Abstract
Chronic active Epstein-Barr virus (CAEBV) infection is characterized by persistent infectious mononucleosis-like symptoms, an unusual pattern of Epstein-Barr virus (EBV) antibodies, detection of the EBV genome in affected tissues or peripheral blood, and chronic illness that cannot be attributed to any other known disease. This is the first reported Korean case of an immunocompetent adult with CAEBV-associated interstitial pneumonitis. A 28-year-old female was admitted with a fever that persisted for 3 weeks. She had multiple lymphadenopathy, hepatosplenomegaly, pancytopenia, and elevated serum aminotransferase levels. Serology for antibodies was positive and chest computed tomography showed diffuse ground glass opacities in both lungs. Histopathology of the lung tissue showed lymphocyte infiltration, and EBV DNA was detected in those lymphocytes using in situ hybridization with an EBV-encoded RNA probe. After 1 month of hospitalization, she improved without specific treatment.
Keywords: Epstein-Barr virus infection, Immunocompetence, Lung diseases, Interstitial pneumonitis
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous virus that can cause both acute and chronic active infections. Primary EBV infection is usually asymptomatic, but it can cause active symptomatic infection, including infectious mononucleosis, which resolves spontaneously after EBV-specific immunity develops [1]. EBV usually remains latent following the primary infection, although in some patients it progresses to chronic active infection characterized by a persistent infectious mononucleosis-like syndrome, which may include fever, persistent hepatitis, extensive lymphadenopathy, hepatosplenomegaly, pancytopenia, high viral loads in peripheral blood, and an unusual pattern of EBV-related antibodies [2]. Recently, Okano et al. [3] reviewed patients with persistent infectious mononucleosis-like syndrome and proposed diagnostic criteria for chronic active EBV (CAEBV) infection.
Chronic active EBV may result in life-threatening complications, such as hemophagocytic syndrome, disseminated intravascular coagulopathy, hepatic failure, coronary artery aneurysm, central nervous system involvement, myocarditis, lymphoma, and hematologic malignancies [2]. Rarely, interstitial pneumonitis occurs as a serious complication in CAEBV patients; here, we report the first such case in Korea. In this case, an immunocompetent adult had a clinical syndrome of CAEBV and interstitial pneumonitis associated with the infiltration of EBV-infected T lymphocytes into the lungs, which implies CAEBV-associated interstitial pneumonitis.
CASE REPORT
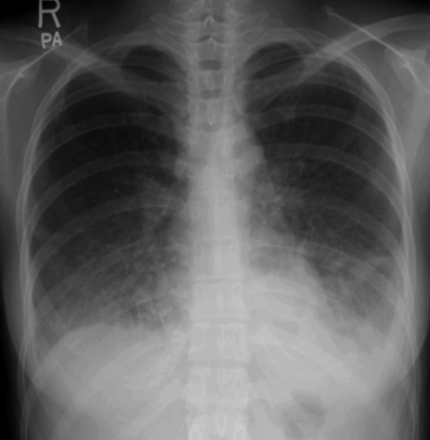
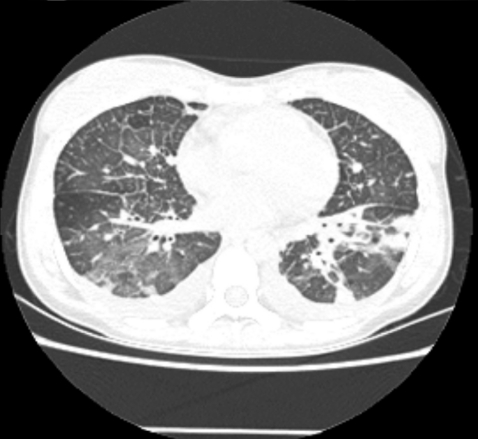
A 28-year-old female was admitted with a persistent fever for 3 weeks and flu-like symptoms. On admission, her temperature was 38.6℃, blood pressure was 130/70 mmHg, and respiration rate was 30/min. Her inguinal lymph nodes were palpable, about 0.5 cm in diameter, tender, and fixed. She had decreased breath sounds in both lower lung fields and hepatosplenomegaly. Her hemoglobin was 13.2 g/dL, platelet count 56,000/µL, and leukocyte count 2,770 units (segmented neutrophils 48.7%, lymphocytes 43%). Her serum bilirubin was 1.3 mg/dL, aspartate transaminase/alanine aminotransferase 610/697 U/L, serum protein 6.3 g/dL, serum albumin 3.5 g/dL, and lactate dehydrogenase 2,327 ng/mL. Blood, urine, and pleural fluid cultures did not grow any bacteria or fungi. The pleural fluid was a transudate. The serologic tests showed EBV viral capsid antigen (VCA)-IgG (+), EBV VCA-IgM (-), EBV- early antigen (EA) (+), EBV nuclear antigen (EBNA) (+), anti-HBs Ab (+), and anti-HCV Ab (-). The EBV-DNA copy number in whole blood, as measured by real-time polymerase chain reaction (RT-PCR), was 946.1 copies/5 µL. The chest radiograph showed diffuse ground glass opacities in both lower lung fields with bilateral pleural effusions (Fig. 1). Computed tomography of the abdomen and chest revealed hepatosplenomegaly and diffuse ground glass opacities and interlobular septal thickening of the lungs (Fig. 2). Flexible bronchoscopy showed normal bronchial anatomy without inflammation of the airway mucosa. Histopathology of inguinal lymph node tissue showed reactive hyperplasia and was negative for EBV DNA. A normocellular marrow with three-lineage hematopoiesis and few small to medium-sized aggregated CD3+ T lymphocytes were observed in a bone marrow aspirate. A conventional PCR technique with an EBV-encoded small RNA (EBER) probe detected EBV DNA in those lymphocytes.
