Abstract
OBJECTIVES
To determine the relationship between opioid consumption and cognitive impairment following hip fracture repair.
DESIGN
Prospective study of consecutive patients.
SETTING
Johns Hopkins Bayview Medical Center; Baltimore, Maryland.
PARTICIPANTS
Two hundred thirty-six patients ≥65 years old undergoing hip fracture repair.
MEASUREMENTS
Elderly patients without preoperative delirium who underwent hip fracture repair between April 2005 and July 2009 were followed for pain, opioid consumption, and postoperative delirium. Patients were tested for delirium with the confusion assessment method preoperatively and mid-morning on postoperative day 2. Pain was assessed by the nursing staff with a numeric 0–10 verbal scale. Opioid analgesia was provided in response to pain at rest to achieve scores of ≤3. Opioid consumption was analyzed with respect to the occurrence of incident postoperative delirium, presence of dementia, and other demographic variables.
RESULTS
Of the 236 patients, 66 (28%) had dementia with 213 (90%) receiving opioids postoperatively; including 55 (83%) demented patients and 158 (93%) non-demented patients. There was no association between the use of any postoperative opioid and incident delirium (P=0.615) in both demented (p=0.333) and non-demented patients (P=0.398). Dementia, but not postoperative delirium, was associated with less opioid use (P<0.001 for dementia; P=0.120 for delirium; P=0.038, for their interaction; Wald χ2 =142.8 with 7 d.o.f.). Furthermore, opioid dose (P≥0.591) on postoperative days 1 and 2 was not predictive of incident delirium. Dementia (P<0.001) and intensive care unit admission (P=0.006), not opioid consumption, were the most important predictors of incident postoperative delirium.
CONCLUSION
Concern for postoperative delirium should not prevent the use of opioid analgesic therapy sufficient to achieve a generally accepted level of comfort in patients with or without preexisting cognitive impairment.
Keywords: delirium, hip fracture, opioids, surgery (complications)
INTRODUCTION
The relationship between opioid analgesic use and postoperative delirium when managing pain in elderly patients following hip fracture repair is poorly defined. For one, there is controversy as to whether opioid administration may itself be a risk factor for postoperative delirium. Although one study showed opioid administration to have an inverse relationship with respect to postoperative delirium,1 others have shown the opposite.2, 3 On the other hand, perioperative pain may be a risk factor for postoperative delirium. In mixed populations of surgical patients, an association has been reported between elevated levels of pain at rest and the development of postoperative delirium.4, 5 Similarly, in elderly patients that have sustained hip fractures, undertreated pain has been associated with an increased incidence of postoperative delirium.1, 6 Given these data, it is unclear whether opioid administration or pain precipitates delirium.
Cognitive impairment is common among hip fracture patients. Up to 21% of patients who require hip fracture repair present with dementia.7 Although the overall prevalence of postoperative delirium in elderly patients after major elective surgery has been estimated to be 10%,8 for procedures such as hip fracture repair the rate of incident postoperative delirium has been reported to be as high as 40%.9 Cognitive impairment may make pain assessment more difficult, and many practitioners express concern regarding evidence that opioid administration may precipitate postoperative delirium.2, 3
Because both opioid administration and elevated pain have been associated with an increased risk of postoperative delirium, and the difficulty with managing pain in patients with dementia, we sought to further define the relationship between opioid consumption and cognitive impairment. Therefore, we measured opioid consumption and pain in patients with and without cognitive impairment on our hip fracture service (HFS), where postoperative pain management is based on an established clinical pathway.
METHODS
The HFS at Bayview Medical Center in Baltimore, Maryland, serves as an interdisciplinary model of care whose function has been described previously.10 According to the HFS clinical pathway, an orthopedic surgeon evaluates patients with suspected hip fractures in the emergency room, confirms the diagnosis, and refers them to a member of the geriatrics team. Orthopedic and geriatrics team members order all necessary laboratory work while the geriatricians oversee the medical evaluation. Then, team members from the departments of Geriatrics, Anesthesiology and Critical Care Medicine, and Orthopedic Surgery jointly render a decision regarding a patient’s fitness for surgery. When a patient is deemed ready for surgery, he/she undergoes surgical hip fracture repair. The goal of the service is repair within 24 hours.
Data are collected prospectively on patients who enter the HFS. A database is maintained on all patients ≥65 years of age who have experienced traumatic hip fracture, are undergoing hip fracture repair, and have given written preoperative consent to the collection and use of their medical information. To determine the postoperative relationship between opioid consumption and incident delirium, 102 patients with preoperative delirium diagnosed via the confusion assessment method (CAM)11 were excluded. After receiving approval from the Institutional Review Board, we studied all patients in the HFS database who had undergone hip fracture repair during the period of April 1, 2005 through July 10, 2009.
During the study period, 317 patients who met age and cognitive criteria presented to the HFS for hip fracture repair. Nine of those were considered ineligible for our study because of inability to speak English. Seventy-two of the remaining 308 patients refused to participate, leaving a study population of 236.
