Abstract
Functional pluripotent characteristics have been observed in specific subpopulations of hepatic cells that express some of the known cholangiocyte markers. Although evidence indicates that specific cytokines, granulocyte-macrophage colony stimulating factors (GM-CSF) and stem cell factor (SCF) may be candidate treatments for liver injury, the role of these cytokines in intrahepatic biliary epithelium remodeling is unknown. Thus, our aim was to characterize the specific cytokines that regulate the remodeling potentials of cholangiocytes after 70% partial hepatectomy (PH). The expression of the cytokines and their downstream signaling molecules was studied in rats after 70% PH by immunoblots, and in small and large murine cholangiocyte cultures (SMCCs and LMCCs) by immunocytochemistry and real-time PCR. There was a significant and stable increase in SCF and GM-CSF levels until 7 days after PH. Real-time PCR analysis revealed significant increases of key remodeling molecules, such as S100A4 and miR-181b after SCF plus GM-CSF administration in SMCCs. SMCCs produced significant amounts of soluble and bound SCF and GM-CSF in response to TGF-β. When SMCCs were incubated with TGF-β plus anti–SCF and GM-CSF antibodies, there was a significant decrease in S100A4 expression. Furthermore, treatment of SMCCs with SCF + GM-CSF significantly increased matrix metalloproteinases (MMP-2 and MMP-9) mRNA as well as miR-181b expression along with a reduction of metalloproteinase inhibitor 3 (TIMP-3). The levels of MMP-2, MMP-9 and miR-181b were also up-regulated in rat liver and isolated cholangiocytes after PH.
CONCLUSION
Our data suggest that altered expression of SCF and GM-CSF following PH can contribute to biliary remodeling (for example post-transplantation) by functional deregulation of activity of key signaling intermediates involved in cell expansion and multipotent differentiation.
Keywords: Colony stimulating factors (CSFs), Stem cell factor (SCF), Granulocyte/macrophage colony stimulating factor (GM-CSF), miR-181, TIMP-3, liver regeneration, biliary remodeling, partial hepatectomy (PH)
Introduction
In addition to playing important roles in the regulation of ductal secretion, cholangiocytes are the target cells of apoptotic, proliferative and regenerative events leading to changes in biliary damage (e.g., after carbon tetrachloride (CCl4) acute administration), hyperplasia (e.g., after bile duct ligation), and regeneration (e.g., after 70% hepatic resection) (1, 2). During liver development both hepatocytes and cholangiocytes arise from common bipotential progenitors called hepatoblasts (3) or hepatic stem cells (4). The hepatic stem cells or hepatoblasts in the liver parenchyma differentiate into hepatocytes, whereas those adjacent to the portal mesenchyme differentiate into cholangiocytes (4, 5). During liver regeneration, the process of hepatic wound healing, a complex cascade of inflammatory signaling, recruits inflammatory cells, stimulates hepatobiliary cell proliferation, directs cell migration and induces vascularization to restore tissue integrity (6). Previous studies related to biliary wound healing focused mainly on the inducers of compensatory biliary proliferation following bile duct insult (7).
Cytokines that are candidates in liver remodeling processes include stem cell factor (SCF), erythropoietin (EPO), granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF); these regulate bone marrow production of circulating red cells, white cells and platelets. These cytokines act on stem cells leading to lineage specific differentiation (8). SCF regulates differentiation of CD34+ stem cells whilst other factors such as EPO, G-CSF and GM-CSF modulate the synthesis of more specific cell types (9). CSFs are involved in hepatic inflammation (via direct effects on the vascular endothelium, and/or neutrophil recruitment and activation) as well as in hepatic repair and regeneration (10).
The potential mechanisms involved in direct actions and/or induction of additional factors that promote liver regeneration and repair are unclear. Multiple studies have suggested that there is an intricate regulatory system involved in hepatic regeneration following injury. Although many cytokines have proliferative effects on hepatobiliary cells both in vitro and in vivo, no single molecule has proven to be a key factor responsible for controlling hepatocyte or proliferation of subpopulations sharing cholangiocyte markers and in vivo remodeling during liver injury. Although evidence suggests that these factors may be candidate treatments for liver injury, either as potential hepatoprotectants or as hepato-reparative agents, the role of one or more of these cytokines in hepatobiliary remodeling after partial hepatectomy is undefined.
Hepatocyte proliferation may be blocked if the tissue injury is too severe (6). During this process, cholangiocytes of the portal ductules and canals of Herring (small tubules lined by epithelium with biliary morphology, which connect the hepatocyte bile canalicular network to the portal biliary ductules) begin expressing hepatocyte-associated transcription factors (11). It has been suggested that cholangiocytes can acquire stem cell phenotypes and subsequently become hepatocytes, restoring liver regeneration when hepatocytes cannot proliferate (12), but an alternative interpretation is that the liver regeneration is derived from hepatic stem cells (4, 13, 14). Furthermore, the hepatic stem cells are located within the canals of Hering and have markers shared with biliary epithelia (4, 13–15), and they expand in disease conditions (14) prior to formation of oval cells, progenitor populations occurring in livers of hosts exposed to oncogenic insults (16). Therefore, hepatic stem cells are one of the most important regenerative alternatives during conditions where hepatocytes fail to proliferate. The current study elucidates the possible role of cytokines mediated remodeling during liver regeneration, especially their synergistic effects on the proliferation of cholangiocytes and their mesenchymal partners, stellate cells (17) from human, rat and mouse bile ducts.
Materials and Methods
Cell lines and cultures
Our small (SMCC) and large (LMCC) murine cholangiocytes were isolated from normal mice (BALB/c) and immortalized by the introduction of the SV40 large-T antigen gene (18). Normal human intrahepatic biliary epithelial cells (HiBEC), human hepatocytes and mediums were obtained from Sciencell Research Laboratories (San Diego, CA). All other cell culture media and supplements were obtained from Invitrogen (Carlsbad, CA).
Animal protocols and 70% hepatectomy model
Male Fisher 344 rats (75–100 g) were purchased from Charles River (Wilmington, MA), The 70% PH surgery was performed according to the classical model of Higgins and Anderson. Tissues were collected and intrahepatic cholangiocytes were isolated from the removed liver tissues as described (1, 2, 19, 20).
Purified Cholangiocytes and Normal Rat Intrahepatic Cholangiocyte Cultures (NRIC)
Virtually pure cholangiocytes were isolated by immunoaffinity separation (1, 2, 19, 20) with a monoclonal antibody (a gift of Dr. R Faris) against an unidentified antigen expressed by all intrahepatic rat cholangiocytes (21). The in vitro experiments were performed in freshly isolated rat cholangiocytes (IRCs) and our polarized NRIC (22).
In Vitro Proliferation and Migration Assay
Commercial available kits were used for the proliferation and migration assays in hepatobiliary cells (Details in Supplementary Information).
Western Blotting
Protein was extracted from cultured cells or homogenized tissues, and Western blots were performed as described (23). Please see the supplemental information for more details.
