Abstract
Objective
To determine the impact of parenteral nutrition (PN) on outcomes in biliary atresia (BA) patients listed for liver transplantation (LTx).
Study Design
We retrospectively reviewed charts of all BA patients from 1990 through 2010 at our institution, s/p hepatoportoenterostomy, ≤ 36 months old, and listed for LTx. Initiation of PN was based on clinical indications.
Results
25 PN and 22 non-PN subjects (74% female) were studied. Median PN initiation age was 7.7 months, mean duration 86 days, and mean PN energy supplied 77 kcal/kg/day. Prior to PN, triceps skinfold thickness (TSF) and mid-arm circumference (MAC) Z-scores were decreasing. After PN, TSF (p=0.003) and MAC (p<0.0001) improved significantly. The PN group had lower MAC and TSF than non-PN at time of LTx listing. Between listing and LTx, MAC and TSF improved in PN and worsened in non-PN such that both groups had the same Z-scores at LTx. PN group had a higher incidence of GI bleeding and ascites pre-LTx, but there was no difference in pre-LTx bacteremia, and post-LTx days in ICU and patient or graft survival.
Conclusions
PN improves nutritional status in malnourished BA patients awaiting LTx, which is associated with post-LTx outcomes comparable to those not requiring PN.
Keywords: cholestasis, anthropometrics, outcomes, triceps skin fold, mid arm circumference
Introduction
Biliary atresia, a progressive fibro-obliterative disease of the extrahepatic and intrahepatic bile ducts, presents with obstructive jaundice within the first 3 months of life and accounts for 30-40% of cases of neonatal cholestasis.(1) Initial management includes surgical intervention with a Kasai hepatoportoenterostomy (HPE), ideally before 45-60 days of age. Even when HPE is performed in a timely fashion, nearly 70-80% of patients with biliary atresia will eventually require liver transplantation, accounting for close to half of all liver transplants performed in children.(1)
Malnutrition, a significant problem for infants with biliary atresia, is caused by early satiety from organomegaly and ascites, malabsorption of dietary lipid from obstructed bile flow, and increased energy expenditure.(2-4) Malnutrition places children at risk for poor clinical outcomes both before and after liver transplant.(5-7) While current literature suggests that more normal nutritional status, as indicated by higher weight and/or length z-scores, portends better clinical outcomes in biliary atresia, the impact of parenteral nutrition supplementation has not been adequately addressed in this disease. In a single case series, PN was initiated in three infants with biliary atresia following failure of enteral therapy.(4) Weight and/or length z-scores have been used to describe nutritional status in biliary atresia, however, mid arm circumference (MAC, a measurement of muscle mass) and triceps skin fold thickness (TSF, a measure of adipose tissue stores) are better measures of nutritional status in children with chronic liver disease.(2, 8-13) We performed a retrospective cohort study to examine the use of parenteral nutrition supplementation and its impact on outcomes of biliary atresia patients cared for in the Pediatric Liver Center at Children’s Hospital Colorado.
Methods
A comprehensive review of medical records was performed at our institution to identify all children with biliary atresia who underwent HPE and were listed for liver transplant before 36 months of age between January 1, 1990 and July 15, 2010. This group of biliary atresia patients was selected because they are at the highest risk for malnutrition and for expiring while on the liver transplant wait-list, and they were believed to be more difficult to nutritionally rehabilitate. Diagnostic criteria for biliary atresia were age <6 months with cholestatic jaundice, and cholangiogram, surgical exploration, and pathology demonstrating partial or complete obstruction of the biliary tree. This study was approved by the Colorado Multiple Institutional Review Board. The clinical endpoint for this study was defined as liver transplantation, death or removal from the liver transplant waiting list.
Medical information was collected from medical records. Demographic data, including race, sex, date of birth, and age at HPE, were collected for each patient. Dietary intake, nutritional indices, physical examination findings, and laboratory data were obtained from the records for each patient within one week of the time of HPE, transplant listing, clinical endpoint (liver transplant, death, or removal from transplant waiting list due to improved clinical status), and initiation of parenteral nutrition (PN). For each patient receiving PN, data including details on the composition of PN were collected at each monthly visit until the clinical endpoint.
Anthropometric data were abstracted from the medical record. Weight, length, and weight/length z-scores were calculated based on the Centers for Disease Control (CDC) growth charts. Triceps skin fold (TSF) thickness and mid arm circumference (MAC) were measured as part of the standard of care at our institution. MAC and TSF were collected using standard technique with Lange calipers by five clinical pediatric dieticians fully trained to measure anthropometrics.(14) MAC was obtained in a single measurement and documented in the medical record. TSF was obtained 3 times for each clinic visit and the average was recorded in the medical record by the clinical pediatric dietician. TSF and MAC z-scores were calculated using results of the United States Ten-State Nutritional Survey.(15) The use of nutritional supplementation (enteral or parenteral), type and amount of oral formula and IV PN formulation used were obtained from the medical record, and total kcal/kg/day was calculated based on the patient’s measured weight and intake. We included dietary intake in the analysis when it was completely documented in the medical record. Information on enteral intake and the composition of PN were collected. For each patient receiving PN, the mean glucose infusion rate (GIR), mean daily dose of intravenous lipids, and mean daily dose of amino acids were calculated. The median GIR, dose of intravenous lipids, and dose of amino acids over the study period were calculated based on aggregate averages for each patient receiving PN. Soy-oil-based intravenous lipid emulsion (Intralipid, Fresenius Kabi, IL, USA) was used in all patients; no patients received fish oil-based intravenous lipid emulsions in this cohort. Amino acid solutions were given as TrophAmine (B. Braun, Bethlehem, PA, USA) or Aminosyn (Hospira, Lake Forest, IL, USA). Standard chemistries and complete blood count were monitored monthly while on PN. Adjustments in PN formulation in cholestatic infants included removal of supplemental manganese and halving of the dose of copper supplementation.
