Abstract
The Arabidopsis calcium-sensing receptor CAS is a crucial regulator of extracellular calcium-induced stomatal closure. Free cytosolic Ca2+ (Ca2+i) increases in response to a high extracellular calcium (Ca2+o) level through a CAS signalling pathway and finally leads to stomatal closure. Multidisciplinary approaches including histochemical, pharmacological, fluorescent, electrochemical, and molecular biological methods were used to discuss the relationship of hydrogen peroxide (H2O2) and nitric oxide (NO) signalling in the CAS signalling pathway in guard cells in response to Ca2+o. Here it is shown that Ca2+o could induce H2O2 and NO production from guard cells but only H2O2 from chloroplasts, leading to stomatal closure. In addition, the CASas mutant, the atrbohD/F double mutant, and the Atnoa1 mutant were all insensitive to Ca2+o-stimulated stomatal closure, as well as H2O2 and NO elevation in the case of CASas. Furthermore, it was found that the antioxidant system might function as a mediator in Ca2+o and H2O2 signalling in guard cells. The results suggest a hypothetical model whereby Ca2+o induces H2O2 and NO accumulation in guard cells through the CAS signalling pathway, which further triggers Ca2+i transients and finally stomatal closure. The possible cross-talk of Ca2+o and abscisic acid signalling as well as the antioxidant system are discussed.
Keywords: ABA signalling, antioxidant system, calcium-sensing receptor, extracellular calcium signalling, guard cells, hydrogen peroxide, nitric oxide
Introduction
Extracellular calcium (Ca2+o) has long been known to promote free cytosolic Ca2+ (Ca2+i) increase and stomatal closure (Schwartz, 1985; MacRobbie, 1992; McAinsh et al., 1995). The calcium-sensing receptor (CAS) was then identified and proven to be involved in this Ca2+o-induced Ca2+i increase (CICI) and stomatal closure (Han et al., 2003; Tang et al., 2007). Furthermore, CAS was reported to localize in the chloroplast thylakoid membrane (Nomura et al., 2008). However, how the guard cells convert the Ca2+o signal through CAS and finally lead to CICI was not well understood.
Hydrogen peroxide (H2O2) was shown to be a second messenger in response to biotic and abiotic perturbations (Fukao and Bailey-Serres, 2004; Laloi et al., 2004). H2O2 also functions as a signalling molecule in abscisic acid (ABA)-induced stomatal movements (Pei et al., 2000). The AtrbohD and AtrbohF (Arabidopsis respiratory burst oxidase homologues D and F) NADPH oxidases double mutant atrbohD/F abolishes the ABA-driven Ca2+i increase and stomatal closure (Kwak et al., 2003). Meanwhile, H2O2 has been found to stimulate Ca2+i transients in regulation of stomatal movement (Allen et al., 2000; Pei et al., 2000). Extracellular calmodulin (ExtCaM), a Ca2+o-activated protein, induces H2O2 generation and the increased H2O2 further elevates stomatal Ca2+i concentration (Chen et al., 2004), suggesting that Ca2+o signalling, H2O2 production, and Ca2+i transients may be orchestrated in guard cells.
Nitric oxide (NO), another key intermediate in ABA and multiple stress responses (Garcia-Mata and Lamattina, 2001, 2002; Neill et al., 2002a), operates as a downstream player of H2O2 signalling in guard cells (Lum et al., 2002; Bright et al., 2006). Interestingly, the Ca2+o-driven NO accumulation in guard cells was reported in recent experiments in Arabidopsis (Li et al., 2009), suggesting the possibility of Ca2+o-driven NO accumulation through H2O2 generation. Like H2O2, an NO-induced Ca2+i increase and stomatal closure have also been found in guard cells of Arabidopsis and Vicia faba (Neill et al., 2002a; Garcia-Mata et al., 2003). Notably, NO was shown to contribute to Ca2+i transients in ABA-induced stomatal closure (Desikan et al., 2002). However, the underlying mechanisms by which NO contributes to the Ca2+o-induced Ca2+i increase and stomatal closure are not clear.
Chloroplasts are a major source of H2O2 and NO production in plant cells (Asada and Takahashi, 1987; Jasid et al., 2006) and also a sensor of environmental stresses through chloroplast redox signalling (Pfannschmidt, 2003). The application of exogenous ABA resulted in H2O2 generation and stomatal closure, which occurs in chloroplasts earlier than within the other regions of guard cells (Zhang et al., 2001). A recent study revealed that chloroplasts play a critical role in CAS-mediated CICI and subsequent stomatal closure in Arabidopsis (Nomura et al., 2008). These results suggest that stomatal movements responding to environmental signals are probably regulated by H2O2 from the chloroplasts. Ca2+o-induced stomatal closure may involve cross-talk between Ca2+o and chloroplast-localized CAS as well as H2O2 in chloroplasts.
Besides what is known about guard cell signalling pathways, other findings also suggested the correlation among Ca2+o, H2O2, NO, and chloroplasts. Ca2+o can increase the activity of NADPH oxidase (NOX) and trigger H2O2 production in maize leaves (Sagi and Fluhr, 2001; Hu et al., 2007; Potocký et al., 2007). Mitogen-activated protein kinase 6 (MPK6) and the prohibitin gene PHB3 were recently identified to function in H2O2-mediated NO synthesis during lateral root formation and in abiotic stress responses (Wang et al., 2010; Wang et al., 2010). Consistently, NO synthesis was shown to be up-regulated by H2O2 in other plant species (Lum et al., 2002; Li et al., 2009). In addition, NO also promoted Ca2+i transients in plant cells in response to biotic and abiotic stresses (Gould et al., 2003; Lamotte et al., 2004, 2006; Vandelle et al., 2006). Similarly to H2O2, using NO-sensitive fluorescent probes, NO synthesis was also evident first in chloroplasts of the mesophyll and across all epidermal cell types including guard cells in response to environmental stimuli (Foissner et al., 2000; Gould et al., 2003; Arnaud et al., 2006).