Real-Time Polymerase Chain Reaction Assays for Mature miRNAs
The microRNA was isolated as described (23), and the expression of specific mature miR-181b was verified by real-time polymerase chain reaction (PCR) analysis using a TaqMan Human MicroRNA Assay kit (Applied Biosystems, Foster City, CA).
ELISA Assay
SCF (SCF ELISA KIT, BioSource™ CA, USA), and GM-CSF (GM-CSF ELISA KIT, BioSource™) ELISAs were performed according to the manufacturer’s instructions.
Statistical Analysis
All data are expressed as mean ± SE. The differences between groups were analyzed by Student’s t-test when two groups were analyzed or ANOVA if more than two groups were analyzed. A P value <0.05 was used to indicate statistically significant differences.
Please see supplementary for more detailed information of this section.
Results
Altered expression of colony stimulating factors and their receptors following partial hepatectomy
Animals underwent 70% PH or sham and were sacrificed at 0, 1, 3, 5 and 7 days after surgery. Liver samples were obtained and analyzed for SCF, GM-CSF, G-CSF and EPO mRNA expression by real-time PCR. Sham animals demonstrated high mRNA levels of SCF, but not GM-CSF, G-CSF and EPO in the liver, suggesting a large baseline hepatic reservoir of SCF (Fig. 1A). There was a significant decline in SCF expression during the first 24 hours after PH, followed by a significant and stable increase in SCF levels up to 7 days after PH (Fig. 1B). Concurrent with this increase in hepatic SCF mRNA levels, there was a significant increase in SCF receptor mRNA levels (Fig. 1C). Meanwhile, a moderate increase of GM-CSF but not receptor mRNA levels was observed after PH. Interestingly, the significant increases of SCF and GM-CSF were also observed in IRCs from total liver after PH (Fig. 1D). No changes in the expression of other CSFs and their receptors such as G-CSF and EPO were observed after PH.
Figure 1. The expressions of colony stimulating factors and their receptors in rat liver after 70% PH.
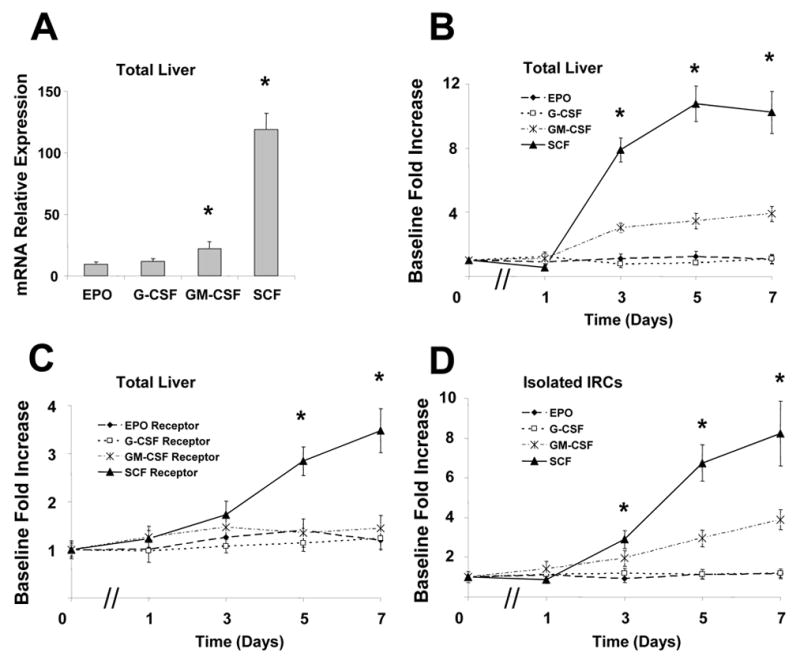
Animals underwent 70% partial hepatectomy (PH) or sham control and liver tissues as well as isolated intrahepatic rat cholangiocytes (IRCs) were obtained at various times postoperatively. RNA was isolated and cDNA generated by reverse transcription using MMLV reverse transcriptase. Basal mRNA expressions of stem cell factor (SCF), erythropoietin (EPO), granulocyte colony-stimulating factor (G-CSF) and granulocyte/macrophage colony stimulating factor (GM-CSF) (Panel A) as well as the alterations of colony stimulating factors (CSFs) (Panel B) and their receptors (Panel C) in total liver tissues, and the expressions of CSF in IRCs (Panel D) after PH were quantified by real-time PCR using SYBR Green as the fluorophore, and expressed relative to GAPDH mRNA expression assessed concurrently in the same samples. Panel A: * p<0.05 when compared with EPO group. Panel B&C: * p<0.05 when compared with sham control group.
CSFs promote the proliferation of primary hepatocytes but not cholangiocytes
We evaluated in vitro the effects of CSF on the proliferation of human and mouse hepatocytes and cholangiocytes. Cells were exposed to media alone or 0.1, 1, 10, 20, 50 ng/ml of CSFs, and proliferation was measured at 72 hours by MTS assays (24). Exposure to 10 and 20 ng/ml SCF and GM-CSF resulted in a significant increase in proliferation of cells of the hepatocytic lineage but not in subpopulations sharing cholangiocyte markers (hepatic stem cells and biliary epithelia) compared with incubation with media alone (Supplementary Fig. 1), suggesting different proliferative roles between cells with hepatocytic markers and those with cholangiocyte traits during regeneration after PH.
Synergistic role of cytokines in proliferation of cells with biliary markers
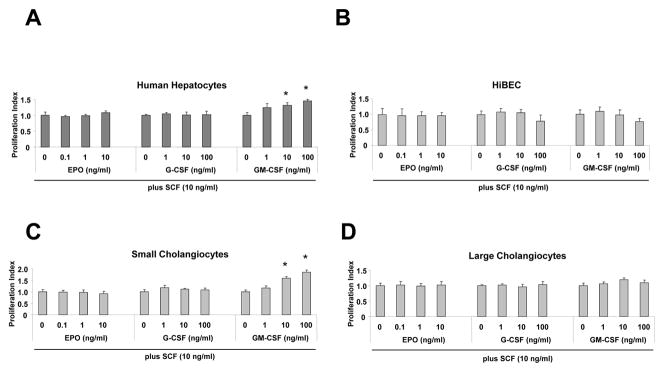
The synergistic effects of SCF in combination with other cytokines have been demonstrated by many studies during the regeneration of different organ systems (25, 26). To determine whether the association between liver regeneration and the biliary epithelium is cytokine dependent, we examined proliferation of cell subpopulations with cholangiocyte markers (hepatic stem cells, biliary epithelia) before or after stimulation with SCF plus GM-CSF, G-CSF and EPO. As shown in Fig. 2, we observed an enhancement of cell proliferation in small murine epithelial subpopulations with biliary markers after stimulation with SCF plus GM-CSF for 72 hours. Addition of this cytokine combination did not change the proliferation index in other human and mouse cholangiocyte subpopulations. These results indicate a regenerative potential in either hepatic stem cells and/or committed biliary epithelial progenitors (small cholangiocytes), after stimulation with SCF plus GM-CSF.