The decision to initiate nutritional supplementation (both enteral and parenteral) was based on the clinical judgment of individual care providers. Enteral supplementation with nasogastric tube feedings was initiated when TSF and MAC z-scores were consistently falling despite maximal attempts with both increasing caloric density and volume of oral feedings. In some patients, z-scores <-2 SD below normal were the indication to initiate NG tube feedings In other cases, in order to prevent severe malnutrition, consistently falling z-scores were the indication to initiate NG feeds. Parenteral nutrition was started when maximal enteral feedings (oral and NG tube), based on both caloric density and volume of feeds, failed to achieve restitution of TSF and MAC z-scores.
Laboratory data obtained from the medical records included serum albumin, total and direct bilirubin, GGT, AST, ALT, alkaline phosphatase, white blood count (WBC), hemoglobin, hematocrit, prothrombin time, and INR. The Pediatric End-Stage Liver Disease (PELD) score was calculated at each time point based on laboratory results and growth parameters.(16)
Medical complications of liver disease and portal hypertension were recorded, including ascites, gastrointestinal bleeding (defined as hematemesis or melena requiring a blood transfusion, medical therapy, or endoscopic intervention), encephalopathy, and bacteremia (defined as one or more positive blood cultures). Portal hypertension was defined clinically as presence of splenomegaly, thrombocytopenia (platelet count <150,000), gastrointestinal bleeding, or ascites. For each patient who received a liver transplant, assigned PELD at the time of transplant, type of transplant (deceased donor whole, split, or reduced liver), number of post-operative days in the Intensive Care Unit (ICU), immunosuppressive drugs used in the first 6 months post-transplant, and patient and graft survival data were collected. All transplants were from a deceased donor as our institution does not routinely perform living-related liver transplants. Immunosuppression medications included: cyclosporine (USAN, Novartis; INN: ciclosporin), tacrolimus (USAN/INN, Astellas), corticosteroids, and mycophenolate mofetil (USAN, Genentech; INN: mycophenolic acid).
Statistical Analysis
Fisher’s exact tests and two sample t-tests were used to examine the differences between the PN and non-PN groups with respect to categorical and continuous variables respectively. Linear mixed effects models with an unstructured covariance structure were used to model the serial continuous outcomes such as MAC and TSF z-scores. Contrasts under this model were used to assess the change in outcomes over time within a group and the difference between two groups. Kaplan-Meier plot and log rank test were used to compare survival after liver transplant between the two groups. Values are expressed as mean ± SE. P value of < 0.05 was considered significant. Pearson correlation was used to determine a correlation between laboratory data (albumin, total bilirubin, and prothrombin time) and weight, length, TSF, or MAC z-score at the time of transplant listing.
Results
One hundred and fifty-nine patients underwent liver transplantation at our institution between January 1, 1990 and July 15, 2010, of whom 62 were listed due to biliary atresia. Fifteen were excluded from this analysis, as 9 did not undergo HPE and 6 were older than 36 months of age at the time of listing for liver transplantation. Therefore, 47 patients with biliary atresia who underwent HPE and were listed for liver transplantation before 36 months of age were included in this study. The patients were divided into two groups based on exposure to PN. The PN (n=25) and non-PN (n=22) groups were similar with respect to demographics, age at HPE, age at listing for liver transplant, and age at clinical endpoint (transplant, death, or removal from transplant waiting list), as shown in Table 1.
Table 1.