Both general and spinal anesthetic techniques were used for patients in this study, and the choice was a function of patient preference. For those who chose general anesthesia, an inhalational technique was used with drug doses individualized for established comorbidities. For those who chose spinal anesthesia, supplemental intravenous sedation during the surgical procedure was provided with some combination of midazolam, propofol, and fentanyl. At the completion of surgery, patients were transferred to either the postanesthesia care unit (PACU) or the intensive care unit (ICU) and, eventually, to the hospital ward. Postoperatively, an interdisciplinary team of physicians from the departments of Orthopedic Surgery, Geriatrics, and Anesthesiology and Critical Care Medicine managed each patient’s recovery until the day of discharge.
Postoperative pain management in the PACU is based on the algorithm of Aubrun et al.12 and was adapted for use with intravenous hydromorphone. Adequate pain relief is defined as a score of ≤3 on a verbal numerical rating scale of 0–10. Briefly, immediately after patients become responsive, they are asked to verbally rate the intensity of their pain at rest (see below for a description of pain measurement). When pain is rated greater than 3 by the patient, intravenous hydromorphone is administered every 5 minutes by 0.1–0.2 mg increments; pain is reassessed every 5 minutes until the pain score is ≤3. A similar algorithm that uses morphine instead of hydromorphone is used in the ICU. After transfer to the hospital ward, a standardized order set for multimodal analgesic therapy is used in which the nursing staff is responsible for assessment and management of pain. After the patient is discharged from the PACU or ICU, pain at rest is assessed every 4 hours and treated with 1–2 mg of morphine intravenously every 5 minutes until adequate pain relief is achieved.13 Approximately 25% of patients initially receive intravenous morphine sulfate via patient-controlled analgesia (PCA) on transfer to the hospital ward. The PCA morphine dose is standardized with boluses of 0.5 to 1 mg, a lockout interval of 6 minutes, and no basal infusion. PCA is discontinued once the patient resumes a regular diet, pain is judged to be under adequate control, and there has been no PCA request for one 8-hour nursing shift.
Transition to oral multimodal pain therapy takes place as soon as it is feasible. Once the patient resumes a regular diet and pain is judged to be under good control with intravenous analgesics, patients are given acetaminophen (500–1000 mg q8h) and/or oxycodone (2.5–10 mg q4h) orally for breakthrough pain.
Measures
Nurses in the PACU, in the ICU, and on the ward were trained to measure resting pain levels using a numeric rating scale. The measurement is made every 15 minutes in the PACU if the patient is awake, and every 4 hours on the hospital ward. The primary means of pain assessment is a determination of pain at rest via a 0–10 verbal numerical rating, where 0 corresponds to no pain and 10 corresponds to the worst pain imaginable. Pain scores are not obtained from intubated patients in the ICU, patients who are nonverbal secondary to cognitive dysfunction, or patients who are otherwise unable to interact meaningfully with the provider. The PACU pain score reported here represents the patient's current pain obtained on discharge from the PACU. Pain scores on the operative day represent average pain since discharge from the PACU. Thereafter, pain scores represent average pain for the prior 24 hours.
The cumulative doses of opioid administered in the operating room, PACU, and ICU, after PACU discharge on the day of surgery, and on postoperative days 1–3 were converted to their equivalent of intravenous morphine sulfate (100 µg of intravenous fentanyl, 2 mg of intravenous hydromorphone, and 30 mg of oral oxycodone were considered equivalent to 10 mg of intravenous morphine sulfate)14, 15 and recorded as milligrams per kilogram.
In addition to the preoperative delirium assessment described above, delirium was assessed midmorning on postoperative day 2 via the CAM by either the attending geriatrician or a trained research nurse.11 The CAM was not routinely performed on postoperative day 1, as measurement could be influenced by residual anesthetic effects.16 If a patient was unable to respond to questions because of external constraints such as intubation with sedation, the CAM was administered after extubation.
The criteria for “probable dementia” was based on a preoperative mini-mental status examination score17 of less than 24 in the absence of delirium, documented history of dementia based on the primary care provider record, or determination of preoperative dementia status by the attending geriatrician during the preoperative evaluation. Regardless of the means of assessing dementia, patients with a positive result were classified as having “probable dementia.”
Statistical Analysis
Unless otherwise indicated, data are reported as the number of events and their percentage for frequency data, as median with upper and lower quartiles for ordinal data, and as mean and standard deviation for continuous data. Group comparisons were analyzed by Fisher’s exact test or the chi-squared test for frequency data, the Mann-Whitney U test for ordinal data, and analysis of variance for continuous data. Logistic regression was used to examine the likelihood of delirium as a function of pain, opioid use, dementia, and other demographic variables. The area under the receiver operating characteristic curve for the predictions of the resulting model (range 0.5–1.0, equivalent to the C-statistic) is also reported. Longitudinally collected continuous data were analyzed by using a generalized estimating equations approach with an identity link function with robust estimation of standard errors. The Wald χ2 test statistic and associated degrees of freedom (d.o.f.) is reported for the longitudinal analysis. All significance values reported are from two-tailed tests. Differences were considered significant at P < 0.05. Statistical analysis was facilitated by Statistica 6.0 (Statsoft, Tulsa, OK) and Stata 10.1 (StataCorp, College Station, TX).