Figure 2. Stem cell factor (SCF) promotes synergistic cellular proliferation in combination with GM-CSF in small murine cells with biliary markers.
Epithelial cells in 96-well plates with 10% fetal bovine serum containing culture medium, and treated with different cytokines for various concentrations as indicated below the panels in combination with SCF (10 ng/ml). After 72 hours, cell proliferation was assessed using MTS assay and the proliferation index derived. The mean ± S.E. from four independent experiments, each in triplicate, at each point is illustrated. GM-CSF in combination with SCF significantly increases cellular proliferation at higher concentrations (≥ 10 ng/ml) in normal human hepatocytes and small murine cholangiocytes (SMCCs). * p <0.05 when compared with control SCF only group.
SCF + GM-CSF induce subpopulations of biliary epithelia to become competent to mitogen signal
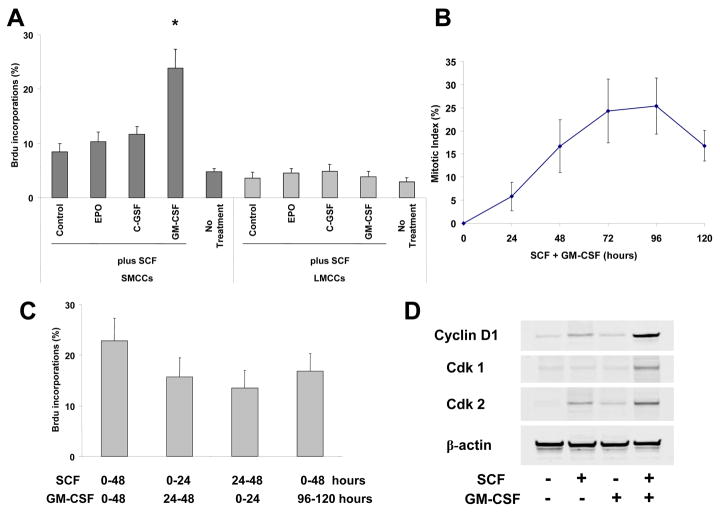
Isolated cholangiocyte subpopulations include cells that are small (7–9 μm) or large (14–15 μm) (2, 20). The small ones comprise the hepatic stem cells and the committed biliary progenitors, whereas the large ones are cholangiocytes (4). These usually acquire long survival potency, maintain certain level of differentiation, and do not respond to single cytokine stimulation. To test the capacity of cholangiocyte subpopulations to proliferate, isolated small (hepatic stem cells and committed biliary epithelia) and large cholangiocytes were exposed for 3 days to different combinations of SCF, EPO, G-CSF and GM-CSF in a medium without fetal calf serum, and DNA synthesis was estimated in these subpopulations (Fig. 3A). In absence of stimulation and in single CSF-treated cultures, low levels of DNA synthesis were detected in both small and large biliary subpopulations. In contrast, combination of SCF plus GM-CSF induced DNA synthesis in at least 30% of small cells with biliary markers at day 3, whereas less than 12% large cholangiocytes incorporated BrdU when stimulated by SCF in presence of GM-CSF. Measurement of mitotic index in cultures stimulated with SCF + GM-CSF for 5 days showed a peak of division at day 4, reaching 25% of small cells with biliary markers (Fig. 3B). Mitotic index matched BrdU incorporation level.
Figure 3. SCF plus GM-CSF promote cell cycle progression in small murine cholangiocytes.
Panel A: SMCCs and large murine cholangiocytes (LMCCs) were stimulated with SCF plus other CSFs (all at 10 ng/ml) for 72 hours, percentages of bromodeoxyuridine (BrdU)-labeled cells were determined by immunocytochemistry after 24-hour BrdU incorporation at day 3. Results represent means ± SE (n = 8). Panel B: SMCCs were stimulated with the combination of SCF/GM-CSF (10 ng/ml) for 5 days. Mitotic index was determined every day after a 24-hour colcemid treatment. Percentages of dividing cells were determined. Values represent means ± SE (n = 6). Panel C: SMCCs were stimulated with (1) combination of SCF/GM-CSF for 48 hours, (2) SCF then GM-CSF for 24 hours each, (3) GM-CSF then SCF for 24 hours each, (4) SCF for 48 hours, followed by 2 days without stimulation and then GM-CSF for 48 hours. BrdU was incorporated during the last 24 hours of treatment, and percentages of BrdU-labeled cells were determined. Results are means ± SE (n = 6). Panel D: Alterations of cell cycle proteins in SCF and/or GM-CSF treated SMCCs. SMCCs were maintained without stimulation or treated with SCF, GM-CSF alone, or SCF/GM-CSF (10 ng/ml) for 3 days. Protein extracts from fractions enriched for cells with biliary markers were analyzed by immunoblotting using antibodies against cyclin D1, Cdk1, Cdk2 and β-actin.
We next tested whether addition of GM-CSF before or after SCF influences replication of small cells with biliary markers. Cultures were co-stimulated with GM-CSF and SCF for 48 hours or stimulated for 24-hour periods with GM-CSF before or after SCF treatment (Fig. 3C). In all cases, cells replicated their DNA, and BrdU incorporation levels were similar. In addition, a pause of 2 days after SCF treatment did not change the responsiveness of cells to GM-CSF.
SCF promotes cell cycle progression to late G1, whereas GM-CSF is necessary for S entry
To further analyze the respective role of combination of the cytokines in cell cycle progression, we studied the expression levels of cyclin D1, Cdk2, and Cdk1 in small and large cholangiocyte subpopulations. Short-term cholangiocyte cultures, known to undergo 1 cell cycle under SCF stimulation, were used as controls.
In untreated and GM-CSF-stimulated cultures, as well as in untreated short-term cultures, cyclin D1 and Cdk1 were not detected (Fig. 3D), suggesting that cells were blocked in G1 upstream from the mitogen-associated restriction point in mid-late G1. In contrast, in GM-CSF plus SCF-stimulated cultures and in SCF-treated short-term cultures, cyclin D1, Cdk2, and Cdk1 expression levels markedly increased, thus confirming the cell progression into S and M phases. These results suggested that SCF alone promoted progression up to late G1, and that GM-CSF stimulation was necessary for the G1/S transition.