Demographics and Clinical Outcomes of Biliary Atresia Patients in Parenteral Nutrition and Non-Parenteral Nutrition Groups
| All Patients | PN Group (n=25) | Non-PN Group (n=22) | |
|---|---|---|---|
| n (%) | n (%) | p value | |
| Sex | |||
| Female | 19 (76%) | 16 (73%) | 1 |
|
| |||
| Ethnicity | |||
| Caucasian | 15 (60%) | 12 (54%) | |
| Hispanic | 8 (32%) | 6 (27%) | |
| Other | 2 (8%) | 4 (18%) | 0.65 |
|
| |||
| Mean (SE) | Mean (SE) | p value | |
|
| |||
| Age at HPE (months) | 2.1 (0.1) | 2.3 (0.2) | 0.48 |
| Age at Listing (months) | 8.9 (1.0) | 9.4 (1.7) | 0.82 |
| Age at Clinical Endpoint (months) | 12.5 (1.2) | 13.6 (1.9) | 0.63 |
|
| |||
| Transplanted Patients | PN group n=22 | Non-PN Group n=20 | |
|
| |||
| Mean (SE) | Mean (SE) | p value | |
|
| |||
| Time from Listing to Transplant (months) | 4 (1.1) | 4 (0.8) | 1 |
| Calculated PELD score at Liver Transplant | 18 (2.6) | 14.2 (2.1) | 0.24 |
| Age at Liver Transplant (months) | 12.9 (1.4) | 12.7 (2.0) | 0.91 |
| Time in ICU after Transplant (days) | 9.1 (1.9) | 8.9 (2.7) | 0.93 |
|
| |||
| n (%) | n (%) | p value | |
|
| |||
| Donor Organ Type | |||
| Cadaveric Whole | 7 (32%) | 8 (40%) | |
| Cadaveric Split | 5 (23%) | 2 (10%) | |
| Cadaveric Reduced | 10 (45%) | 10 (50%) | 0.6 |
| Immunosuppression | |||
| Cyclosporine* | 14 (64%) | 16 (80%) | 0.31 |
| Tacrolimus* | 8 (36%) | 4 (20%) | 0.31 |
| Corticosteroids** | 22 (100%) | 20 (100%) | 1 |
| Mycophenolate Mofetil** | 10 (45%) | 7 (35%) | 0.54 |
| Number of Patients Re-Transplanted | 1 (5%) | 2 (9%) | 0.49 |
Initial post-transplant calcineurin inhibitor
number (%) of patients receiving medication during the first 6 months post-transplant
PN = parenteral nutrition
HPE = hepatoportoenterostomy
PELD = Pediatric End-Stage Liver Disease
Characteristics of the PN Group
Of the 25 patients who received PN, 18 (72%) were receiving nasogastric (NG) tube feedings at the time of PN initiation and had failed to show significant improvement on NG supplementation despite maximum tolerated enteral therapy. The mean duration of NG supplementation was 2.9 ± 0.5 months at the time of PN initiation and NG supplementation accounted for a mean of 71% of total energy intake. Energy density of formula or fortified breastmilk was a mean of 28 kcal/oz (range 20-44 kcal/oz) and mean enteral kcal/kg/day was 118 (range 40-180) at the time of listing for transplantation. One patient received nasojejunal supplementation at the time of transplant listing. Seven patients (28%) began PN without initiation of NG supplementation due to presentation with severe malnutrition at the time of referral to our center with mean MAC z-score -2.5 and mean TSF z-score -3.2 (4 patients), decreased skin fold thickness despite adequate documented oral intake of 160 kcal/kg/day (1 patient), post-surgical ileus (1 patient), and hypoglycemia (1 patient). PN was initiated in all during an inpatient hospitalization and was then transitioned to home care.
The median age at PN initiation was 7.7 months (range: 1.9 to 26 months) and mean duration of PN supplementation was 86 ± 18 days. At the time of clinical endpoint (transplant, death, or removal from transplant waiting list), mean energy supplied by PN was 77 ± 5.5 kcal/kg/day. Median glucose infusion rate was 13.2 (range 6.1-17.2), median intravenous lipid dose was 2.7 gm/kg/day (range 1.7-3.9 gm/kg/day), and median intravenous amino acid dose was 2 gm/kg/day (range 0.7-3.2 gm/kg/day). Two patients received intravenous lipid only (mean 2.5 gm/kg/day).
Although prior to PN initiation, indices of nutritional status (weight, length, TSF, and MAC z-scores) were decreasing, these stabilized or improved following PN administration. From the start of PN administration to the clinical endpoint, mean TSF z-score increased from -2.5 ± 0.2 to -1.8 ± 0.2 (p=0.003), and mean MAC z-score increased from -2.2 ± 0.2 to -1.4 ± 0.2 (p<0.0001). Twenty-two (88%) of the patients in the PN group received a liver transplant. All transplants were deceased donor organs (32% whole, 23% split, and 45% reduced), and mean age at the time of liver transplantation was 12.9 ± 1.4 months. Details of immunosuppression over the first six months post-transplant are provided in Table 1. Three patients died prior to receiving a liver transplant.
Characteristics of the Non-PN Group
Of the 22 patients in the non-PN group, 6 (27%) received NG feeds. NG supplementation accounted for a mean of 56% of total energy intake in these patients at the time of the clinical endpoint (transplant, death, or removal from transplant waiting list). Mean duration of NG supplementation was 3.4 ± 0.7 months. In two subjects, NG feeds were initiated prior to listing, and in 4 patients, NG feeds were initiated after listing but before the clinical endpoint. Weight z-score (-1.0 ± 0.2 at HPE, -1.4 ± 0.3 at time of transplant listing, and -1.6 ± 0.4 at time of clinical endpoint), length z-score (-1.0 ± 0.2 at time of HPE, -1.2 ± 0.2 at time of transplant listing, and -1.4 ± 0.3 at time of clinical endpoint) and TSF z-score (-1.4 ± 0.2 at time of HPE, -1.5 ± 0.3 at time of transplant listing, and -1.6 ± 0.3 at time of clinical endpoint) did not significantly change from time of HPE to the clinical endpoint. MAC scores significantly worsened in this group from the time of transplant listing to clinical endpoint. MAC z-scores were -1.2 ± 0.3 at the time of HPE, -0.8 ± 0.3 at the time of transplant listing, and -1.3 ± 0.2 at the time of clinical endpoint, p=0.03. Twenty (91%) of the patients in the non-PN group received a liver transplant. All transplants were deceased donor organs (40% whole, 10% split, and 50% reduced), and mean age at the time of liver transplantation was 12.7 ± 2.0 months. Details of immunosuppression over the first six months post-transplant are provided in Table 1. Two patients were removed from the transplant list because of significant improvement in clinical status.