RESULTS
The demographic characteristics and postoperative course of patients in the study cohort are summarized in Tables 1, 2, and 3, which compare patients who did and did not experience postoperative delirium and those who did and did not present with probable dementia. Table 1 demonstrates that patients with probable dementia were older and sicker than those without, as evidenced by higher ASA and Charlson comorbidity scores. The differences in ASA status were due to the presence of more patients with mild systemic disease (ASA status 2) in the non demented group. Patients who developed postoperative delirium had a higher Charlson comorbidity score, and were more likely to have probable dementia than those who did not develop postoperative delirium. No differences were observed in probable dementia or incidence of postoperative delirium with respect to whether spinal or general anesthesia was used (Table 2). As shown in Table 3, patients with probable dementia and those experiencing postoperative delirium were less likely than cognitively intact patients to receive PCA on arrival to the hospital ward. The use of PCA had no effect on either opioid consumption (P = 0.333) or pain scores (P = 0.621). No differences were observed in the number of patients who reported severe pain (pain score>6) on one or more postoperative days with respect to delirium or dementia (Table 3). Patients who were cognitively intact and did not develop delirium reported severe pain (pain score >6) on at least one postoperative day 12.5% of the time compared with 13.9% for those who were cognitively intact and did become delirious (P not significant). Probable dementia was not associated with infectious or cardiovascular complications. However, patients who developed delirium were more likely to sustain cardiovascular complications (Table 3).
TABLE 1.
Preoperative Factors
| Probable Dementia | Delirium | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Overall (n=236) |
no (n=170) |
yes (n=66) |
P-Value¶ | no (n=176) |
yes (n=60) |
P-Value¶ |
| Age (yrs) | 81.5 (7.1) | 80.6 (7.3) | 83.8 (6.0) | 0.002* | 81.0 (7.1) | 83.0 (6.9) | 0.060 |
| Male/Female (n/n) |
67/169 | 47/123 | 20/46 | 0.748 | 45/131 | 22/38 | 0.135 |
| Probable dementia (n) |
66 (28.0%) | N/A | N/A | N/A | 32 (18.2%) | 34 (56.7%) | <0.001* |
| Postoperative delirium (n) |
60 (25.4%) | 26 (15.3%) | 34 (51.5%) | <0.001* | N/A | N/A | N/A |
| ASA status (range: 1–5) |
3 (3–3) | 3 (3–3) | 3 (3–3) | 0.005* | 3 (3–3) | 3 (3–3) | 0.075 |
| Modified Charlson Co-Morbidity Index Score (range: 2–15) 32 |
6.5 (3.9) | 6.0 (4.0) | 7.8 (3.3) | 0.001* | 6.1 (3.8) | 7.7 (3.9) | 0.005* |
Mean (±s.d.) for continuous data, median (lower quartile, upper quartile) for ordinal data, and number (percent of total) for frequency data. Asterisks indicate p<0.05.
TABLE 2.
Intraoperative Anesthetic Drug Doses
| Probable Dementia | Delirium | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n=236) |
no (n=170) |
yes (n=66) |
P-Value¶ | no (n=176) |
yes n=60) |
P- Value¶ |
|
| Spinal/General (n/n) | 136/100 | 101/69 | 35/31 | 0.383 | 106/70 | 30/30 | 0.176 |
| Opioid dose (morphine equivalents, mg/kg)a Overall |
0.21 (0.16) | 0.22 (0.17) | 0.20 (0.15) | 0.605 | 0.21 (0.17) | 0.21 (0.14) | 0.935 |
| Spinal Anesthesia | 0.15 (0.14) | 0.16 (0.15) | 0.13 (0.12) | 0.319 | 0.15 (0.15) | 0.13 (0.11) | 0.477 |
| General Anesthesia | 0.30 (0.14) | 0.30 (0.15) | 0.29 (0.14) | 0.679 | 0.30 (0.15) | 0.29 (0.13) | 0.622 |
| Propofol dose (mg/kg) Overall |
4.1 (5.2) | 4.4 (5.6) | 3.5 (4.0) | 0.286 | 4.0 (5.3) | 4.5 (5.1) | 0.538 |
| Spinal Anesthesia | 5.8 (5.9) | 6.1 (6.4) | 5.1 (4.5) | 0.415 | 5.6 (6.0) | 6.7 (5.6) | 0.394 |
| General Anesthesia | 1.2 (1.1) | 1.3 (1.2) | 1.1 (0.7) | 0.632 | 1.2 (1.2) | 1.3 (0.9) | 0.808 |
| Number receiving midazolam (n) Overall |
50 (21.2%) | 45 (26.8%) | 5 (7.6%) | 0.001* | 43 (25.3%) | 7 (11.7%) | 0.044* |
| Spinal Anesthesiab | 26 (20.6%) | 23 (22.8%) | 3 (8.6%) | 0.082 | 24 (22.6%) | 2 (6.7%) | 0.065 |
| General Anesthesiab | 24 (24.0%) | 22 (31.9%) | 2 (6.5%) | 0.005* | 19 (27.1%) | 5 (16.7%) | 0.315 |
| Midazolam dose (mg/kg)c Overall |
.026 (.012) | .026 (.012) | .024 (.014) | 0.706 | .026 (.011) | .023 (.015) | 0.524 |
| Spinal Anesthesia | .027 (.012) | .029 (.011) | .017 (.011) | 0.088 | .029 (.011) | .023 (.025) | 0.545 |
| General Anesthesia | .024 (.012) | .023 (.012) | .035 (.012) | 0.196 | .024 (.012) | .023 (.014) | 0.899 |
Mean (±s.d.) for continuous data, median (lower quartile, upper quartile) for ordinal data, and number (percent of total) for frequency data. Asterisks indicate p<0.05.