Contribution to regenerative potentials by cytokines in small cells with biliary markers
Our previous studies have demonstrated that small cholangiocytes (originated from small bile duct branches, and resistant to CCl4-induced apoptosis) (2, 20) de novo proliferate to compensate for the loss of large biliary mass (2, 4). It is possible that the combination of SCF and GM-CSF may alter the induction of remodeling potentials in cholangiocytes during liver regeneration. Alkaline phosphatase activity has been used as a biomarker for tissue repair and remodeling. We next compared StemTAG™ alkaline phosphatase activity in mouse cholangiocytes after stimulation with SCF, GM-CSF, or a combination of the two. As shown in Supplementary Fig. 2A, AP activity was scarcely detected 72 hours after stimulation with SCF or GM-CSF alone in small and large murine cells with biliary markers. In contrast, a more intense AP staining was observed when small cells with biliary markers were stimulated with SCF plus GM-CSF combination. The mRNA levels of SCF receptor were equally decreased after stimulation with either SCF alone or SCF combined with GM-CSF. In addition, the GM-CSF receptor mRNA levels were similar between stimulation with SCF alone and SCF plus GM-CSF (Supplementary Fig. 2B). These results indicate that enhanced remodeling potential of small cells with biliary markers after stimulation with SCF plus GM-CSF is due to the activation of downstream signaling pathways rather than increased levels of CSF receptors. Together, the data suggest that altered tissue repair capabilities with SCF plus GM-CSF in small mouse cells with biliary markers, but not in the presence of either SCF or GM-CSF alone.
Characterization of tissue remodeling marker S100A4 in Cytokine-treated cholangiocytes
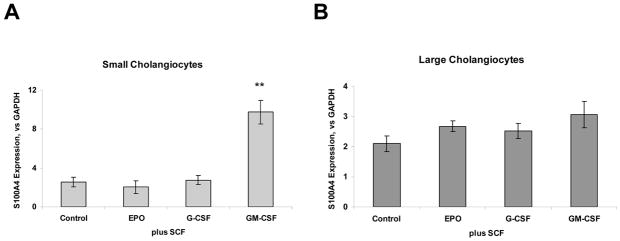
S100A4, a member of the S100 family of proteins, is of particular interest as a marker of chronic tissue remodeling. It plays an important role in matrix remodeling by up-regulating the expression of matrix metalloproteinases. We assessed the effect of cytokine combinations on S100A4 mRNA expression. Quantitative real-time PCR analysis revealed a 3.8 ± 0.2-fold increased expression of S100A4 after SCF plus GM-CSF administration in small cells with biliary markers compared to SCF alone (p<0.01), suggesting a significant remodeling event after treatment (Fig. 4A–B). Interestingly, the same treatment combination also induced a moderate increase of S100A4 protein expression in human intrahepatic cholangiocytes (Fig. 4C, bottom panel). In contrast, no significant changes were observed in S100A4 expression in small and large cells with biliary markers by SCF or GM-CSF alone or other SCF combinations.
Figure 4. SCF regulates mesenchymal marker S100A4 in combination with GM-CSF in small murine cholangiocytes.
Total RNA was isolated from SMCCs (Panel A) and LMCCs (Panel B) treated with CSFs + SCF and quantitative real-time PCR for mesenchymal marker–S100A4 was performed using a Superarray qPCR Assay kit. Quantitative data representing the mean and SE from 3 experiments performed in triplicate are presented in the bar graph. The expression of S100A4 was normalized to that of the GAPDH gene control. SCF promotes synergistic mesenchymal transition or transformation in combination with GM-CSF only in small murine cells with biliary markers. The differences between any of the other groups were not significant. Panel C: Immunocytochemistry for S100A4 and CK-19 was performed in SMCCs and HiBECs. An increase in S100A4 expression is observed in both cell lines after SCF/GM-CSF treatment (10 ng/ml, 72 hours). **p < 0.01 compared with expression in SCF only group.
Involvement of SCF and GM-CSF in TGF-β dependent signaling in cholangiocytes
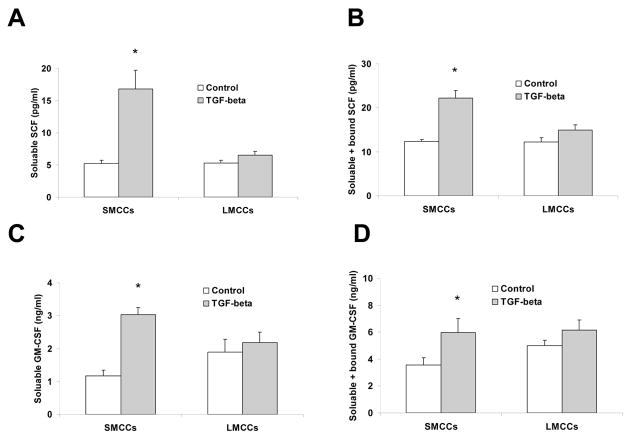
TGF-β has been associated with intracellular matrix deposition and hepatic/biliary tissue repair/damage (27). It is a cytokine that stimulates mesenchymal proliferation, inhibits epithelial growth and is important in organogenesis. Since prior studies have suggested that TGF-β is produced in response to partial hepatectomy (28), we next determined whether TGF-β stimulates SCF and GM-CSF production and release. SMCC and LMCC in vitro were stimulated with 10 ng/ml TGF-β or media alone and were harvested after 72 hours of incubation. Supernatants or supernatants plus cells were collected for SCF and GM-CSF measurement by ELISA assay. Supernatant levels of SCF/GM-CSF were used to estimate levels of soluble SCF/GM-CSF; for quantitation of soluble plus bound SCF/GM-CSF, supernatants plus cells were sonicated and SCF/GM-CSF levels in this solution were used as an estimate of soluble plus bound SCF/GM-CSF. Small mouse cholangiocytes produced significant amounts of both soluble and soluble plus bound SCF in response to TGF-β when compared to controls whereas large cholangiocytes only showed a slightly increase at the time point studied (Fig. 5A–D).
Figure 5. TGF-β stimulates SCF and GM-CSF production and release in SMCCs.