Comparisons of PN and Non-PN Groups
The median time from transplant listing to PN initiation was 5 days. PN was initiated as early as 52 days before transplant listing and as late as 380 days after listing. There were no differences between PN and non-PN subjects with respect to the intervals from HPE to transplant listing, from transplant listing to the clinical endpoint (transplant, death, or removal from transplant waiting list), and from transplant listing to liver transplant. There was no significant difference in percentage of subjects who received PN before and after implementation of the PELD scoring system in 2002.(16) Calculated PELD scores were similar between the PN and non-PN groups at HPE and time of liver transplant listing (Table 2).
Table 2.
Laboratory, Nutritional, and PELD Score Data in PN vs. Non-PN Groups
| At time of HPE | At time of Liver Transplant Listing | At Clinical Endpoint | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PN | Non-PN | P | PN | Non-PN | P | PN | Non-PN | P | |||||||
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | ||||
| Albumin (g/dL) | 19 | 3.3 (0.1) | 22 | 3.6 (0.1) | 0.15 | 24 | 2.8 (0.1) | 22 | 3.2 (0.1) | 0.03 | 24 | 2.8 (0.1) | 18 | 3.1 (0.2) | 0.31 |
| Total bilirubin (mg/dL) | 19 | 9.7 (1.0) | 22 | 8.7 (1.0) | 0.54 | 24 | 12.1 (1.4) | 22 | 11.8 (1.9) | 0.86 | 24 | 21.9 (2.2) | 18 | 13.7 (2.8) | 0.04 |
| Direct bilirubin (mg/dL) | 19 | 7.1 (0.9) | 22 | 6.9 (0.8) | 0.79 | 21 | 8.4 (1.0) | 18 | 7.6 (1.3) | 0.82 | 22 | 14.5 (1.7) | 15 | 10.6 (2.6) | 0.5 |
| AST (U/L) | 19 | 293 (65) | 22 | 186 (61) | 0.25 | 24 | 154 (17) | 22 | 181 (16) | 0.38 | 24 | 452 (27) | 18 | 189 (196) | 0.92 |
| ALT (U/L) | 19 | 194 (29) | 22 | 163 (27) | 0.43 | 24 | 112 (19) | 22 | 171 (18) | 0.03 | 24 | 153 (24) | 18 | 148 (34) | 0.73 |
| GGT (U/L) | 18 | 1032 (161) | 17 | 958 (166) | 0.56 | 21 | 528 (151) | 16 | 940 (167) | 0.04 | 18 | 210 (60) | 11 | 502 (66) | 0.0007 |
|
| |||||||||||||||
| WBC (x103/μL) | 19 | 13.6 (1.2) | 22 | 14.5 (1.2) | 0.68 | 23 | 13.4 (1.2) | 20 | 14.8 (1.3) | 0.26 | 24 | 12.0 (1.6) | 18 | 13.8 (2.2) | 0.57 |
| Platelets (x103/μL) | 18 | 485 (34) | 22 | 515 (31) | 0.65 | 24 | 202 (26) | 19 | 330 (28) | 0.001 | 24 | 99 (17) | 18 | 207 (18) | <0.0001 |
| Hemoglobin | 17 | 16.8 (3.3) | 20 | 11.4 (3.0) | 0.25 | 24 | 10.4 (0.3) | 20 | 11.3 (0.3) | 0.09 | 23 | 9.5 (0.3) | 16 | 11.7 (0.4) | <0.0001 |
| Prothrombin time (sec) | 19 | 14.1 (0.7) | 22 | 12.8 (0.6) | 0.14 | 24 | 14.8 (0.7) | 22 | 14.4 (0.9) | 0.6 | 24 | 18.5 (0.9) | 18 | 14.6 (1.1) | 0.01 |
|
| |||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
|
|
|||||||||||||||
| % Weight z-score <-2 | 25 | 8% | 22 | 18% | 0.4 | 25 | 40% | 22 | 36% | 1.0 | 25 | 36% | 22 | 32% | 1.0 |
| % Length z-score <-2 | 25 | 4% | 22 | 9% | 0.6 | 25 | 20% | 22 | 18% | 1.0 | 25 | 36% | 22 | 23% | 0.36 |
| % TSF z-score <-2 | 14 | 7% | 6 | 0% | 1.0 | 20 | 70% | 14 | 21% | 0.01 | 13 | 38% | 10 | 30% | 1.0 |
| % MAC z-score <-2 | 13 | 31% | 6 | 17% | 1.0 | 20 | 40% | 14 | 21% | 0.29 | 13 | 23% | 10 | 10% | 0.6 |
|
|
|||||||||||||||
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | ||||
|
|
|||||||||||||||
| Calculated PELD score | 19 | 8.9 (0.9) | 22 | 7.1 (0.9) | 0.12 | 24 | 12.5 (1.3) | 22 | 10.7 (1.8) | 0.22 | 24 | 19.6 (2.0) | 18 | 12.0 (2.7) | 0.04 |
Liver transplant, death, or removal from transplant waiting list.
Statistically significant (p<0.05) values are bolded.