All opioids were converted to their equivalent dose of intravenous morphine sulfate using 100 µg of intravenous fentanyl, 2 mg of intravenous hydromorphone, and 30 mg of oral oxycodone as equivalent to 10 mg of intravenous morphine sulfate14, 15. PACU=Post Anesthesia Care Unit; POD=PostOperative Day
The percentages for those receiving midazolam are with respect to the subgroup (e.g. spinal or general anesthesia.
Averaged only over the subset of the population receiving midazolam.
TABLE 3.
Postoperative Factors
| Probable Dementia | Delirium | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n=236) |
no (n=170) |
yes (n=66) |
P-Value¶ | no (n=176) |
yes (n=60) |
P-Value¶ | |
| Number receiving any opioids (n) | 213 (90%) | 158 (93%) | 55 (83%) | 0.047* | 160 (91%) | 53 (88%) | 0.615 |
| Number using PCA (n)a | 60 (25.4%) | 50 (29.4%) | 10 (15.2%) | 0.030* | 54 (30.7%) | 6 (10.0%) | 0.001* |
| Opioid dose, cumulative for PACU through POD-3 (morphine equivalents, mg/kg)b | 0.62 (0.77) | 0.71 (0.82) | 0.36 (0.55) | 0.005* | 0.66 (0.82) | 0.49 (0.59) | 0.176 |
| Pain averaged over POD0-POD3 (0–10) | 2.4 (1.9) | 2.7 (1.9) | 1.5 (1.8) | <0.001* | 2.6 (1.9) | 1.7 (1.8) | 0.007* |
| Severe Pain (>6/10, n) | 29 (12.3%) | 24 (14.1%) | 5 (7.6%) | 0.192 | 24 (13.6%) | 5 (8.3%) | 0.365 |
| Number admitted to ICU (n) | 53 (22.5%) | 35 (20.6%) | 18 (27.3%) | 0.299 | 31 (17.6%) | 22 (36.7%) | 0.004* |
| Stay in ICU (days)c | 4.3 (4.8) | 4.3 (5.5) | 4.1 (3.2) | 0.870 | 3.3 (2.5) | 5.7 (6.7) | 0.071 |
| Number receiving erythrocyte transfusion (n) | 136 (57.6%) | 94 (55.3%) | 42 (63.6%) | 0.304 | 96 (54.5%) | 40 (66.7%) | 0.130 |
| Erythrocytes transfusion (units of packed erythrocytes)c | 1.9 (1.1) | 1.9 (1.1) | 1.8 (1.2) | 0.768 | 1.8 (1.0) | 2.1 (1.4) | 0.103 |
| Number experiencing infectious complications (n)d | 73 (30.9%) | 51 (30.0%) | 22 (33.3%) | 0.640 | 49 (27.8%) | 24 (40.0%) | 0.105 |
| Number experiencing cardiovascular complications (n)e | 49 (20.8%) | 31 (18.2%) | 18 (27.3%) | 0.152 | 27 (15.3%) | 22 (36.7%) | 0.001* |
Mean (±s.d.) for continuous data, median (lower quartile, upper quartile) for ordinal data, and number (percent of total) for frequency data. Asterisks indicate p<0.05.
PCA=Patient Controlled Analgesia
All opioids were converted to their equivalent dose of intravenous morphine sulfate using 100 µg of intravenous fentanyl, 2 mg of intravenous hydromorphone, and 30 mg of oral oxycodone as equivalent to 10 mg of intravenous morphine sulfate14, 15. PACU=Post Anesthesia Care Unit; POD=PostOperative Day
Averaged only over the subset of the population receiving admitted to the Intensive Care Unit or receiving erythrocyte transfusion.
Infectious complications include urinary tract infection and pneumonia
Cardiovascular complications include congestive heart failure, myocardial infection, and new arrhythmia.