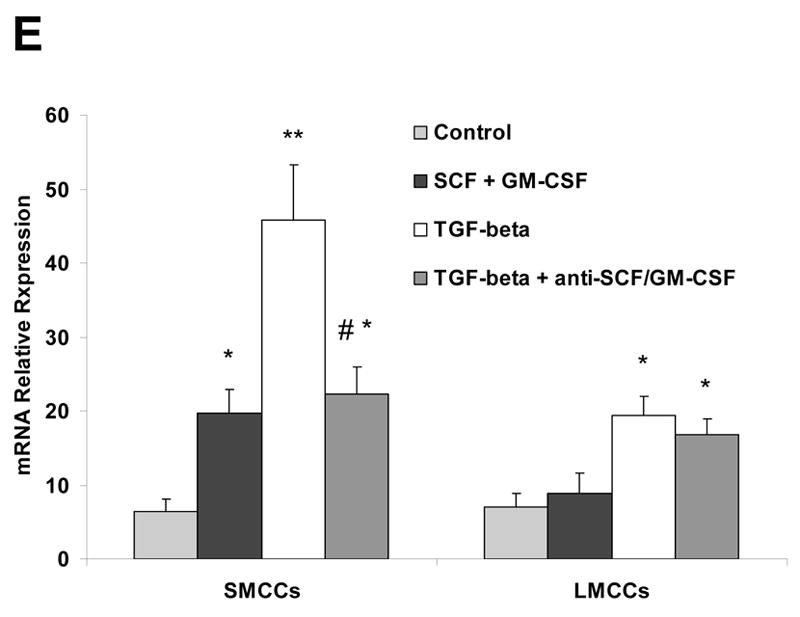
SMCC and LMCC mouse cells were stimulated with 10 ng/ml TGF-β or media alone. Supernatants and supernatants plus cells were harvested at 72 hours and SCF and GM-CSF levels were measured by ELISA. Supernatant SCF and GM-CSF levels were used to determine levels of soluble SCF and GM-CSF (Panel A and C). For quantitation of soluble + bound SCF and GM-CSF (Panel B&D), supernatants plus cells were sonicated and SCF and GM-CSF levels in this solution were measured as an estimate of soluble + bound SCF. There were no significant differences noted in levels of soluble compared with soluble + bound SCF in cells incubated in media alone. There were significant increases in soluble SCF and GM-CSF levels at 72 hours time-point from cells treated with TGF-β in SMCCs (*P < 0.05 vs. media alone). In addition, levels of soluble + bound SCF and GM-CSF were significantly increased in SMCCs after TGF-β treatment compared with treatment with media alone (*P < 0.05 vs. media alone). Furthermore, levels of soluble plus bound SCF and GM-CSF were significantly increased compared with levels of soluble SCF alone in SMCCs after TGF-β treatment at 72 hours. Data are expressed as the mean ± SE. Panel E: Anti-SCF+GM-CSF partially blocked TGF-β –induced changes in small cells with biliary markers. SMCCs and LMCCs were incubated with 10 ng/ml TGF-β with anti–SCF and GM-CSF antibodies (10 μg/l) or control anti-serum for 72 hours, total RNA was isolated and quantitative real-time PCR for mesenchymal marker – s100A4 was performed using a Superarray qPCR Assay kit. Quantitative data representing the mean and SE from 3 experiments. The expression of s100A4 was normalized to that of the GAPDH gene control. A significant decrease in S100A4 mRNA expression was noted in SMCCs treated with TGF-β with anti–SCF and GM-CSF antibodies compared to control TGF-β group, suggesting that SCF plus GM-CSF are involved in TGF-β–induced changes in this system. *P < .05, **P < .01 relative to control; #P < .05 compared with expression in control TGF-β group.
Since TGF-β is a well-known S100A4 inducer and because the above experiments suggest that TGF-β can induce SCF/GM-CSF production and release, the next experiments were designed to evaluate whether SCF+GM-CSF and TGF-β-induced S100A4 expression in small cholangiocytes occurs via a related pathway. At a dose of 10 ng/ml, both TGF-β and SCF+GM-CSF induced significant cell remodeling after 72 hours of incubation, as measured by incorporation of S100A4 mRNA expression (Fig. 5E). Next, the effects of SCF plus GM-CSF blockade in the presence of TGF-β were measured. When small cholangiocytes were incubated with 10 ng/ml TGF-β plus anti-SCF and GM-CSF antibodies (10 μg/l), a significant decrease in S100A4 mRNA expression was noted, suggesting that SCF plus GM-CSF play an important role in TGF-β-induced remodeling in this system. Meanwhile, when large cholangiocytes were incubated with the same combinations, only a slight decrease in S100A4 expression level was observed which did not reach statistical significance (Fig. 5E).
SCF + GM-CSF enhance miR-181 targeting of TIMP-3
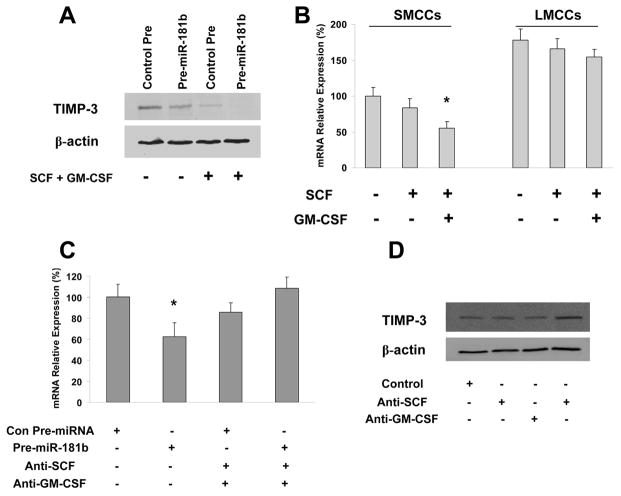
Our recent data suggested that microRNA-181 is critical for hepatic cell remodeling and differentiation and target 3′-UTR of tissue inhibitor of metalloproteinases-3 (TIMP-3) leading to TIMP-3 mRNA degradation (29). TIMP-3 has unique domains that interact with extracellular matrix components and, unlike the other TIMPs, is mainly bound to tissue matrix (30). As miR-181 has been shown to be regulated by TGF-β, we performed experiments to determine whether SCF + GM-CSF impact miR-181 targeting of TIMP-3 in SMCCs. To this end, small cholangiocytes were transfected with Pre-miR-181b or control Pre-miRNA in combinations with the treatments of SCF plus GM-CSF; the cell lysates were obtained to determine the levels of TIMP-3 protein. As shown in Figure 6A, the levels of TIMP-3 protein and mRNA were reduced in small cells with biliary markers and co-transfected with the miR-181b expression plasmid (Pre-miR-181b); SCF plus GM-CSF further reduced TIMP-3 protein. The level of TIMP-3 expression in small cells with biliary markers was lower than in LMCCs (Fig. 6B). Importantly, TIMP-3 was almost fully degraded in cells simultaneously treated with SCF plus GM-CSF and transfected with miR-181b expression vectors; the ability of miR-181b targeting of TIMP-3 degradation was offset when SCF and GM-CSF was knocked down by specific antibodies (Fig. 6C). As shown in Figure 6D, the levels of TIMP-3 protein in anti-SCF plus GM-CSF treated cells were also higher than in control small cells with biliary markers. Therefore, SCF plus GM-CSF accelerates miR-181b-induced TIMP-3 protein degradation, whereas anti-SCF plus GM-CSF counteracts this process. It is of note that TIMP-3 protein is almost absent when SCF plus GM-CSF and miR-181b were treated/transfected simultaneously. These findings suggest that the level of TIMP-3 in SMCCs is regulated by SCF + GM-CSF and miR-181b.
Figure 6. SCF/GM-CSF facilitates miR-181b-induced TIMP-3 degradation.
Panel A and C: SMCCs were transfected with control or miR-181b precursors with or without co-treatment with SCF/GM-CSF (10 ng/ml, 72 hours). Panel A is western blotting with TIMP-3 antibody and β-actin was used as internal control, whereas Panel C is real-time PCR analysis for TIMP-3 mRNA level and GAPDH was used as internal control. Panel B: Total RNA from SMCCs and LMCCs treated with different combination of SCF/GM-CSF indicated in the bottom were subjected to real-time PCR analyses for TIMP-3 mRNA level. GAPDH was used as internal control. The mRNA expression values relative to control SMCCs group were shown (n=6). Panel D: Western blotting using TIMP-3 antibodies in SMCCs treated with different combination of SCF/GM-CSF for 72 hours as indicated in the bottom. β-actin was used as the loading control. * p < 0.05 relative to no treatment/control group.
