:1635–44. doi: 10.1002/hep.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem Cells. 2009;27:290–9. doi: 10.1634/stemcells.2008-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, Zeng N, Bayan JA, Ding W, Wang KS, French S, Birnbaum MJ, Rountree CB, Stiles BL. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139:2170–82. doi: 10.1053/j.gastro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–45. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B, Yang GH, Ji Y, Fan J. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–62. doi: 10.1136/gut.2008.176271. [DOI] [PubMed] [Google Scholar]
- 65.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met is a therapeutic target for personalized treatment in Hepatocellular carcinoma. Hepatology. 2011 doi: 10.1002/hep.24450. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie Z, Choong PF, Poon LF, Zhou J, Khng J, Jasinghe VJ, Palaniyandi S, Chen CS. Inhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasion. Cancer Chemother Pharmacol. 2008;62:949–57. doi: 10.1007/s00280-008-0684-z. [DOI] [PubMed] [Google Scholar]
- 67.Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG, Iredale JP. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–51. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 69.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, Thorgeirsson SS. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 70.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–53. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 71.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang SY, Robell K, Kahana JA, Geske RS, Kleymenova EV, Choudhry AE, Lai Z, Leber JD, Minthorn EA, Strum SL, Wood ER, Huang PS, Copeland RA, Kumar R. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–74. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 72.Jatiani SS, Baker SJ, Silverman LR, Reddy EP. JAK/STAT Pathways in Cytokine Signaling and Myeloproliferative Disorders: Approaches for Targeted Therapies. Genes Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker SJ, Reddy EP. Targeted inhibition of kinases in cancer therapy. Mt Siai J Med. 2010;77:573–86. doi: 10.1002/msj.20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–84. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- 75.Chu KJ, Lai EC, Yao XP, Zhang HW, Lau WY, Fu XH, Lu CD, Shi J, Cheng SQ. Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation--a systematic review and meta-analysis. Asian J Surg. 2010;33:120–6. doi: 10.1016/S1015-9584(10)60021-8. [DOI] [PubMed] [Google Scholar]
- 76.Lu SC. Where are we in the chemoprevention of hepatocellular carcinoma? Hepatology. 2010;51:734–6. doi: 10.1002/hep.23497. [DOI] [PubMed] [Google Scholar]
- 77.Machida K. TLRs, Alcohol, HCV, and Tumorigenesis. Gastroenterol Res Pract. 2010;2010:518674. doi: 10.1155/2010/518674. [DOI] [PMC free article] [PubMed] [Google Scholar]