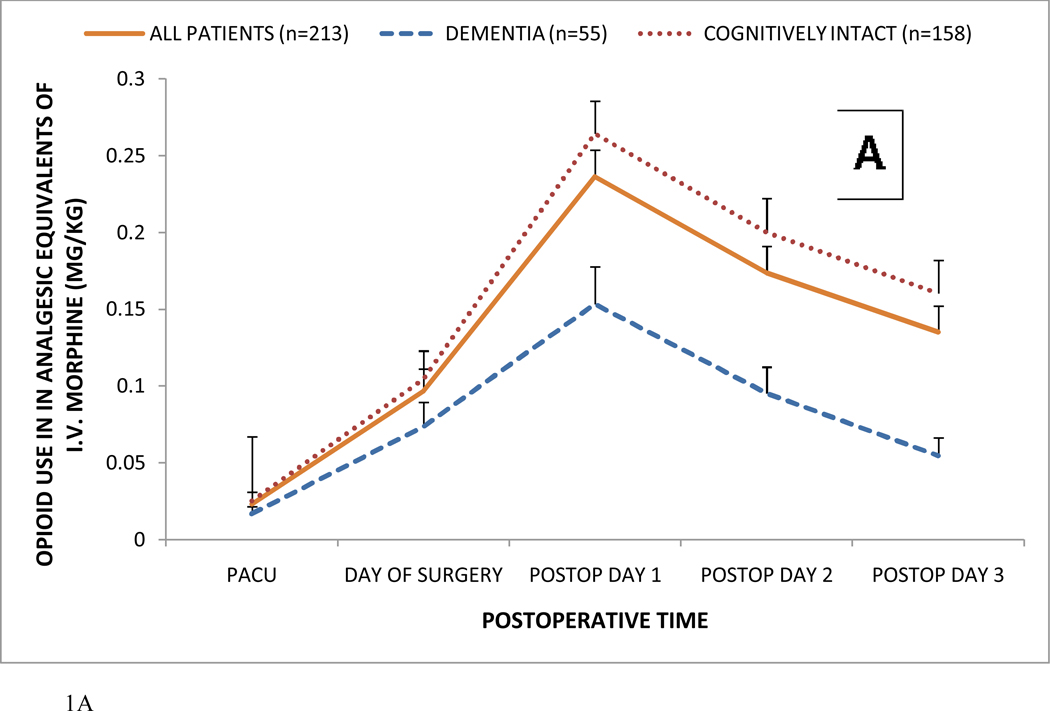
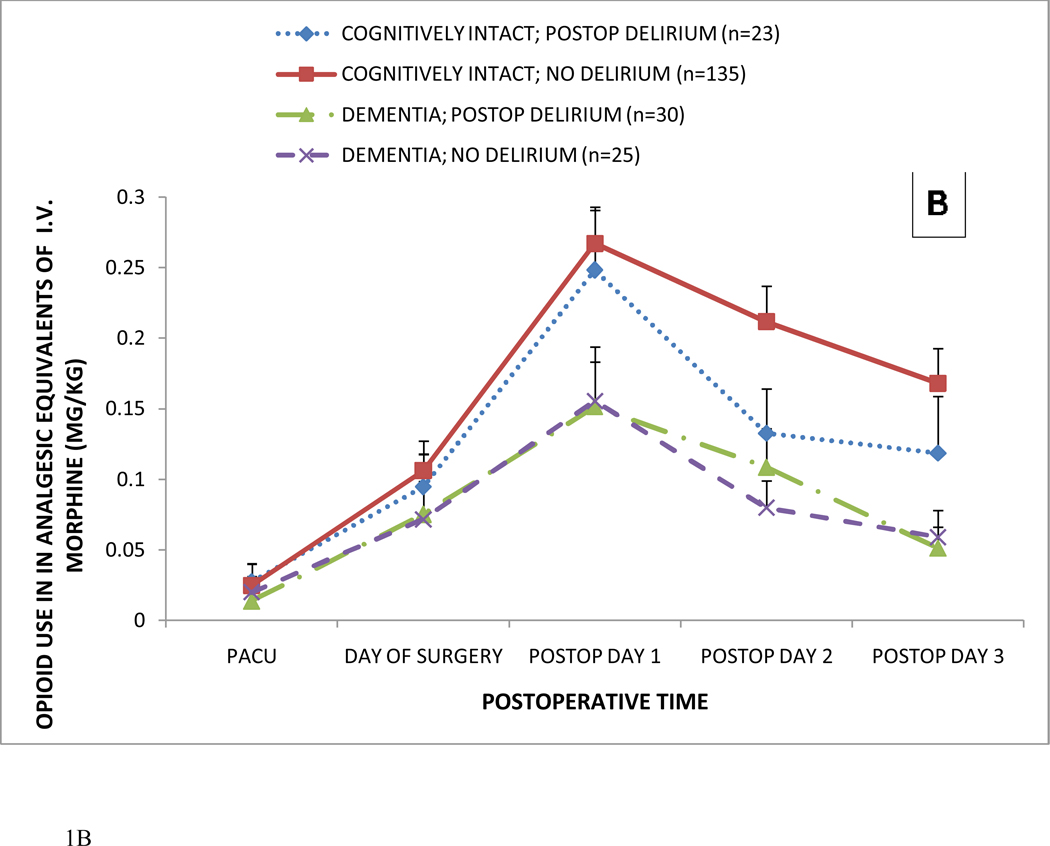
Of the 236 patients, 213 (90%) received opioids postoperatively; including 55 (83%) demented patients and 158 (93%) non-demented patients (p=0.047, Table 3). There was no association between the use of any postoperative opioid and incident delirium (p=0.615) in both demented (p=0.333) and cognitively intact patients (p=0.398). Figure 1 shows postoperative opioid consumption in hip fracture patients receiving any postoperative opioid. In Figure 1A, postoperative opioid consumption is shown in patients with and without probable dementia. In Figure 1B, postoperative opioid consumption is shown as a function of probable dementia, postoperative delirium, neither, or both (mean ± SEM). In the total patient population (n=236), postoperative delirium was not associated with alterations in opioid consumption. As revealed by longitudinal analysis, probable dementia, but not postoperative delirium, was associated with significantly less opioid use (P<0.001 for dementia; P=0.120 for delirium; P=0.038, for their interaction; Wald χ2 =142.8 with 7 d.o.f.). Furthermore, opioid dose (P≥0.591) on postoperative days 1 and 2 was not predictive of incident delirium. Postoperative delirium did not affect pain scores (P = 0.236), while patients with probable dementia exhibited lower pain scores (P < 0.001). Thus, dementia, with or without delirium, was associated with lower opioid consumption and pain scores.