In this report, the Apollo 4000 system with H2O2- and NO-selective electrochemical sensors was used to detect H2O2 and NO production in epidermis or chloroplasts. Different tools were also utilized to characterize functionally the role of H2O2 and NO as well as the contribution of the CAS signalling pathway in Ca2+o-induced Ca2+i increase and stomatal closure, including CAS antisense lines (CASas) and Arabidopsis mutants defective in H2O2 or NO synthesis (e.g. the H2O2 synthetic enzymatic mutant atrbohD/F and a mutant indirectly impaired in NO synthesis Atnoa1), a histochemical technique, and H2O2-and NO-specific fluorescent dyes. The transcriptional activities of Arabidopsis cytosolic antioxidant enzymes such as copper/zinc superoxide dismutase 1 (CSD1), ascorbate peroxidase 1 (APX1), and glutathione-disulphide reductase (ATGR1) in leaves were also investigated for the involvement of the antioxidant system in Ca2+o-induced H2O2 generation. A functional relationship among CAS, H2O2, and NO as well as the antioxidant system was established in Ca2+o-dependent guard cell signalling.
Materials and methods
Plant materials and growth conditions
Arabidopsis plants of the wild type and various mutants were grown in mixture matrix (turves:vermiculite=1:1) with a 16 h light and 8 h dark cycle under a photon flux rate of 200 μM m−2 s−1 at 22 °C, 70% relative humidity. Fully expanded Arabidopsis leaves of ∼5 weeks old were harvested for immediate use. Seeds of CASas, atrbohD/F, and Atnoa1 mutants (background Col-0) were all obtained from Duke University. These mutants were further confirmed by reverse transcription-PCR analysis with specific primers on total RNA extracted from leaves (Supplementary Fig. S1A, C available at JXB online). Primer sequences used for PCR are shown in Supplementary Table S1. CAS antisense lines were identified by western blot analysis using anti-CAS antibody as described (Han et al., 2003) (Supplementary Fig. S1B).
Stomatal bioassay
Stomatal assays were performed essentially as described by Desikan et al. (2002). Abaxial epidermal strips from similar rosette leaves were floated in 10 mM MES buffer (pH 6.15) containing 50 mM KCl and 50 μM CaCl2 for 2 h under light conditions to open the stomata before the addition of various compounds. Following this, 2 mM CaCl2, 10 μM H2O2, or 60 μM sodium nitroprusside (SNP) was added to the buffer and incubated for another 2 h to assay stomatal closure. To study the effect of catalase (CAT), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide potassium salt (cPTIO), or neomycin on stomatal closure, epidermal strips were transferred to and incubated in MES buffer containing 2 mM CaCl2 plus 100 U ml−1 CAT, 100 μM cPTIO, or 100 μM neomycin for 2 h.
For time-resolved measurements of Ca2+o-, H2O2-, or SNP-induced stomatal closure, abaxial epidermal strips with open stomata were incubated in various buffers for different times as indicated in the figure legends. Stomatal apertures were determined as the ratio of width to length using image analysis computer software (SigmaScan Pro5) and were presented as the percentage with respect to untreated control or zero time as the standard.
Fluorescent imaging by microscopy
The variations of H2O2 and NO in guard cells were examined by loading 2′,7′-dichlorofluorescein diacetate (H2DCFDA) and 3-amino,4-aminomethyl-2′,7′-difluorofluorescein diacetate (DAF-FM DA) as described (Suhita et al., 2004; Gonugunta et al., 2008). Abaxial epidermal strips with open stomata were transferred to MES buffer with 50 μM H2DCFDA or 10 μM DAF-FM DA for 15 min in darkness at room temperature and then rinsed with MES buffer three times before various treatments. Fluorescence was observed under a Motic AE31 fluorescence microscope (Speed Fair Co., Ltd, Hong Kong, excitation filter, 488 nm and emission, 535 nm) with a digital video camera (Motic MHG-100B, Speed Fair Co., Ltd, Hong Kong). Images were acquired from fluorescence between different samples, genotypes, and during time-courses at identical illumination intensity, camera gain, and exposure time in this experiment. The fluorescent pixel intensities of subtracting the basal signal from guard cells were analysed using MetaMorph 7.5 software. H2DCF fluorescence and chlorophyll autofluorescence in guard cells were further visualized using a laser scanning confocal microscope (LEICA TCS SP2) at an excitation wavelength of 488 nm. H2DCF fluorescence was detected from 495 nm to 535 nm, whereas chlorophyll autofluorescence was detected between 630 nm and 730 nm.
Subcellular localization of H2O2 in guard cells
The histochemical localization of H2O2 based on generation of electron-dense cerium perhydroxide precipitates was performed as described (Bestwick et al., 1997; Pellinen et al., 1999). Tissue fragments (2×5 mm2) from leaves incubated in MES buffer with or without 2 mM CaCl2 or 10 μM ABA were excised and kept in 50 mM MOPS buffer containing 5 mM CeCl3 at pH 7.2 under vacuum for 20 min. After being double fixed with 2.5% glutaraldehyde and 1.0% OsO4, the CeCl3-treated sections were dehydrated in an ascending ethanol series and embedded with the abaxial epidermis clinging to the surface of the embedding block. Blocks were progressively dehydrated in a graded acetone series and polymerized. Blocks of abaxial epidermis were sliced into 70–90 nm sections on a ultramicrotome, and mounted on uncoated copper grids. Sections of the guard cells were observed using transmission electron microscopy (TEM; JEM-2100HC, JEOL, Japan).
Preparations of leaf epidermis and chloroplasts
Arabidopsis leaf epidermis was extracted as described by Pandey et al. (2002). The epidermis peels, which were pale green and ∼1 mm2 in size, were ready for H2O2 or NO monitoring using a four-channel free radical Apollo 4000 analyser (World Precision Instruments, Sarasota, FL, USA). The intactness of epidermal cells in the epidermal fragments was evaluated using Evans blue dye as described (Mergemann and Sauter, 2000).