PN = parenteral nutrition
HPE = hepatoportoenterostomy
TSF = triceps skin fold
MAC = mid-arm circumference
PELD = Pediatric End-Stage Liver Disease
Clinical Endpoint = transplant, death, or removal from transplant waiting list
Nutritional intake of patients receiving PN was compared to the non-PN group. Sixty nine percent of the cohort had complete documentation of dietary intake and were included in this analysis. The remaining 31% had incomplete data available for calculation of enteral intake (50% of these were breastfed, 20% were on a “regular diet,” 30% received an undocumented amount of formula) and were excluded from further analyses. The non-PN group was missing more dietary records than the PN group (40% vs. 17%, p = 0.01). Mean total energy intake (kcal/kg/day) in PN and non-PN groups at the time of HPE (143 ± 10 vs. 150 ± 10), transplant listing (121 ± 7 vs. 111 ± 7), and clinical endpoint (104 ± 7 vs. 103 ± 8) were similar (p=NS). Although total kcal/kg/day at the time of clinical endpoint was the same in both groups, subjects in the PN group received a majority (63%) of their energy intake from parenteral nutrition and 37% of their energy intake from enteral nutrition (Figure 1).
Figure 1.
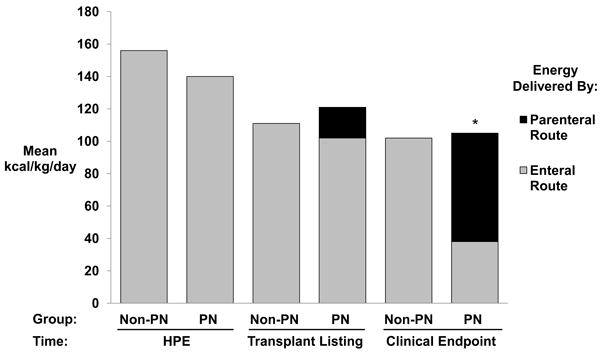
Energy Intake in PN vs. Non-PN Groups. Total energy intake was similar between PN and Non-PN groups at time of HPE, time of transplant listing, and time of clinical endpoint. There was a significant difference in energy delivered by parenteral route in the PN group at the time of clinical endpoint compared with the Non-PN group at the clinical endpoint (*p=0.002). PN=parenteral nutrition, HPE = hepatoportoenterostomy, Clinical Endpoint = liver transplant, death, or removal from transplant waiting list.
Measures of muscle mass (MAC) and adipose tissue stores (TSF) are believed to better reflect nutritional status than weight and weight/length measurements in infants and children with chronic liver disease because of the contribution of an enlarged liver and/or spleen and the presence of ascites and fluid retention that may inflate the weight for calculation of the growth parameters.(2, 8-13) Therefore, MAC and TSF z-scores were used as primary measures of nutritional status. At the time of HPE, MAC (-1.4 ± 0.2 vs. -1.2 ± 0.3) and TSF (-1.4 ± 0.1 vs. -1.4 ± 0.2) z-scores were similar in PN vs. non-PN groups, p=NS (Figure 2). At the time of transplant listing, the PN group had lower MAC z-scores compared with the non-PN group (-1.7 ± 0.2 vs. -0.8 ± 0.3, p=0.01). Between listing and clinical endpoint, MAC z-scores improved in the PN group (-1.7 ± 0.2 to -1.4 ± 0.2, p=0.02) but worsened in the non-PN group (-0.8 ± 0.3 to -1.3 ± 0.2, p = 0.03). Following transplant listing, the PN group had an average monthly rate of change in MAC z-score of +0.30 z-score units per month while the non-PN group had a monthly change in rate of -0.15 z-score units per month (p=0.0002). At the time of transplant listing, the PN group had lower TSF z-scores compared with the non-PN group (-2.2 ± 0.2 vs. -1.5 ± 0.3, p=0.001). Between listing and clinical endpoint, TSF z-scores improved significantly in the PN group (-2.2 ± 0.2 to -1.8 ± 0.2, p =0.006) and were unchanged in the non-PN group (-1.5 ± 0.2 to -1.6 ± 0.1, p=NS). The average monthly rate of change in TSF z-score from listing to clinical endpoint was +0.25 and +0.02 z-score units respectively for PN and non-PN groups (p=0.04). At clinical endpoint, there was no significant difference between the two groups with respect to MAC and TSF z-score. Similar patterns of change were seen in weight, length, and weight/length z-scores; however, the differences between groups were not statistically significant.
Figure 2.
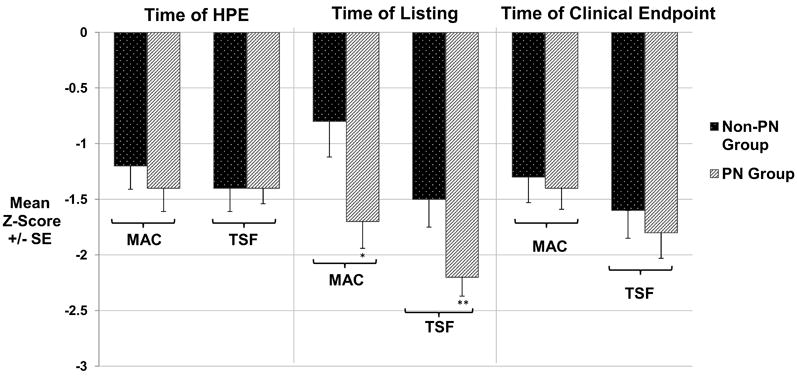
Comparison of MAC and TSF Thickness between PN and Non-PN Groups. MAC and TSF thickness were similar in PN and Non-PN groups at the time of HPE. However, by the time of listing for transplantation MAC and TSF were significantly lower in the PN group compared to the Non-PN group (*p=0.01 and **p=0.001). At the time of clinical endpoint, because both MAC and TSF had improved in the PN group, there was no difference in MAC and TSF between the PN and Non-PN groups. MAC=mid-arm circumference, TSF=triceps skin fold, PN=parenteral nutrition, Clinical Endpoint=transplant, death, or removal from transplant waiting list.