Alteration of matrix remodeling enzymes – MMPs in cholangiocytes after treatments of SCF + GM-CSF
Partial hepatectomy triggers hepatocyte/proliferation of subpopulations sharing biliary epithelial markers/apoptosis, and hepatic/biliary matrix remodeling, all of which are important events in the regenerating liver. Matrix metalloproteinases (MMPs) are a family of zinc-containing neutral proteinases that are involved in matrix remodeling in both normal and pathophysiological processes. The onset of extracellular matrix production after PH correlates with a peak in the expression of TGF-β messenger RNA, which stimulates collagen synthesis. To confirm the functional relevance of SCF and GM-CSF-dependent modulation of regeneration, we assessed the expression of MMPs involved in cell remodeling. Treatment of small mouse cholangiocytes with SCF+GM-CSF significantly increased MMP-2 and MMP-9 mRNA expressions when compared to SCF or GM-CSF alone. Compared with the controls, the expression of both MMP-2 and MMP-9 was increased in TGF-β treated small cholangiocytes by 2.1- ± 0.4-fold and 3.6- ± 0.7-fold, respectively. Furthermore, the expression of both MMP-2 and MMP-9 were decreased after addition of antibodies of SCF + GM-CSF in TGF-β treated small cells with biliary markers (Fig. 7). The up-regulations of MMP-2, MMP-9 as well as S100A4 were also observed in rat liver tissues as well as in IRCs 3 day after partial hepatectomy using the specific PCR Array kit from SABiosciences (#PAHS-033A; Fig. 8A–C), along with the significant increase of miR-181b level and the reduced TIMP-3 expression by Zymogen Gel Assay and real-time PCR analysis (Fig. 8D). Our findings provide evidence of a link between SCF plus GM-CSF and the expression of mediators of cell remodeling in biliary cell subpopulations and lines. These data suggest that altered expression of SCF and GM-CSF following PH can contribute to biliary remodeling by functional deregulation of activity of key signaling intermediates.
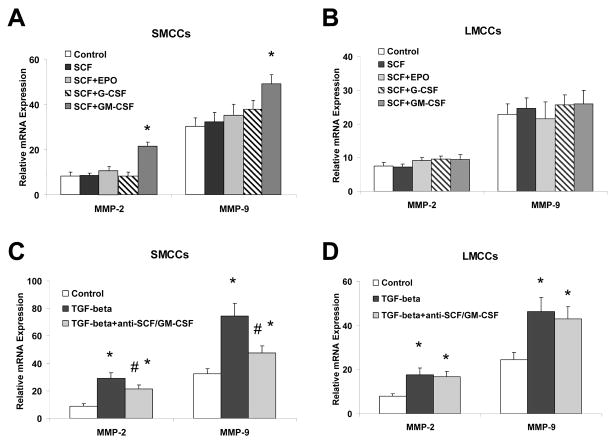
Figure 7. SCF plus GM-CSF modulate mRNA expression of matrix metalloproteinases (MMPs).
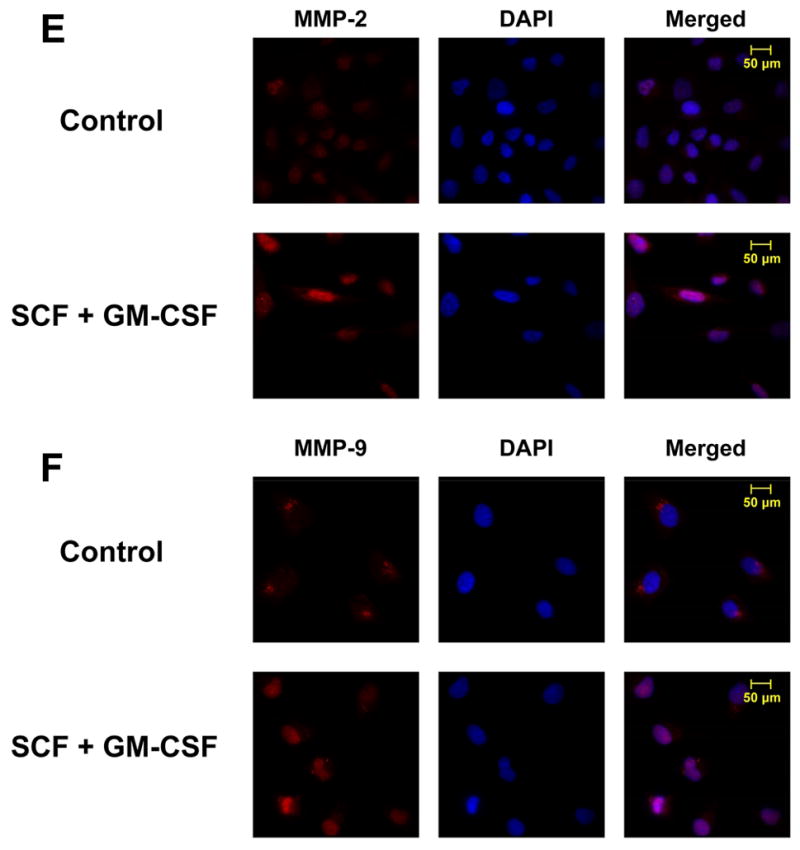
Panel A and B: LMCCs and SMCCs were treated with SCF and SCF plus EPO, G-CSF, GM-CSF or controls. Quantitative real-time PCR was performed for MMP-2, MMP-9, and GAPDH mRNA expression. SCF plus GM-CSF treatment increases MMP-9 mRNA expression in SMCCs. *P < .05 relative to SCF only group. Panel C and D: SMCCs and LMCCs were incubated with 10 ng/ml TGF-β with anti–SCF and GM-CSF antibodies (10 μg/l) or control antiserum for 72 hours, total RNA was isolated and quantitative real-time PCR for MMP-2 and MMP-9 was performed using a Superarray qPCR Assay kit. The expression of MMPs was normalized to that of the GAPDH gene control. Inhibition of SCF plus GM-CSF reduced both MMP-2 and MMP-9 mRNA expression in SMCCs compared with controls. Panel E and F: Immunocytochemistry for MMP-2 (panel E) and MMP-9 (Panel F) was performed in SMCCs. An increase in MMP-2 expression along with the enhanced expression of MMP-9 is observed in SCF/GM-CSF treated cholangiocytes. *p < 0.05, **p < 0.01 relative to control.