Arabidopsis chloroplasts were isolated using the method of Aronsson and Jarvis (2002). The integrity of the isolated chloroplasts was estimated to be >85% (Supplementary Fig. S2 at JXB online). The chloroplast pellets were finally resuspended in HMS buffer containing 50 mM HEPES, 3 mM MgSO4, 0.3 M sorbitol, pH 7.2. The chloroplast counts were determined microscopically using a haemocytometer.
H2O2 and NO measurement by the Apollo 4000 system
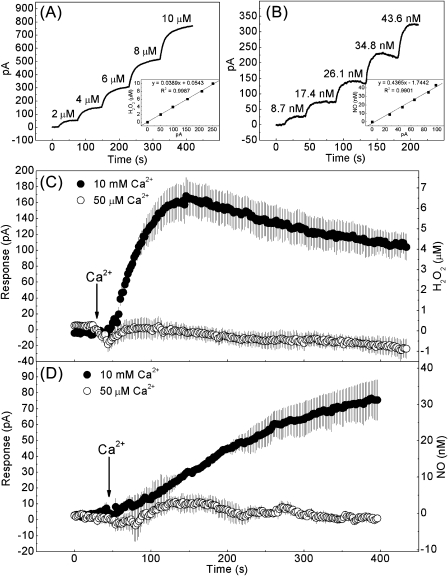
The H2O2- and NO-selective electrochemical sensors ISO-HPO and ISO-NOP (WPI, Sarasota, FL, USA), widely used for animals, plants, microalga, and subcellular organelles (Bouchard and Yamasaki, 2008; de Oliveira et al., 2008; D’Agostino et al., 2009; Pandolfi et al., 2010), were respectively connected to the Apollo 4000 system (World Precision Instruments) and adapted for real-time detection of H2O2 and NO from Arabidopsis leaf epidermis or chloroplast. Both sensors consist of a combination of an internal H2O2- or NO-sensing pair of working and counter (reference) electrodes. Each sensor fits inside a replaceable stainless steel membrane sleeve filled with an electrolyte solution and is separated from the outside environment by an H2O2- or NO-selective membrane that covers the end of the stainless steel. The poise voltage of each sensor was set to 400 mV for H2O2 or 865 mV for NO detection. Direct current from each sensor presents the environmental H2O2 or NO concentration and the data are recorded on a PC connected to the Apollo 4000 system. The H2O2 or NO sensors were inserted into a water-jacketed chamber containing 3 ml of MES buffer with epidermal peels from 0.5 g of Arabidopsis rosette leaves or 1 ml of HMS buffer. A circulating bath was used to keep the temperature constant at 30 °C and the sample was mixed at a low rate using a magnetic bar controlled by a magnetic stirrer. To observe Ca2+o-induced H2O2 and NO production from the epidermis, 10 mM or 50 μM CaCl2 (in MES buffer) was supplemented when the current signal became stable. For H2O2 detection from chloroplasts, a chloroplast suspension of 3×106 individuals was transferred to HMS buffer with or without 10 mM CaCl2. The H2O2 sensor was calibrated in a set of known H2O2 standards (0–10 μM range) while the NO sensor was calibrated with S-nitroso-N-acetyl-penicillamine (SNAP) in 0.1 M CuCl2 (0–50 nM range of NO) as described by the manufacturer (Fig. 1A, B).
Fig. 1.
Electrochemical detection of H2O2 and NO production from Arabidopsis epidermis in response to Ca2+o. (A, B) Calibration of the H2O2- and NO-selective electrochemical sensors ISO-HPO (A) and ISO-NOP (B). The current output jumped rapidly after each addition of H2O2 of known concentration or SNAP in CuCl2 solution that produced a known concentration of NO. The calibration curve is shown in the inset. (C, D) Trace obtained upon supplementation with 10 mM (upper trace) or 50 μM (lower trace) CaCl2 in epidermal peel suspensions using ISO-HOP (C) and ISO-NOP (D). Each trace represents the average of three samples (±SE). The arrow shows the time point at which CaCl2 was applied.
Fluorometric detection of H2O2 and NO production from chloroplasts
The fluorometric method used for the detection of NO from barley root mitochondria suspensions, as described recently by Gupta and Kaiser (2010), was adapted for measuring H2O2 and NO production from chloroplasts. For all experiments, chloroplasts were first incubated in HMS buffer with 50 μM H2DCFDA or 10 μM DAF-FM DA for 1 h in darkness. Chloroplasts were then rinsed twice by centrifugation at 2500 g for 4 min each, resuspended with HMS buffer, and kept in darkness until analysed. To study concentration-dependent Ca2+o induction of H2O2 and NO production, H2DCF- or DAF-FM-pre-loaded chloroplasts were incubated in HMS buffer with 0–2 mM CaCl2 for 10 min. For time-related analysis, 2 mM CaCl2 was applied for the time points ranging from 0 min to 10 min. All experiments were performed at a controlled temperature of 30 °C. The fluorescence of 5×104 ml−1 chloroplasts was detected using a fluorescence spectrophotometer (Cary Eclipse, Varian) with 495±5 nm and 515±5 nm as the excitation and emission wavelengths, respectively.
Real-time quantitative PCR analysis
Detached leaves were incubated in MES buffer containing 50 μM or 2 mM CaCl2 for 0–24 h under light at 22 °C. Total RNA was extracted from leaves collected at various time points given in the figure legends using the TRIZOL Reagent (Invitrogen Inc., CA, USA). For real-time quantitative PCR, first-strand cDNA was synthesized using M-MLV reverse transcriptase (Takara Bio Inc., Japan) with an oligo d(T)18 primer. The resulting cDNAs were used as templates for subsequent PCRs which were performed on the Rotor-Gene™ 6000 real-time analyser (Corbett Research, Mortlake, Australia) in standard mode with FastStart Universal SYBR Green (ROX, Roche Ltd., Mannheim, Germany) according to the manufacturer’s protocol. All cycling conditions were as follows: 10 min at 94 °C; 40 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, followed by a melting curve program (55–99 °C, with a 5 s hold at each temperature). The primers were designed according to known sequences of Arabidopsis CSD1, APX1, and ATGR1 genes (GenBank accession nos AT1G08830, AT1G07890, and AT3G24170, respectively) acquired from NCBI. The primers used for amplification are listed in Supplementary Table S2 at JXB online, and the products were checked by melting curve analysis. Amplified products were cloned into PMD-18T vector (Takara) and subjected to sequencing analyses. The mean mRNA expression level was normalized using the ΔΔCt method described by Livak and Schmittgen (2001) with Actin2 as the internal control.