At the time of HPE, the PN and non-PN groups had similar baseline laboratory studies (Table 2). At the time of transplant listing, patients on PN had lower mean serum albumin (2.8 vs. 3.2 g/dL, p=0.03), GGT (528 vs. 940 units/L, p =0.04), and platelet counts (202 vs. 330 × 103/μL, p = 0.001). At the time of clinical endpoint, mean serum albumin (2.8 vs. 3.1 g/dL, p=NS) was similar in the PN and the non-PN group, however the PN group had higher serum total bilirubin (21.9 vs. 13.8 mg/dL, p=0.04), more prolonged prothrombin time (18.5 vs. 14.6 sec, p=0.01), and lower GGT (210 vs. 502 units/L, p=0.0007).
Weight, length, TSF, and MAC z-scores were compared to serum albumin, total bilirubin, and prothrombin time at the time of transplant listing. TSF z-score was weakly associated with serum albumin (R2=0.34, p = 0.05) and weight z-score was weakly associated with prothrombin time (R2=0.32, p = 0.03). All other associations were not significant.
Medical outcomes were compared between the PN and non-PN groups from time of HPE until clinical endpoint. The PN group had a higher incidence of GI bleeding (60.0% vs. 14%, p = 0.002) and development of ascites (76.0% vs. 36.4%, p=0.009) than the non-PN group. There was no significant association between presence of a central line and the incidence of a positive blood culture (48% of patients with a central line developed bacteremia compared to 20% of patients without a central line, p = 0.09). There was no significant association between PN use and incidence of a positive blood culture (52% of PN group developed bacteremia compared with 41% of non-PN group, p = 0.89).
There were no deaths in the non-PN group and 3 deaths in the PN group (1 each from gastrointestinal bleeding, fungal sepsis, and respiratory arrest) prior to transplant (p=NS). The mean age at liver transplant, time from listing to transplant, mean calculated PELD score at the time of transplant, graft type, and type of immunosuppression used during the first six months post-transplant were similar between PN and non-PN groups (Table 1). Among transplanted patients, there were no differences between PN and non-PN groups in days spent in the Intensive Care Unit post-transplant (9.2 vs. 8.9 days), graft survival, patient survival and re-transplantation rates (Figure 3 and Table 1).
Figure 3.
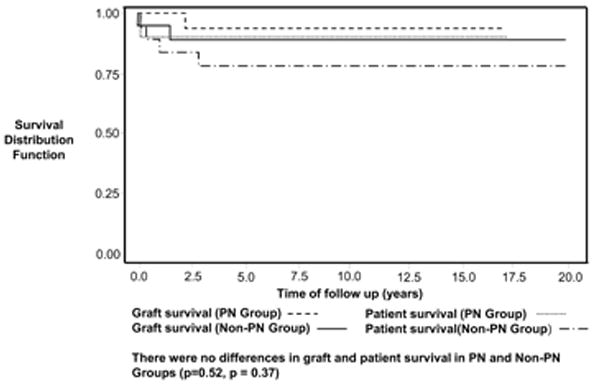
Post-transplant Patient and Graft Survival in PN vs. Non-PN Groups. Patient (log rank test p=0.37) and graft (log rank test p=0.52) survival was similar in both PN and Non-PN groups following liver transplantation (log rank test p=0.37).
Discussion
This is the first study to report the impact of parenteral nutrition on outcomes in biliary atresia patients with end-stage liver disease. Institution of PN in malnourished biliary atresia patients restores many patients to the same nutritional status observed in those patients managed with enteral nutrition alone. Outcomes after liver transplantation were similar in the two groups, despite more advanced liver disease at the time of transplant in the PN group. These data suggest that the beneficial effects of PN on nutritional status may have contributed to better outcomes following liver transplantation than would have been expected had the patients remained in their severely compromised nutritional state.
Malnutrition, a significant problem for children with biliary atresia, can be difficult to assess as ascites, organomegaly, and peripheral edema may confound interpretation of their weight and weight/length measurements.(2, 13) Weight z-scores and weight/length percentiles have been shown to overestimate nutritional status in patients with biliary atresia. MAC (a measure of muscle mass) and TSF (a measure of adipose stores) more accurately assess the state of malnutrition in these patients and were utilized as the primary markers of nutritional status in this study.(2, 8-13)
Decreased oral intake, early satiety, fat malabsorption, and increased energy expenditure due to a hypermetabolic state all likely contribute to malnutrition in biliary atresia patients.(17) As mean energy intake (111 kcal/kg/day) in the PN groups was well below estimated caloric needs (131 kcal/kg/day), with signs of progressive malnutrition (lower MAC and TSF z-scores), parenteral nutrition was initiated to improve nutritional status while the patients awaited liver transplantation.(3) Within the PN group, 64% had already failed to show improved nutritional status following the initiation of NG tube feeding supplementation, thus PN was considered the only viable option. With the addition of PN, the total energy intake was maintained in the PN group at the same level as the non-PN group at clinical endpoint. PN administration reversed the trend of falling TSF z-scores in the PN group and restored values to those observed in the non-PN group by the time of the clinical endpoint. At transplant listing and time of the clinical endpoint, although total energy intake was similar in both groups, MAC and TSF z-scores had improved only in the PN group. It is likely that the PN group would have had continued deterioration of nutritional status following transplant listing had PN not been instituted.