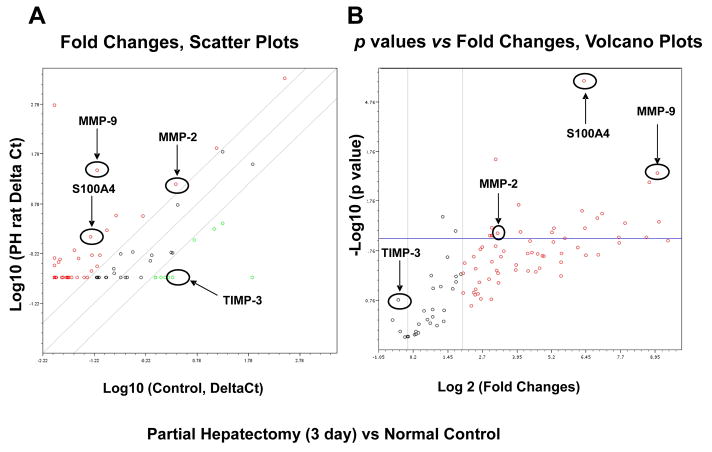
Figure 8. Alterations of MMP-2, MMP-9, TIMP-3, miR-181b and S100A4 in rat liver and isolated intrahepatic rat cholangiocytes after partial hepatectomy.
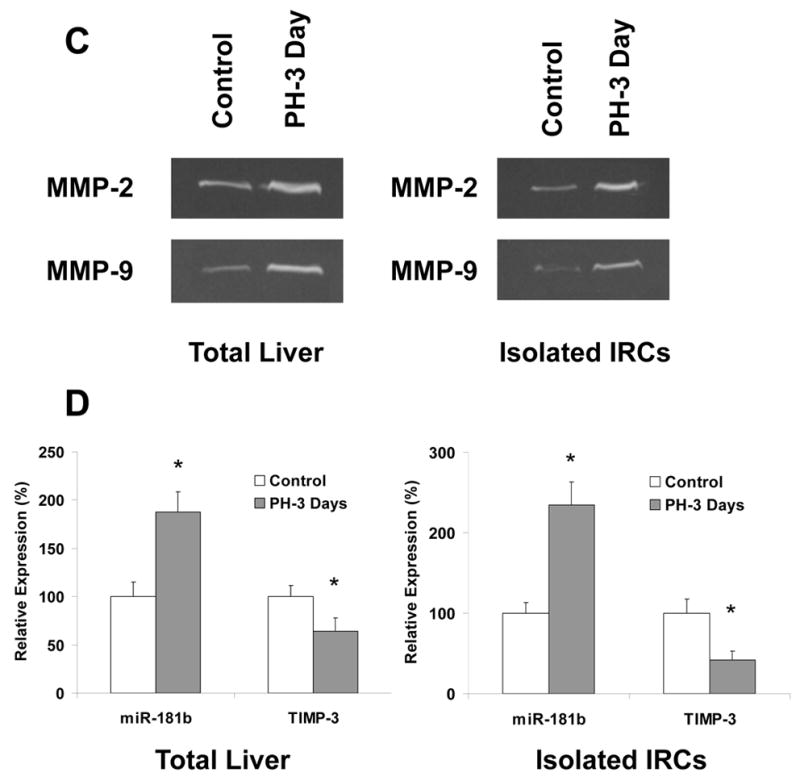
Rats underwent 70% hepatectomy or sham control and liver tissue was obtained for Specific PCR Array assay (PAHS-033A, SABiosciences) at 72 hours postoperatively. We choose this PCR array assay since this is the only 84-gene group PCR array, which includes S100A4 and other remodeling molecules. Panel A and B: Total RNAs from liver tissues/isolated IRCs of control and partial hepatectomy were characterized in technical triplicates, and the relative expression levels and p values for each gene in the related samples are plotted against each other in the Scatter and Volcano Plots, depicting the relative expression levels (Log10) for selected genes in treated versus control panels (Panel A), or in p values vs. fold changes (Panel B). Genes for which the difference in expression levels was greater than a factor of three and/or p<0.05 are shown out of the cut-off lines on the graph. MMP-2, MMP-9 and S100A4 are among the top up-regulated genes in partial hepatectomy group when compared to sham control rats with p value < 0.05. Panel C and D: Total RNAs from liver tissues/isolated IRCs of control and partial hepatectomy (3 days) were subjected to MMP Zymogen Gel Assay (Panel C) and Real-Time PCR assay (Panel D) respectively. MMP-2, MMP-9 and miR-181b were significantly upregulated, whereas TIMP-3 was reduced in total liver tissues as well as IRCs after partial hepatectomy (72 hours). * p < 0.05 relative to controls.
Discussion
Although a role for cholangiocytes in liver regeneration has been proposed, the molecular mechanisms by which cytokines modulate hepatobiliary remodeling and subsequently contribute to hepatic repair are unknown. We show that SCF and GM-CSF expressions are significantly increased after PH compared to normal controls. Moreover, we demonstrate that combination of SCF plus GM-CSF stimulates specific cholangiocyte subpopulations in proliferation, remodeling, migration, and associated mesenchymal cell activation via TGF-β dependent signaling and downstream effects involving the expression of miR-181b as well as matrix remodeling enzyme TIMP-3, MMP-2 and MMP-9. The identification of SCF plus GM-CSF as the important regulators of remodeling events in vitro emphasizes an essential synergistic role of these cytokines in mediating hepatic regeneration and repair, and provides insight into the contribution of altered remodeling in recovering the severe liver injury.
Cholangiocytes and hepatocytes share embryologic origins. This common heritage contributes traits carried into adulthood. If specific cells exist within the adult liver with multipotential capacity, they should be able to differentiate into either hepatocytes or cholangiocytes in particular circumstances such as when late lineage stage hepatocytes are lost or their regenerationis blocked. Plasticity between intrahepatic cholangiocytes and hepatocytes has been assumed (17, 31) suggesting that terminally differentiated cells of one lineage are able to directly differentiate into another lineage or undergo transdifferentiation (32). However, the data are interpretable also as expansion and differentiation of a stem cell population (4). The extent to which liver stem cells mediate liver regeneration has been hotly debated. One of the primary reasons for this controversy is the use of multiple definitions for the hepatic stem cell (33). Recent studies have demonstrated that meticulously isolated and rigorously characterized gallbladder epithelial cells cultured under defined in vitro conditions acquire hepatocyte-like properties, such as the ability to synthesize bile acids and take up low-density lipoprotein, without expression of oval cell or hematopoietic stem cell markers (12). The recent discovery of biliary tree stem cell populations (34) provides again an alternative interpretation of expansion and differentiation of stem cells as opposed to transdifferentiation. Therefore, specific subpopulations of cells that express some of the known cholangiocyte markers, can be hypothesized to contain a multipotent stem cell population when exposed to certain environmental conditions. Our studies have suggested that such cells could attain functional pluripotent characteristics after synergistic treatment of SCF and GM-CSFs, and subsequently they could be used to repopulate damaged livers.