Results
Ca2+o induces H2O2 and NO production in guard cells of Arabidopsis epidermis
To assess the effects of Ca2+o on promoting H2O2 and NO production from Arabidopsis epidermis, electrochemical sensors ISO-HPO and ISO-NOP were used to detect H2O2 and NO, respectively, in epidermal peels. Calibrations of both sensors were performed, and changes in the concentration of H2O2 and NO were then calculated based on a linear relationship between H2O2 or NO and the corresponding current over the current ranges 0–250 pA or 0–100 pA (Fig. 1A, B). The specificity of both sensors was also confirmed by further experiments showing that CAT or cPTIO could, respectively, remove the H2O2 or NO signal, while CAT or cPTIO themselves could not affect this signal (Supplementary Fig. S3 at JXB online). To initiate the reaction, a high or low concentration of CaCl2 was added to the suspension of epidermal peels. Upon high CaCl2 (10 mM) supplementation, H2O2 production from the epidermis monitored by a H2O2 electrochemical sensor rapidly increased to nearly 7 μM within 100 s and then reached a plateau (Fig. 1C). While high CaCl2 caused a gradual increase of NO in epidermis, no significant change occurred after adding a low CaCl2 concentration to a suspension of epidermal peels (Fig. 1D). In addition, NO synthesis seems to lag behind that of H2O2 when the epidermis is exposed to Ca2+o signal.
In order to determine whether the detected production of H2O2 and NO was mainly released from guard cells, the H2O2 and NO targets in Arabidopsis epidermal peels in response to Ca2+o were investigated. The endogenous H2O2 and NO in 10 mM CaCl2-pre-incubated epidermal peels were determined by using the H2O2 and NO fluorescent probes H2DCFDA and DAF-FM DA. Intensive green fluorescence for both probes was observed in guard cells rather than in epidermal cells (Supplementary Fig. S4A, B at JXB online). However, the fact that no fluorescence was observed from epidermal cells was not due to death during the preparation of epidermal fragments, because ∼80% of epidermal cells were still alive when estimated using Evans blue dye (Supplementary Fig. S4C). These results indicated that the detected H2O2 and NO were mainly produced from guard cells of extracted epidermis when exposed to Ca2+o.
Chloroplasts are the cellular source for H2O2 production in Ca2+o-exposed guard cells
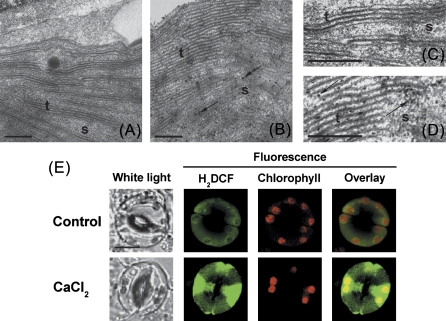
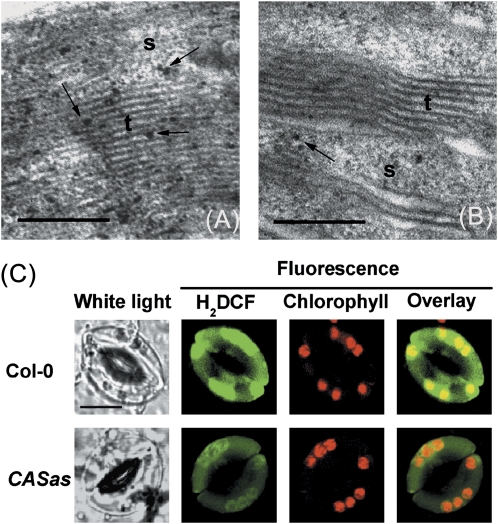
It is widely accepted that chloroplasts in guard cells of most higher plants are potential sources of H2O2 (Wang and Song, 2008). In the present experiments, chloroplasts of guard cells on abaxial epidermal sections were selected for study of Ca2+o-induced H2O2 production using the CeCl3 staining method. After exposure to 2 mM CaCl2, H2O2 accumulation was visible using TEM as black precipitate spots on thylakoids and in stroma of chloroplasts (Fig. 2A–D), similar to the results seen for methyl viologen-induced H2O2 generation in spongy chloroplasts (Pellinen et al., 1999). Interestingly, intensive green fluorescence of H2DCF from chloroplasts was also detected in 2 mM CaCl2-exposed guard cells (Fig. 2E) compared with the control. ABA was suggested to lead to the formation of H2O2 in chloroplasts and stomatal closure (Zhang et al., 2001). Therefore, experiments were carried out to examine whether there is downstream signalling cross-talk between Ca2+o and ABA. Consistently, ABA-driven H2O2 accumulation in chloroplasts of guard cells was also observed (Supplementary Fig. S5 at JXB online).
Fig. 2.
Ca2+o-induced H2O2 accumulation in guard cell chloroplasts from Arabidopsis. (A, B) TEM images of chloroplasts in guard cells with CeCl3 staining from a leaf section incubated in 50 μM (A) or 2 mM (B) CaCl2. (C) Further detail of A. (D) Further details of B. The black spots in the TEM images represent H2O2 forming electron-dense cerium perhydroxide precipitates. Examples of individual precipitates are shown by arrows. s, stroma; t, thylakoids. Bar=200 nm. (E) Confocal images of changes in H2DCF fluorescence intensity from guard cell chloroplasts of the wild type in response to 50 μM (control) and 2 mM CaCl2. H2DCF, chlorophyll, and overlay fluorescence images from guard cell are shown. Bar=10 μm. (This figure is available in colour at JXB online.)