At transplant listing, albumin levels and platelet counts were significantly lower in the PN compared to the non-PN group, reflecting malnutrition and more severe liver disease. Among patients receiving PN, albumin levels stabilized or improved after listing. Platelet counts, however, continued to fall, and serum bilirubin and prothrombin time rose after transplant listing in the PN group, suggesting progression of chronic liver failure and portal hypertension. Moreover, at clinical endpoint the serum bilirubin and prothrombin time were higher and platelet counts significantly lower in the PN vs. non-PN group. Thus, despite worsening liver function, PN administration was successful in improving or stabilizing nutritional status in these infants with end-stage biliary atresia.
Malnourished biliary atresia patients are at higher risk of poor pre-transplant outcomes.(5, 18) Length and weight z-scores >-2 are associated with better outcomes in biliary atresia patients.(5) Once listed for liver transplant, malnutrition (length or weight z-scores <-2) is associated with increased need for intensive care unit monitoring pre- transplant and mortality.(16) Decreasing weight and length z-scores and serum albumin are also risk factors for death pre- transplant.(6) In our PN group, TSF, MAC and albumin levels were decreasing prior to PN administration, and stabilized/increased following PN. Prior to transplant, the PN group had a higher incidence of GI bleeding and ascites compared with the non-PN group. Although this most likely represents more advanced liver disease and portal hypertension in the PN group, parenteral nutrition, with subsequent expansion of vascular volume, may have also contributed. The administration of PN was not, however, associated with increased bacteremia or pre-transplant mortality. In summary, based on expected worse pre-transplant outcomes in BA patients who are severely malnourished,(5, 6, 16) we speculate that the pre-transplant complications of liver disease would have been even worse in the PN group had they not achieved improved nutritional status provided by PN administration.
Malnourished biliary atresia patients are also at increased risk for significant post-transplant complications. Growth failure at the time of liver transplantation confers increased risk for graft failure and post-transplant death.(6, 7, 18) In our study, PN improved MAC and TSF z-scores to > -2 at clinical endpoint. Although weight z-scores also improved, these findings are difficult to interpret given the frequency of ascites and fluid retention in this population. Following transplant, both PN and non-PN groups had similar clinical outcomes (days in ICU post-transplant, need for re-transplant, graft and patient survival). We postulate that without PN driven improvements in nutritional status, this group would have had poorer post-transplant outcomes compared to the non-PN group.(6, 7, 18)
Although this study addresses the importance of nutrition in biliary atresia, there are several limitations. The retrospective nature of this study resulted in incomplete nutritional data at all time points for some subjects (e.g., those that were breast fed). Although bacteremia rates were similar, we were unable to normalize bacteremia events to number of days with intravenous catheters in place because of inadequate documentation of line removal dates in the medical record. In addition, although pre-transplant mortality was similar between the PN and non-PN group, the possibility of a Type 2 error exists. We acknowledge that the PN and non-PN groups were not clinically equivalent (the PN group had more advanced disease and more severe portal hypertension), thus limiting to some extent the comparison of outcomes of these two groups. However, the more advanced liver disease in the PN group would have biased this group towards worse clinical outcomes, which were not observed.
It should be noted that, despite improved nutritional status in patients receiving PN, their liver disease appeared to progress more rapidly after PN initiation (based on higher bilirubin, lower platelets, longer prothrombin time, and higher calculated PELD) in the setting of a significantly lower GGT, compared to the non-PN group. PN-associated liver disease is well-documented in premature infants and infants with intestinal failure requiring PN supplementation(19) but has not been described in BA patients. Our PN patients received doses of soy-based intravenous lipids in the range implicated in the pathogenesis of liver injury in infants with intestinal failure.(20-22) Thus, it is possible that PN may have contributed to the more rapid progression of cholestasis in the PN group. Lipid sparing techniques and use of omega-3 fatty acid-based intravenous lipid preparations, shown to reverse PN-associated cholestasis(20-22), might be considered for BA patients in the future to minimize this possibility.
In conclusion, malnutrition is a serious clinical problem in patients with biliary atresia awaiting liver transplantation. This study provides evidence that PN can be effectively utilized in biliary atresia patients with advanced liver disease and portal hypertension to improve nutritional status while awaiting liver transplantation. These data also suggest that PN may stabilize or improve the clinical outcomes both pre- and post-liver transplant by virtue of improvement in nutritional status. The potential effect of PN on the pace of liver function worsening in BA patients requires additional investigation. Based on the outcomes documented in this study, we suggest that PN should be considered a useful option for infants and children with end-stage liver disease and severe malnutrition awaiting liver transplantation.