Inadequate liver regeneration is still an unsolved problem in major liver resection and living donor liver transplantation. The studies have implicated the usage of cytokines as the exogenous stimulators of liver regeneration in various animal models of liver resection and liver transplantation (35). SCF and GM-CSF have been demonstrated to affect cellular differentiation and proliferation in various types of cells besides hepatobiliary epithelial cells. SCF enhances growth and differentiation when combined with other cytokines (36). SCF and GM-CSF induces synergistic proliferation and differentiation in myeloid progenitor cells including the megakaryoblastic cell line MO7e (37). Synergistic effects are of critical importance biologically, because hematopoietic stem cells and early progenitor cells require growth factors in combination for self-renewal and differentiation. Moreover, in the bone marrow microenvironment the physiological actions of SCF occur in combination with other growth factors and extracellular matrix proteins. The combination of SCF plus GM-CSF promoting remodeling within the liver supports the possibility of TGF-β-dependent mechanisms contributing to synergistic hepatic repair in response to SCF and GM-CSF.
Our current study indicates that there are relatively high constitutive SCF and GM-CSF levels within the liver during regeneration. SCF and GM-CSF are normally found as the transmembrane molecules under homeostatic conditions and are solubilized after inflammatory stimuli to induce the proper enzyme release to cleave it from the cell surface. This procedure may allow a substantial SCF and GM-CSF reservoir to be accumulated on the cell surface, ready for release upon the environmental condition changes. We hypothesize that solubilized SCF and GM-CSF released during liver regeneration could interact via their receptors with the surrounding specific subpopulations of cells with biliary markers populations, functioning as a remodeling agent within the damaged tissue. Although hepatobiliary homeostasis and regeneration likely involves multiple complex mechanisms and pathways, our current data strongly suggests that SCF and GM-CSF plays significant synergistic roles in reestablishing the homeostasis of the biliary system.
The expression of downstream mediators of biliary remodeling could be modulated by SCF plus GM-CSF. Therapeutic strategies to increase SCF plus GM-CSF may be potentially useful to rebuild the hepatobiliary system after liver injury. Knowledge of specific processes such as biliary proliferation, migration, remodeling and mesenchymal transition that are regulated by CSFs, and the identification of critical targets for SCF and GM-CSF, provides novel insights into the mechanisms in the development and remodeling of the intrahepatic biliary epithelium.
Supplementary Material
Acknowledgments
This study was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award and NIH grants DK58411 and DK76898 to Dr. Alpini.
Abbreviations
- CSFs
Colony stimulating factors
- EPO
erythropoietin
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- miRNA
microRNA
- LMCC
large murine cholangiocytes
- MMP
matrix metalloproteinase
- PH
partial hepatectomy
- S100A4
S100 calcium binding protein A4
- SCF
Stem cell factor
- TIMP-3
tissue inhibitor of metalloproteinases-3
- SMCC
small murine cholangiocytes
- TGF-β
Transforming growth factor-β
References
- 1.LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 2.LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 3.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 4.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 6.Chatzipantelis P, Lazaris AC, Kafiri G, Nonni A, Papadimitriou K, Xiromeritis K, Patsouris ES. CD56 as a useful marker in the regenerative process of the histological progression of primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:837–842. doi: 10.1097/MEG.0b013e3282fdf66f. [DOI] [PubMed] [Google Scholar]
- 7.Komori A, Nakamura M, Fujiwara S, Yano K, Fujioka H, Migita K, Yatsuhashi H, et al. Human intrahepatic biliary epithelial cell as a possible modulator of hepatic regeneration: Potential role of biliary epithelial cell for hepatic remodeling in vivo. Hepatol Res. 2007;37 (Suppl 3):S438–443. doi: 10.1111/j.1872-034X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 8.Bath PM, Sprigg N. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. 2006;3:CD005207. doi: 10.1002/14651858.CD005207.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Akel S, Petrow-Sadowski C, Laughlin MJ, Ruscetti FW. Neutralization of autocrine transforming growth factor-beta in human cord blood CD34(+)CD38(−)Lin(−) cells promotes stem-cell-factor-mediated erythropoietin-independent early erythroid progenitor development and reduces terminal differentiation. Stem Cells. 2003;21:557–567. doi: 10.1634/stemcells.21-5-557. [DOI] [PubMed] [Google Scholar]
- 10.Ren X, Hu B, Colletti L. Stem cell factor and its receptor, c-kit, are important for hepatocyte proliferation in wild-type and tumor necrosis factor receptor-1 knockout mice after 70% hepatectomy. Surgery. 2008;143:790–802. doi: 10.1016/j.surg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuver R, Savard CE, Lee SK, Haigh WG, Lee SP. Murine gallbladder epithelial cells can differentiate into hepatocyte-like cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;293:G944–955. doi: 10.1152/ajpgi.00263.2006. [DOI] [PubMed] [Google Scholar]
- 13.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008;48:1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- 15.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 16.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Yao HL, Cui CB, Wauthier E, Barbier C, Costello MJ, Moss N, et al. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology. 2010;52:1443–1454. doi: 10.1002/hep.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 19.Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 22.Alpini G, Phinizy JL, Glaser S, Francis H, Benedetti A, Marucci L, LeSage G. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066–1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 23.Meng F, Henson R, Wehbe-Janek H, Smith H, Ueno Y, Patel T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem. 2007;282:8256–8264. doi: 10.1074/jbc.M607712200. [DOI] [PubMed] [Google Scholar]
- 24.Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray P, Krishnamoorthy N, Oriss TB, Ray A. Signaling of c-kit in dendritic cells influences adaptive immunity. Ann N Y Acad Sci. 2010;1183:104–122. doi: 10.1111/j.1749-6632.2009.05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ordog T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 28.Thenappan A, Li Y, Kitisin K, Rashid A, Shetty K, Johnson L, Mishra L. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology. 2010;51:1373–1382. doi: 10.1002/hep.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng F, Glaser S, Francis H, DeMorrow S, Han Y, Passarini JD, Stokes A, et al. Functional Analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y, Winkler IG, Barbier V, Sims NA, Hendy J, Levesque JP. Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirica AE, Williams TW. Appearance of ductular hepatocytes in rat liver after bile duct ligation and subsequent zone 3 necrosis by carbon tetrachloride. Am J Pathol. 1992;140:129–136. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 33.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinale V, Wang Y, Carpino G, Alvaro D, Reid L, Gaudio E. Multipotent stem cells in the biliary tree. Ital J Anat Embryol. 2010;115:85–90. [PubMed] [Google Scholar]
- 35.Karpoff HM, D’Angelica M, Blair S, Brownlee MD, Federoff H, Fong Y. Prevention of hepatic tumor metastases in rats with herpes viral vaccines and gamma-interferon. J Clin Invest. 1997;99:799–804. doi: 10.1172/JCI119226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNiece IK, Langley KE, Zsebo KM. The role of recombinant stem cell factor in early B cell development. Synergistic interaction with IL-7. J Immunol. 1991;146:3785–3790. [PubMed] [Google Scholar]
- 37.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.