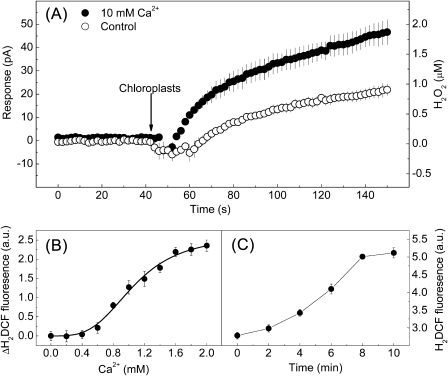
It was then investigated whether Ca2+ could directly cause H2O2 generation from chloroplasts of guard cells in vitro. Purified chloroplasts were transferred to HMS buffer with or without (control) 10 mM CaCl2, and the H2O2 signal was detected by the H2O2 sensor ISO-HPO. Here, chloroplasts in suspension buffer containing no CaCl2 can also be detected to produce H2O2 (Fig. 3A). This is because transferring the stable chloroplasts to a new suspension buffer will also cause H2O2 generation without any stimulation until they are stable again. However, the chloroplasts incubated in HMS with 10 mM CaCl2 responded much more strongly than the control within 100 s (Fig. 3A), suggesting that Ca2+ enhanced H2O2 production in chloroplasts. Using a fluorescence spectrophotometer, a Hill curve could be fitted to the data of CaCl2 dose-dependent H2O2 production (Fig. 3B) with a Hill coefficient of ∼1.1. The activation time course of 2 mM CaCl2 could be described by a simple sigmoid equation, with an activation time constant of 6 min (Fig. 3C). However, no pronounced increase in NO from chloroplasts was observed when exposed to 0–10 mM CaCl2 (Supplementary Fig. S6 at JXB online), indicating no correlation between Ca2+ and NO production in chloroplasts. It is well known that chloroplasts are the major source of H2O2 in plants, and H2O2 production in chloroplasts from guard cells or mesophyll cells in response to various stimuli has been well documented (Pellinen et al., 1999; Zhang et al., 2001), suggesting that the chloroplasts from guard cells and mesophyll cells are identical in their function to produce H2O2. Although total chloroplasts were used to study Ca2+-induced H2O2 production, the results may also represent Ca2+-induced H2O2 production in chloroplasts from guard cells. These data demonstrated that H2O2, but not NO, was produced in chloroplasts in response to a lower concentration of Ca2+ (∼1.1 mM) within a few minutes.
Fig. 3.
Effect of Ca2+ on H2O2 generation in isolated Arabidopsis chloroplasts. (A) The upper trace represents the rapid H2O2 production in chloroplasts incubated with 10 mM CaCl2, and the lower trace represents less production of H2O2 in chloroplasts incubated without CaCl2 (control). Data are averages of three samples ±SE. (B) Averaged increases in relative H2DCF fluorescence in chloroplasts plotted as a function of applied CaCl2 for 10 min (n=8, ±SE). Data were fitted to the Hill equation. (C) The trace shows the time-course of H2O2 production in chloroplasts upon 2 mM CaCl2 supplementation. H2DCF fluorescence was measured from the time at which CaCl2 was provided (n=8, ±SE).
H2O2 and NO generation are essential for Ca2+o-induced stomatal closure
The effects of Ca2+o, H2O2, or the NO donor SNP on stomatal closure have been demonstrated before (Pei et al., 2000; Neill et al., 2002a; Han et al., 2003). Therefore, the question was asked as to whether H2O2 and NO generation are required for Ca2+o-induced stomatal closure. The fluorescent probes of H2O2 and NO enabled determination of the kinetics of H2O2 and NO changes in guard cells upon exposure to Ca2+o (Fig. 4A, B, D, E). According to Allen et al. (2001), 1–10 mM Ca2+o can lead to stomatal closure. To prolong the response of guard cells to Ca2+o, a low concentration (2 mM) of Ca2+o was used in this experiment. In the presence of Ca2+o, an alteration in H2O2 or NO production in the wild type was visible by 15 min, reaching saturation at 25 min, and then slowly decreased, compared with the untreated control. These results were analogous to ABA-driven H2O2 and NO formation in guard cells (Suhita et al., 2004; Gonugunta et al., 2008). Here, time-resolved measurements of Ca2+o-, H2O2-, and SNP-induced stomatal closure were also performed (Fig. 4C). Exposure to H2O2 or SNP induced a striking decrease in stomatal aperture after 20 min. Comparably, with Ca2+o treatment, stomatal aperture decrease steeply after 30 min, which was a 15 min lag behind Ca2+o-induced H2O2 and NO production. Thus, H2O2 or SNP appeared to result in a more rapid decrease in stomatal aperture than Ca2+o, suggesting the possibility that H2O2 and NO generation are required for stomatal closure in response to Ca2+o.
Fig. 4.
H2O2 and NO productions occur before stomatal closure induced by Ca2+o in Arabidopsis. (A, B) Kinetics of the increase in H2DCF (A) and DAF-FM (B) fluorescence intensities in guard cells of both wild type and CASas in response to 2 mM Ca2+o (n=50, ±SE). The relative changes in H2O2 and NO production were expressed using the fluorescence at time zero as the standard (100%). (D, E) Examples of H2DCF (D) and DAF-FM (E) fluorescence images of guard cells from A and B, respectively. Bar=50 μm. (C) Kinetics of 2 mM Ca2+o-, 10 μM H2O2-, and 60 μM SNP-induced stomatal closure of both the wild type and CASas (n=100, ±SE). Stomatal apertures (width to length) were measured as the percentage using zero time as the standard (100%). (This figure is available in colour at JXB online.)