Figure 1.
Postoperative opioid consumption in hip fracture patients receiving any postoperative opioid.
Panel A: Postoperative opioid consumption in hip fracture patients with and without probable dementia (mean ± SEM).
Panel B: Postoperative opioid consumption as a function of probable dementia, postoperative delirium, neither, or both (mean ± SEM).
In the total patient population, postoperative delirium was not associated with alterations in opioid consumption. As revealed by longitudinal analysis, probable dementia, but not postoperative delirium, was associated with significantly less opioid use (P<0.001 for dementia; P=0.120 for delirium; P=0.038, for their interaction; Wald χ2 =142.8 with 7 d.o.f.). Furthermore, opioid dose (P≥0.591) on postoperative days 1 and 2 was not predictive of incident delirium. All opioids were converted to their analgesic equivalent of intravenous (i.v.) morphine sulfate as described in the text.
When dementia and ICU admission were entered in all of the logistic models, logistic regression revealed that probable preoperative dementia and postoperative ICU admission were the most important risk factors for development of incident delirium (Table 4). The observation of delirium on postoperative day 2 was not related to opioid consumption or pain scores on postoperative days 1 and 2 (Table 4). Demographic variables such as age, gender, and illness severity; and intraoperative variables such as type of anesthesia and dose of midazolam, propofol, or fentanyl did not further identify any subgroup at risk of developing postoperative delirium.
Table 4.
Logistic regression to determine the additional predictive value of postoperative pain and opioid use with respect to incident delirium on the second postoperative day.
| Variables in addition to ICU Admission and Dementia |
Overall χ2 |
Overall Area under ROC. curve** |
P (Overall) | Dementia* | P Dementia |
ICU Admission* |
P ICU Admission |
Additional variable* |
P (Additional Variable) |
|---|---|---|---|---|---|---|---|---|---|
| None | 37.9 | 0.74 | <0.001 | 5.86 (3.04,11.31) | <0.001 | 2.71 (1.33,5.53) | 0.006 | NA | NA |
| Pain-POD1 | 25.2 | 0.73 | <0.001 | 4.34 (2.03, 9.28) | <0.001 | 2.78 (1.26, 6.11) | 0.011 | 0.90 (0.77, 1.06) | 0.203 |
| Pain-POD2 | 25.0 | 0.75 | <0.001 | 4.25 (1.98, 9.12) | <0.001 | 2.19 (0.98, 4.93) | 0.056 | 0.89 (0.74, 1.07) | 0.220 |
| Opioid-POD1 | 38.0 | 0.74 | <0.001 | 6.00 (3.07, 11.74) | <0.001 | 2.76 (1.34, 5.68) | 0.006 | 1.27 (0.35, 4.68) | 0.711 |
| Opioid-POD2 | 37.8 | 0.75 | <0.001 | 5.70 (2.92, 11.15) | <0.001 | 2.58 (1.26, 5.27) | 0.009 | 0.63 (0.11, 3.49) | 0.591 |
Odds ratio (95% confidence interval)
Area under receiver operating characteristic (ROC) curve (range 0.5–1.0, equivalent to the C-statistic) for predictions of logistic model.
NA = not applicable; POD = postoperative day.
Some data regarding opioid consumption and pain scores was missing because of a failure to obtain or record it, or because the patient was discharged before the end of the study period. The number of patients who had missing opioid consumption or pain score data because of discharge did not differ between those who were cognitively intact and those with delirium, dementia, or both (P ≥ 0.272). The number of missing data points per patient (mean ± SD) after accounting for discharge was 0.06 ± 0.24 for opioid consumption and 0.64 ± 1.07 for pain scores. Opioid consumption and pain scores during hospitalization did not differ between patients with intact data sets and those with one or more missing data points. They also did not differ with respect to the number of missing data points when we took into consideration those missing data for any reason and those missing data after accounting for discharge before the end of the study period (P ≥ 0.558).
DISCUSSION
This study found no association between the use of any postoperative opioid and incident delirium in both demented and cognitively intact patients after hip fracture repair. Postoperative delirium was not associated with the amount of opioid consumed. Furthermore, opioid dose on postoperative days 1 and 2 was not predictive of incident delirium. In our target population of HFS patients, dementia and ICU admission, not opioid consumption, were most predictive of incident postoperative delirium. Therefore, concern about precipitating postoperative delirium should not prevent the use of opioid analgesic therapy sufficient to achieve a generally accepted level of comfort in elderly patients with or without preexisting cognitive impairment.