Figure 1.
Diffuse ground glass opacity in both lower lung fields with bilateral pleural effusions.
Figure 2.
Diffuse ground glass opacity and interlobular septal thickening of the lungs.
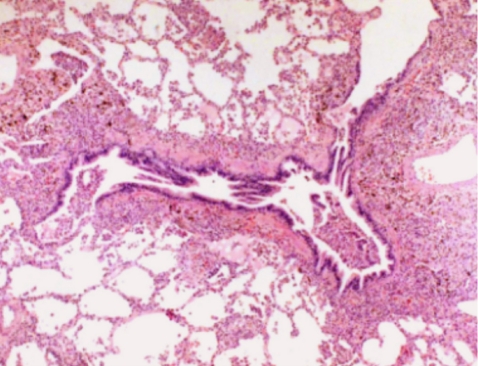
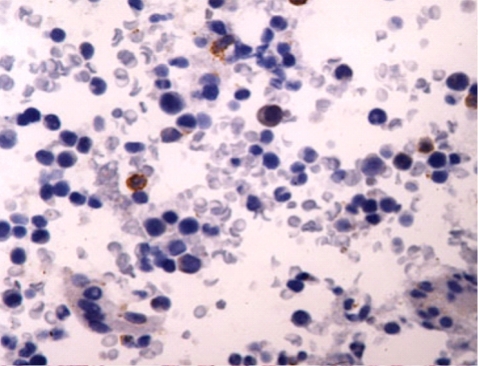
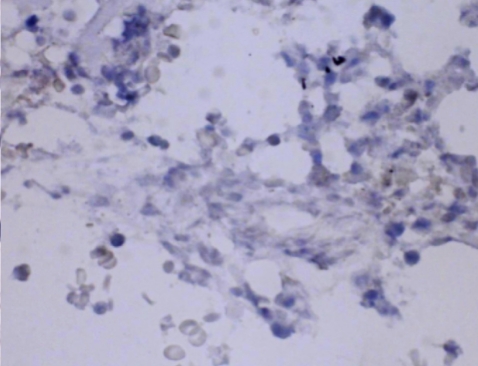
Six days after admission, the patient was transferred to the intensive care unit because of impending respiratory failure. At this point, a lung biopsy was performed with video-assisted thoracoscopic surgery. Histopathologically, the lung tissue showed infiltration of the alveolar septum and peribronchial interstitium by small to large lymphocytes (Fig. 3). The infiltrate was composed predominantly of CD3+ and CD56- T lymphocytes on immunohistochemistry (Fig. 4). EBV DNA was detected by in situ hybridization using an EBER probe on the lymphocytes (Fig. 5) and the TCRγ gene arrangement showed a polyclonal pattern.
Figure 3.
Infiltration of small to large lymphocytes into the alveolar septum and peribronchial inter-stitium (H&E, × 400).
Figure 4.
Numerous CD3-positive T lymphocytes (immunohistochemistry, × 400).
Figure 5.
Intracellular Epstein-Barr virus (EBV) DNA detected in lymphoid cells (EBV-encoded RNA in situ hybridization, × 400).
Twenty-six days after admission, her symptoms and signs had resolved spontaneously and she was discharged from the hospital. She was followed for 18 months. Six months after discharge, her EBV-DNA copy number had increased to 6,936 copies/5 µL, but she remained well clinically over the entire follow-up period.