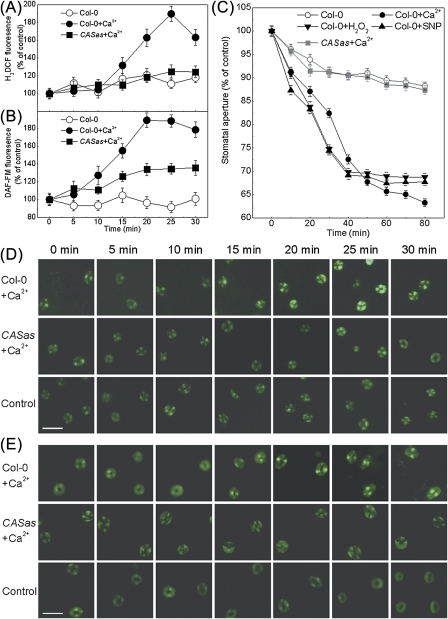
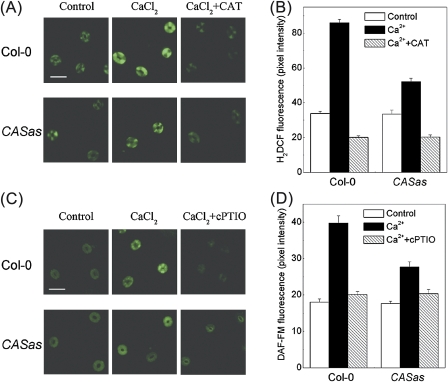
The H2O2 scavenger CAT and NO scavenger cPTIO were further used to study their effects on Ca2+o-induced H2O2 and NO production as well as stomatal closure. Ca2+o-induced NO production in guard cells has been shown by using NO-specific fluorescent dyes (Garcia-Mata and Lamattina, 2007; Li et al., 2009). Here a significant increase in H2DCF or DAF-FM fluorescence was also observed in guard cells in Ca2+o-treated epidermal peels (P <0.001), demonstrating the Ca2+o-induced H2O2 and NO production in guard cells (Fig. 5). However, pre-treatment of guard cells with CAT or cPTIO significantly suppressed H2O2 or NO generation and stomatal responses to Ca2+o in the wild-type plants (Col-0) (P <0.001) (Fig. 5; Table 1). In line with this result, the guard cells from atrbohD/F and Atnoa1 were less sensitive to Ca2+o (P <0.001) (Table 1), indicating a requirement for H2O2 and NO synthesis for Ca2+o-induced stomatal closure. Furthermore, pre-treated epidermal peels with H2O2 or SNP can induce stomatal closure in the wild type (P <0.001); however, both atrbohD/F and Atnoa1 stomata did close in response to SNP (P <0.001) while Atnoa1 stomata were less responsive to H2O2 compared with the wild type and atrbohD/F (P <0.001) (Table 1). Since H2O2 unidirectionally caused NO generation within 60 s in guard cells while NO had no effect on H2O2 production (Bright et al., 2006; Li et al., 2009), it is assumed that Ca2+o-induced NO generation is H2O2 dependent.
Fig. 5.
CAS regulates Ca2+o-induced H2O2 and NO production in guard cells of Arabidopsis. (A, B) Guard cells pre-loaded with 50 μM H2DCFDA in the wild type and CASas were incubated in MES buffer (control), 2 mM CaCl2, or 2 mM CaCl2 plus 100 U ml−1 CAT for 20 min in darkness. H2DCF fluorescence images and intensities of the guard cells were recorded in A and B, respectively. (C, D) Guard cells pre-loaded with 10 μM DAF-FM DA in the wild type and CASas were incubated in MES buffer (control), 2 mM CaCl2, or 2 mM CaCl2 plus 100 μM cPTIO for 20 min in darkness. DAF-FM fluorescence images and intensities of the guard cells were recorded in C and D, respectively. The data are expressed as the average ±SE (n=100). Bar=50 μm. (This figure is available in colour at JXB online.)
Table 1.
The effects of CAT or cPTIO on Ca2+-, H2O2-, or SNP-induced stomatal closure in various Arabidopsis genotypes
| Treatment | Stomatal aperture of each genotypea |
|||
| Col-0 | CASas | atrbohD/F | Atnoa1 | |
| None (control) | 100.0±1.0 | 102.3±1.2 | 100.7±1.2 | 101.3±1.1 |
| 2 mM Ca2+ | 58.9±0.7 | 103.2±1.3 | 83.5±1.2 | 84.6±1.1 |
| 2 mM Ca2++100 U ml−1 CAT | 86.2±1.1 | 101.1±1.4 | NDb | ND |
| 2 mM Ca2++100 μM cPTIO | 96.5±1.0 | 101.7±1.2 | ND | ND |
| 10 μM H2O2 | 66.0±0.7 | 71.3±1.0 | 59.9±0.8 | 87.7±1.2 |
| 60 μM SNP | 67.4±0.8 | 74.0±1.0 | 65.4±0.8 | 66.2±0.8 |
Stomatal aperture from Col-0 without any treatment is taken as 100%. Results are averages ±SE derived from analyses of stomatal apertures from 100 guard cells of at least three plants for each genotype.
ND, not determined.
CAS mediates H2O2 and NO generation during Ca2+o-induced stomatal closure
Earlier studies have shown that stomatal movement is modulated by the Ca2+o level through CAS while Ca2+o-induced stomatal closure is abolished in CASas lines (Han et al., 2003; Tang et al., 2007). Consistently, the results demonstrate that Ca2+o fails to induce stomatal closure in the CASas mutants (P <0.001), and CASas plants pre-treated with CAT or cPTIO remain impaired in Ca2+o-induced guard cell responses (P >0.05) (Table 1). However, the stomatal bioassay showed that exogenous H2O2 or SNP induced stomatal closure in both Col-0 and CASas plants (P <0.001) (Table 1). Further, the involvement of CAS in Ca2+o-induced H2O2 and NO production was investigated. It was found that H2DCF or DAF-FM fluorescence intensity was impaired in CASas plants after Ca2+o treatment (P <0.001) (Figs 4A, B, D, E, 5). Consistently, fewer black precipitate spots of H2O2 were seen in CASas guard cell chloroplast (Fig. 6B) than in the wild type (Fig. 6A) in response to Ca2+o. However, no precipitate spots of H2O2 were found in the cytoplasm, while some were detected on the plasma membrane of both wild-type and CASas guard cells in response to Ca2+o (Supplementary Fig. S7 at JXB online), which was due to the activation of plasma membrane NOX by Ca2+o (Sagi and Fluhr, 2001; Potocký et al., 2007). Although a small amount of NOX-driven H2O2 production somewhat promotes Ca2+o-induced stomatal closure, H2O2 production in chloroplasts is more important for this process because chloroplasts and CAS are crucial for Ca2+o-induced stomatal closure (Han et al., 2003; Nomura et al., 2008) and because Ca2+o-induced H2O2 production was mainly seen in chloroplast (Fig. 2E). Meanwhile, H2DCF fluorescence intensity was impaired in CASas guard cell chloroplasts after the exposure to Ca2+o (Fig. 6C). These results indicate that CAS affects events upstream of H2O2-dependent NO generation, thereby triggering stomatal closure.