Our study revealed no relationship between opioid consumption and postoperative delirium, supporting the findings of Lynch et al.,4 who reported no relationship between opioid dose and postoperative delirium. In contrast, Leung et al.5 reported elevated opioid consumption in elderly surgical patients who received postoperative PCA and became delirious. Their finding suggests a contribution of opioid drugs to delirium production and would be consistent with some of the established psychopharmacologic effects of opioids.18 An association between opioid administration and postoperative delirium has been reported, but the doses that mediate this effect have been difficult to define.2 In hospitalized cancer patients, exposure to the equivalent of >90 mg of morphine per day increased the risk of delirium by 40%.19 In patients electively undergoing total hip arthroplasty, higher doses of PCA-administered opioids (11.9 mg hydromorphone in the first 48 postoperative hours) were associated with a higher incidence of postoperative delirium.3 In contrast to these prior studies, patients in the current study received cumulative opioid doses well below those reported to be associated with postoperative delirium. Based on reported data concerning the opioid doses necessary to elicit delirium and the generally effective pain control experienced by the cohort that we studied, it is not surprising that opioid dose was not associated with postoperative delirium in the current study.
In our target population of HFS patients, dementia and ICU admission, but not opioid consumption, were associated with incident postoperative delirium. Dementia has clearly been established as an important risk factor for delirium in hip fracture patients.20–22 ICU-acquired delirium is a well defined phenomenon.23 ICU admission has been identified as a precipitating risk factor for delirium24 and is consistent with our group’s experience with elderly patients undergoing hip fracture repair.9 In cognitively intact hip fracture patients, Morrison et al.1 found a relationship between higher pain scores, inadequate opioid administration, and postoperative delirium. However, the pain scores of patients in our study were markedly different from those reported by Morrison et al.1 They reported that 21% of cognitively intact patients experienced severe pain whereas we found that patients without dementia reported severe pain (pain score >6) at least once during the first three postoperative days only 12.5% of the time if they experienced postoperative delirium and 13.9% of the time if they did not. Morrison et al.1 found that inadequate analgesia, defined as a cumulative morphine dose of less than 10 mg during the first three postoperative days, increased the risk of developing delirium. In our study, the average cumulative opioid dose in patients who were cognitively intact was the equivalent of 14 mg of morphine. Using Morrison’s definition,1 analgesia was adequate in our cohort as evidenced by both opioid dose and pain scores, and this would preclude demonstrating a relationship between decreased opioid consumption, increased pain scores, and delirium.
Even with the postoperative analgesic regimen used in our HFS clinical pathway, opioid consumption differed substantially between patients with and without probable dementia. Similarly, Adunsky et al.6 reported that in hip fracture patients, each 5-point decrease in mini-mental status examination was associated with a 2.1-fold greater likelihood of receiving smaller opioid doses postoperatively. Our observation that patients with probable dementia reported less postoperative pain is also consistent with studies showing that Alzheimer’s disease is associated with a decrease in reported pain.25 Certainly, the decreased opioid use by patients with probable dementia in the current study is consistent with the decrease in reported pain levels when compared to those who were cognitively intact preoperatively.
The current study revealed no association between rest pain and delirium. We found no evidence to indicate that delirious patients were reporting or experiencing higher levels of pain. Several investigators have reported elevations in pain with delirium.4, 5 However, the pain levels reported in those prior studies were higher than those of the current study. It is unclear whether the difference in pain scores can be attributed to a younger population (66 years4 and 74 years5 versus 82 years in the current study) or a difference in surgical populations. Elevations in pain threshold with aging have been reported by many investigators.26 In addition, surgical procedures vary with respect to the intensity of the associated postoperative pain.27 Our study included only hip fracture patients whereas previous investigations were performed with mixed surgical populations.4, 5 Recently, investigators have demonstrated that hip fracture patients almost uniformly report no postoperative pain at rest.28 Similarly, in our study the mean pain score over the first three postoperative days was 2.4 ± 1.9.
Our study had several limitations. Postoperative delirium was defined in this study by criteria for delirium presented in the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition29 as assessed by the CAM. We assessed delirium at the preoperative evaluation and on postoperative day 2. Because we evaluated postoperative delirium only on the second postoperative day, we were unable to determine the relationship between opioid consumption and postoperative delirium on a longitudinal basis. Previous studies have shown an association between postoperative delirium and pain at rest on postoperative day 2, but not at later time points.4 This association is consistent with the observation that postoperative delirium usually presents at around 24 hours postoperatively and resolves in most patients within 48 hours.30 In addition, our data indicate that pain scores peaked on postoperative day 1 and then started to decline. This progression suggests that we are unlikely to have observed pain-associated effects on postoperative delirium at a later postoperative time. Recent studies examining the relationship between postoperative delirium and pain have used the CAM on both postoperative days 1 and 2.5 The data indicate that we may have missed transient episodes of mild delirium on postoperative day 1, but given our postoperative delirium criteria, we were able to diagnose the clinically important episodes.31 Our patients with probable dementia may not have reported pain reliably secondary to cognitive impairment and, therefore, received less opioid analgesia. Missing pain scores may have affected our results and could be informative. Despite the number of missing pain scores, values for opioid consumption were consistently reported, and opioid consumption indirectly reflects underlying pain. Furthermore, our analysis demonstrated that pain and opioid consumption did not differ between patients whose data sets were intact and those with one or more missing data points or with respect to the number of missing data points.
In conclusion, dementia and ICU admission, not opioid consumption, were the most important predictors of delirium on postoperative day 2. Our results indicate that in our hip fracture population, opioid dosage is not related to incident delirium. Therefore, opioid administration within the confines of our HFS pain management algorithm should not be limited by concern for precipitating delirium.