Acknowledgments
Funding: Supported in part by Ruth L. Kirschstein National Research Service Award (#5T32DK067009), Cystic Fibrosis Foundation Clinical Fellowship Award (#GEIDER08B0), and NIH/NCRR Colorado CTSA Grant UL1 RR025780. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
List of Abbreviations
- PN
parenteral nutrition
- LTx
liver transplantation
- HPE
hepatoportoenterostomy
- TSF
Triceps Skin Fold
- MAC
Mid-arm Circumference
- Z
Z-Score
- GGT
gamma-glutamyl transpeptidase
- AST
aspartate aminotransferase
- ALT
alanine transaminases
- WBC
white blood cell count
- INR
international normalized ratio
- PELD
Pediatric End-Stage Liver Disease
- NG
nasogastric
- GI
gastrointestinal
- ICU
intensive care unit
- GIR
glucose infusion rate
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
References
- 1.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37(1):4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sokol RJ, Stall C. Anthropometric evaluation of children with chronic liver disease. Am J Clin Nutr. 1990;52(2):203–8. doi: 10.1093/ajcn/52.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Greer R, Lehnert M, Lewindon P, Cleghorn GJ, Shepherd RW. Body composition and components of energy expenditure in children with end-stage liver disease. J Pediatr Gastroenterol Nutr. 2003;36(3):358–63. doi: 10.1097/00005176-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Guimber D, Michaud L, Ategbo S, Turck D, Gottrand F. Experience of parenteral nutrition for nutritional rescue in children with severe liver disease following failure of enteral nutrition. Pediatr Transplant. 1999;3(2):139–45. doi: 10.1034/j.1399-3046.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 5.DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, et al. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology. 2007;46(5):1632–8. doi: 10.1002/hep.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, et al. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr. 2005;147(2):180–5. doi: 10.1016/j.jpeds.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 7.Dick AA, Perkins JD, Spitzer AL, Lao OB, Healey PJ, Reyes JD. Impact of obesity on children undergoing liver transplantation. Liver Transpl. 16(11):1296–302. doi: 10.1002/lt.22162. [DOI] [PubMed] [Google Scholar]
- 8.Yuksekkaya HA, Cakir M, Tumgor G, Baran M, Arikan C, Yagci RV, et al. Nutritional status of infants with neonatal cholestasis. Dig Dis Sci. 2008;53(3):803–8. doi: 10.1007/s10620-007-9917-y. [DOI] [PubMed] [Google Scholar]
- 9.Roggero P, Cataliotti E, Ulla L, Stuflesser S, Nebbia G, Bracaloni D, et al. Factors influencing malnutrition in children waiting for liver transplants. Am J Clin Nutr. 1997;65(6):1852–7. doi: 10.1093/ajcn/65.6.1852. [DOI] [PubMed] [Google Scholar]
- 10.Schneider AC, Pinto RB, Silveira TR. Nutritional risk and malnutrition determination by anthropometry in cirrhotic children and adolescents. Arq Gastroenterol. 2007;44(4):345–9. doi: 10.1590/s0004-28032007000400012. [DOI] [PubMed] [Google Scholar]
- 11.Hehir DJ, Jenkins RL, Bistrian BR, Blackburn GL. Nutrition in patients undergoing orthotopic liver transplant. JPEN J Parenter Enteral Nutr. 1985;9(6):695–700. doi: 10.1177/0148607185009006695. [DOI] [PubMed] [Google Scholar]
- 12.Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, et al. The nature of malnutrition in children with end-stage liver disease awaiting orthotopic liver transplantation. Am J Clin Nutr. 1992;56(1):164–8. doi: 10.1093/ajcn/56.1.164. [DOI] [PubMed] [Google Scholar]
- 13.Arvay JL, Zemel BS, Gallagher PR, Rovner AJ, Mulberg AE, Stallings VA, et al. Body composition of children aged 1 to 12 years with biliary atresia or Alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;40(2):146–50. doi: 10.1097/00005176-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt PK, Carter JE. Static and dynamic differences among five types of skinfold calipers. Hum Biol. 1990;62(3):369–88. [PubMed] [Google Scholar]
- 15.Frisancho AR. Triceps skin fold and upper arm muscle size norms for assessment of nutrition status. Am J Clin Nutr. 1974;27(10):1052–8. doi: 10.1093/ajcn/27.8.1052. [DOI] [PubMed] [Google Scholar]
- 16.McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74(2):173–81. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pierro A, Koletzko B, Carnielli V, Superina RA, Roberts EA, Filler RM, et al. Resting energy expenditure is increased in infants and children with extrahepatic biliary atresia. J Pediatr Surg. 1989;24(6):534–8. doi: 10.1016/s0022-3468(89)80500-7. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd RW, Chin SE, Cleghorn GJ, Patrick M, Ong TH, Lynch SV, et al. Malnutrition in children with chronic liver disease accepted for liver transplantation: clinical profile and effect on outcome. J Paediatr Child Health. 1991;27(5):295–9. doi: 10.1111/j.1440-1754.1991.tb02541.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman SS, Gondolesi GE, Fishbein TM. Parenteral nutrition associated liver disease. Semin Neonatol. 2003;8(5):375–81. doi: 10.1016/S1084-2756(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 20.Fallon EM, Le HD, Puder M. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant. 15(3):334–40. doi: 10.1097/mot.0b013e3283394879. [DOI] [PubMed] [Google Scholar]
- 21.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cober MP, Teitelbaum DH. Prevention of parenteral nutrition-associated liver disease: lipid minimization. Curr Opin Organ Transplant. 15(3):330–3. doi: 10.1097/MOT.0b013e328338c2da. [DOI] [PubMed] [Google Scholar]