Fig. 6.
Ca2+o-induced H2O2 accumulation in Arabidopsis guard cell chloroplasts is impaired in CASas. (A) TEM images showing the 2 mM CaCl2-induced H2O2 accumulation in guard cell chloroplasts with CeCl3 staining in the wild type. (B) Limited black precipitate spots in chloroplast of CASas guard cell with CeCl3 staining exposed to 2 mM CaCl2. The black spots in the TEM images represent H2O2 forming electron-dense cerium perhydroxide precipitates. Examples of individual precipitates are shown by arrows. s, stroma; t, thylakoids. Bar=200 nm. (C) Confocal images of H2DCF fluorescence intensity from guard cell chloroplasts of the wild type and CASas in response to 2 mM CaCl2. H2DCF, chlorophyll, and overlay fluorescence images from guard cells are shown. The scale bar represents 10 μm. (This figure is available in colour at JXB online.)
Ca2+o induces changes in transcript levels of cytosolic antioxidant enzymes in Arabidopsis
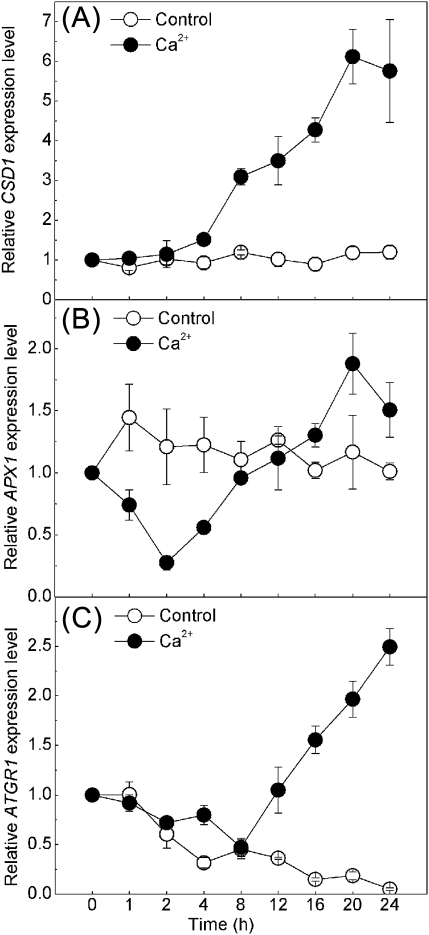
In this study, transient H2O2 production induced by Ca2+o as well as stomatal closure induced by Ca2+o within 2 h have been demonstrated. Previous work showed that ABA, CaCl2, H2O2, or SNP could increase the gene expression of cytosolic antioxidant enzymes such as superoxide dismutase, CAT, APX, and glutathione reductase in maize plants (Hu et al., 2005, 2007; Zhang et al., 2006; Sang et al., 2008). Based on these findings, it was decided to determine whether the cytosolic antioxidant system functions in H2O2 metabolism of Ca2+o stimulation. The expression of CSD1, which encodes a cytosolic superoxide dismutase that catalyses the conversion of O2- to H2O2 (Kliebenstein et al., 1998), started to increase at 4 h of 2 mM CaCl2 treatment and reached a maximum at 20 h (Fig. 7A). APX1 and ATGR1, which encode cytosolic ascorbate peroxidase and glutathione-disulphide reductase, respectively, in the metabolism of H2O2 (Neill et al., 2002b), were expressed in leaves after 2 mM CaCl2 treatment, with a decrease during the first 4–8 h, followed by an increase after 8 h (Fig. 7B, C). For the control, the expression of CSD1 and APX1 remained unchanged during the 24 h period without CaCl2 treatment, while ATGR1 transcription declined gradually in MES buffer under light. Although the expression of antioxidant genes in stomatal cells is hard to detect, the cytosolic antioxidant system might function as a mediator in Ca2+o and H2O2 signalling in guard cells.
Fig. 7.
The transcription level of three cytosolic antioxidant enzymes in Arabidopsis leaves challenged with Ca2+o. qRT-PCR analysis of time-resolved relative expression levels of CSD1, APX1, and ATGR1 in leaves incubated with MES buffer (control) or with 2 mM CaCl2 during 24 h is shown in (A), (B), and (C), respectively. The transcript levels at 0 h of incubation were set at 1. Error bars indicate the SE from three independent repeats.
Discussion
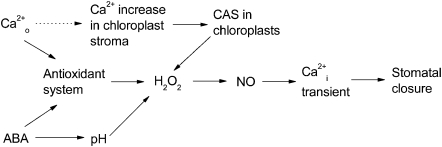
In this study, the focus was on Ca2+o-induced stomatal movement and the involvement of CAS, H2O2, NO, and the antioxidant system in the process. By combining the present data with results from other studiess, a model was built describing the Ca2+o signal transduction pathway, which cross-talks with ABA signalling, leading to stomatal closure (Fig. 8). This model allowed the systematic understanding of the molecular mechanism of guard cells in sensing environmental cues.
Fig. 8.
Schematic representation of the possible signalling cascade leading to the stomatal closure by Ca2+o and ABA. This model integrates the present data as well as results from previous studies described in the ‘Discussion’. The cascades for which the evidence is either ambiguous or still lacking are indicated by dotted arrows, while the well-established events (directly or indirectly) are represented by solid arrows.