ACKNOWLEDGMENT
Funding support from NIA RO1 AG033615.
Frederick Sieber: Receive research Grant for an investigator-initiated research from NIA.
Hochang Lee: Received research grant for an investigator-initiated research from GSK, and served as a consultant for Labopharm. Also, served as a member/consultant of a data monitoring board for a clinical trial sponsored by Pfizer.
Sponsor’s Role:
No sponsor for this manuscript. Sponsor for all of the collaborative projects described within the manuscript were NIH institutes.
Footnotes
Author Contributions:
FS,SM,HL,AG: Concept, design of manuscript, data analysis and interpretation, prep of manuscript and serial revisions.
REFERENCES
- 1.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–1522. [PubMed] [Google Scholar]
- 3.Marino J, Russo J, Kenny M, et al. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:29–37. doi: 10.2106/JBJS.H.00079. [DOI] [PubMed] [Google Scholar]
- 4.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Leung JM, Sands LP, Paul S, et al. Does postoperative delirium limit the use of patient-controlled analgesia in older surgical patients? Anesthesiology. 2009;111:625–631. doi: 10.1097/ALN.0b013e3181acf7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adunsky A, Levy R, Mizrahi E, et al. Exposure to opioid analgesia in cognitively impaired and delirious elderly hip fracture patients. Arch Gerontol Geriatr. 2002;35:245–251. doi: 10.1016/s0167-4943(02)00044-4. [DOI] [PubMed] [Google Scholar]
- 7.Zakriya KJ, Christmas C, Wenz JF, Sr, et al. Preoperative factors associated with postoperative change in confusion assessment method score in hip fracture patients. Anesth Analg. 2002;94:1628–1632. doi: 10.1097/00000539-200206000-00050. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen LS, JT M. Central nervous system dysfunction after anesthesia in the geriatric patient. Anesthesiol Clin North America. 2000;18:59–70. doi: 10.1016/s0889-8537(05)70149-8. [DOI] [PubMed] [Google Scholar]
- 9.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jonge KE, Christmas C, Andersen R, et al. Hip fracture service-an interdisciplinary model of care. J Am Geriatr Soc. 2001;49:1737–1738. doi: 10.1046/j.1532-5415.2001.49292.x. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 12.Aubrun F, Monsel S, Langeron O, et al. Postoperative titration of intravenous morphine in the elderly patient. Anesthesiology. 2002;96:17–23. doi: 10.1097/00000542-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Aubrun F, Bunge D, Langeron O, et al. Postoperative morphine consumption in the elderly patient. Anesthesiology. 2003;99:160–165. doi: 10.1097/00000542-200307000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Patanwala AE, Duby J, Waters D, et al. Opioid conversions in acute care. Ann Pharmacother. 2007;41:255–266. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 15.Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 16.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Hill JL, Zacny JP. Comparing the subjective, psychomotor, and physiological effects of intravenous hydromorphone and morphine in healthy volunteers. Psychopharmacology (Berl) 2000;152:31–39. doi: 10.1007/s002130000500. [DOI] [PubMed] [Google Scholar]
- 19.Gaudreau JD, Gagnon P, Roy MA, et al. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109:2365–2373. doi: 10.1002/cncr.22665. [DOI] [PubMed] [Google Scholar]
- 20.Juliebo V, Bjoro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354–1361. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 21.Galanakis P, Bickel H, Gradinger R, et al. Acute confusional state in the elderly following hip surgery: Incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–355. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 22.Bitsch M, Foss N, Kristensen B, et al. Pathogenesis of and management strategies for postoperative delirium after hip fracture: A review. Acta Orthop Scand. 2004;75:378–389. doi: 10.1080/00016470410001123. [DOI] [PubMed] [Google Scholar]
- 23.Vasilevskis EE, Ely EW, Speroff T, et al. Reducing iatrogenic risks: ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010;138:1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena S, Lawley D. Delirium in the elderly: A clinical review. Postgrad Med J. 2009;85:405–413. doi: 10.1136/pgmj.2008.072025. [DOI] [PubMed] [Google Scholar]
- 25.Scherder EJ, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Lancet Neurol. 2003;2:677–686. doi: 10.1016/s1474-4422(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Raja SN. Assessment and management of chronic pain in the elderly. In: Sieber FE, editor. Geriatric Anesthesia. First ed. New York: McGraw-Hill; 2007. pp. 319–336. [Google Scholar]
- 27.Chandani A. Assessment and management of acute pain in the elderly. In: Sieber FE, editor. Geriatric Anesthesia. First ed. New York: McGraw-Hill; 2007. pp. 303–315. [Google Scholar]
- 28.Foss NB, Kristensen MT, Palm H, et al. Postoperative pain after hip fracture is procedure specific. Br J Anaesth. 2009;102:111–116. doi: 10.1093/bja/aen345. [DOI] [PubMed] [Google Scholar]
- 29.Fourth Edition Text Revision (DSM-IV-TR) 4th ed. Washington, D.C.: American Psychiatric Publishing, Inc.; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 30.Duppils GS, Wikblad K. Acute confusional states in patients undergoing hip surgery. A prospective observation study. Gerontology. 2000;46:36–43. doi: 10.1159/000022131. [DOI] [PubMed] [Google Scholar]
- 31.Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]