DISCUSSION
In 1978, Virelizier et al. [4] reported the case of a young female patient with very high IgG antibody titers to VCA and EA, accompanied by EBNA-positive cells in the blood and lymph nodes. Additional reports described a similar illness in another young female in 1984 [5] and in seven Japanese patients in 1986 [6]. In 1988, Straus [7] proposed diagnostic criteria for this syndrome, which he called severe and chronic EBV infection. In 2005, Okano et al. [3] has subsequently proposed another set of diagnostic criteria for this condition, which he called CAEBV infection syndrome, based on a review of the literature and their further clinical experience.
The proposed diagnostic criteria included the following: 1) persistent or recurrent infectious mononucleosis-like symptoms; 2) an unusual pattern of EBV antibodies with elevated anti-VCA and anti-EA, or detection of the EBV genome in affected tissues including the peripheral blood; and 3) chronic illness that cannot be explained by any other known disease processes at the time of diagnosis [3]. Our case met all of these criteria.
In our case, both immunohistochemistry and in situ hybridization with an EBER probe enabled us to detect numerous CD3+ T lymphocytes that were infiltrating the lungs and contained the EBV genome in their nuclei. In CAEBV, unlike classic infectious mononucleosis, T lymphocytes or NK cells contain the EBV genome rather than B cells. In addition, most patients with CAEBV have defective EBV-specific cytotoxic T cells, NK cells, and lymphokine-activated killer activity, which involve the defective production of several cytokines, such as interferon gamma and interleukin-1. The etiology of CAEBV remains unclear; however, it is possible that defective EBV replication in T/NK cells and aberrant EBV-infected T/NK cell proliferation are major factors in the development of CAEBV [2,6].
The major clinical features of CAEBV are fever, hepatosplenomegaly, liver dysfunction, pancytopenia, lymphadenopathy, hypersensitivity to mosquito bites, skin rash, and uveitis [3]. In addition, CAEBV often results in lifethreatening complications, such as hemophagocytic syndrome, disseminated intravascular coagulopathy, hepatic failure, coronary artery aneurysm, central nervous system involvement, myocarditis, and interstitial pneumonitis [2].
The literature describes three pulmonary manifestations associated with EBV infection: hilar/mediastinal lymphadenopathy, pleural effusion, and interstitial pneumonitis. Few reports describe pulmonary parenchymal involvement as a complication of acute or chronic active EBV infection in immunocompetent patients [8,9]. A report on two children with CAEBV stated that the histopathology of their lung tissues showed interstitial infiltration of mature lymphocytes, which spread into the interalveolar septa, and similar to our case, EBV-positive T lymphocytes were detected throughout the alveolar septae and vascular lumens [9].
We report an immunocompetent adult who had a clinical syndrome of CAEBV and interstitial pneumonitis and pleural effusion associated with the infiltration of EBV-infected T lymphocytes into the lungs. This is the first known case of CAEBV-associated interstitial pneumonitis in Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H. Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol. 2006;16:251–261. doi: 10.1002/rmv.505. [DOI] [PubMed] [Google Scholar]
- 3.Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80:64–69. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]
- 4.Virelizier JL, Lenoir G, Griscelli C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet. 1978;2:231–234. doi: 10.1016/s0140-6736(78)91744-0. [DOI] [PubMed] [Google Scholar]
- 5.Joncas JH, Ghibu F, Blagdon M, Montplaisir S, Stefanescu I, Menezes J. A familial syndrome of susceptibility to chronic active Epstein-Barr virus infection. Can Med Assoc J. 1984;130:280–284. [PMC free article] [PubMed] [Google Scholar]
- 6.Okano M, Sakiyama Y, Matsumoto S, Mizuno F, Osato T. Unusual lymphoproliferation associated with chronic active Epstein-Barr virus infection. AIDS Res. 1986;2(Suppl 1):S121–S123. [PubMed] [Google Scholar]
- 7.Straus SE. The chronic mononucleosis syndrome. J Infect Dis. 1988;157:405–412. doi: 10.1093/infdis/157.3.405. [DOI] [PubMed] [Google Scholar]
- 8.Ankermann T, Claviez A, Wagner HJ, Krams M, Riedel F. Chronic interstitial lung disease with lung fibrosis in a girl: uncommon sequelae of Epstein-Barr virus infection. Pediatr Pulmonol. 2003;35:234–238. doi: 10.1002/ppul.10244. [DOI] [PubMed] [Google Scholar]
- 9.Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis: clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med. 1986;104:636–643. doi: 10.7326/0003-4819-104-5-636. [DOI] [PubMed] [Google Scholar]