Chloroplasts are the potential sites of CAS-mediated H2O2 generation in guard cells in response to Ca2+o stimulation
Chloroplasts were postulated to regulate stomatal movement as a sensor of environmental stresses (Wang and Song, 2008). Further evidence was provided for the chloroplast control of Ca2+o-induced Ca2+i transients and stomatal closure (Nomura et al., 2008). H2O2 generation in chloroplasts occurred much earlier than within the other regions of guard cells after the application of exogenous ABA (Zhang et al., 2001). Consistent with this, TEM and confocal data indicate that ABA- and Ca2+o-induced stomatal closure were both accompanied by H2O2 accumulation in chloroplasts (Fig. 2; Supplementary Fig. S5 at JXB online), suggesting a common route for Ca2+o and ABA signalling mediated by H2O2 generation in chloroplasts. In the present experiments, it was found that a low concentration of Ca2+ could directly cause H2O2 generation in vitro within a few minutes (Fig. 3). Therefore, it is possible that Ca2+o-induced H2O2 accumulation in chloroplasts requires an early limited increase of Ca2+i which might be induced by the activation of membrane calcium channels.
CAS, a putative Ca2+-binding protein localized in chloroplast thylakoid membranes (Han et al., 2003; Nomura et al., 2008), elicits a cascade of intracellular signalling events including the phospholipase C–inositol 1,4,5-triphosphate (PLC–IP3) pathway, and mediates CICI (Tang et al., 2007). This signalling cascade is probably activated by a Ca2+ increase in chloroplast stroma. It was noted that a large Ca2+ increase in chloroplast stroma followed by Ca2+i transients was detected when tobacco leaves were transferred from light to darkness (Sai and Johnson, 2002). However, Ca2+o-induced Ca2+i transients were disrupted in CASas plants (Han et al., 2003; Nomura et al., 2008; Weinl et al., 2008). These results suggested that elevation of the stroma Ca2+ level might be required for the CAS-mediated CICI signalling pathway. An early limited increase in Ca2+i regulated by the membrane calcium channel in response to Ca2+o may meet the need for the stromal Ca2+ increase required for CAS activation in chloroplasts. The observation that Ca2+o-induced H2O2 accumulation in chloroplast was impaired in CASas suggests that chloroplast H2O2 may function downstream of CAS in the Ca2+o signalling pathway (Fig. 5A, B). In the studies of Han et al. (2003) and Nomura et al. (2008), Ca2+o induced a rapid (the first step) but impaired cytoplasmic Ca2+ increase in CASas, and the prolonged cytoplasmic Ca2+ oscillations (the second step) were significantly disrupted compared with the wild type. However, they are normal in terms of ABA-driven stomatal closure (Nomura et al., 2008). Based on these findings, it is proposed that Ca2+o causes biphasic Ca2+i transients. The first step is an early increase of Ca2+i that activates CAS in chloroplasts, followed by the second step for H2O2 generation and long-term Ca2+i transients that also act downstream of ABA signalling through a different pathway such as cytosolic alkalinization (Suhita et al., 2004) and ABA receptors. In contrast to H2O2, no firm evidence for Ca2+-induced NO production in chloroplasts has been obtained (Supplementary Fig. S6 at JXB online). Therefore, it is possible that NO is produced around the chloroplasts through H2O2 diffusion, considering the requirement for H2O2 in ABA-induced NO generation (Bright et al., 2006).
Temporal sequence of CAS-mediated CICI and stomatal closure
As described (Allen et al., 1999, 2000, 2001; Han et al., 2003; Weinl et al., 2008), 1–10 mM Ca2+o can stimulate an initial Ca2+i spiking within 30 s, followed by a long-term Ca2+i transient which was blocked in CASas. However, 1–2 mM Ca2+o-induced stomatal closure was visible by ∼30 min as described by Weinl et al. (2008) and in the present work (Fig. 4C), while 10 mM Ca2+o caused a much more rapid reduction in stomatal aperture (Allen et al., 2001). According to these reports, 2 mM or 10 mM Ca2+o were also used as an experimental stimulus to probe the guard cell signalling pathway in this study. The long-term Ca2+i transients regulated by CAS seem more likely to lead to the final stomatal closure. What happens to the guard cells is still unknown from the initial Ca2+i spiking at 30 s to the stomatal closure at 30 min except for long-term Ca2+i transients when exposed to 1–2 mM Ca2+o, and how the guard cells maintain the long-term Ca2+i transients during this 30 min and perform closure that is regulated by CAS remained unclear before. H2O2 at 100 μM was shown to cause one or two separate transients of Ca2+i, leading to stomatal closure (Allen et al., 2000). Therefore, there is enough time (within 30 min) for guard cells to perform CAS-mediated H2O2 production to maintain Ca2+i transients.
The data indicate that Ca2+o-driven H2O2 and NO generation, which were inhibited in CASas, could be detected within 50–100 s (Fig. 1C, D) and increased steeply at ∼15 min in guard cells, earlier than Ca2+o-induced stomatal closure (Fig. 4) but later than Ca2+o-driven H2O2 production from chloroplasts at low concentration (Fig. 3). Interestingly, H2O2 and SNP had more rapid effects than Ca2+o on stomatal closure (Fig. 4C), supporting the speculation that Ca2+o induces Ca2+i transients and stomatal closure through CAS regulation as well as H2O2 and NO generation. As for the order of H2O2 and NO generation, it was shown by Bright et al. (2006) that H2O2→NO seemed to be more acceptable. In the present work, the electrochemical sensors were used for H2O2 and NO detection from the epidermis and chloroplasts. Ca2+o-induced H2O2 and NO production was observed from guard cells or chloroplasts (Figs 1, 3A). Moreover, more rapid generation of H2O2 than NO suggests that NO may act downstream of H2O2. However, the time point when Ca2+o induced visible H2O2 and NO production was not basically in line when comparing the two approaches. The exact reasons for such a discrepancy in real-time H2O2 and NO detection from guard cells are not sure clear. This could be due to the varied sensitivity of each method, which, however, also resulted in an identical conclusion supporting the Ca2+o signal transduction model.
ABA was shown to promote Ca2+i transients, leading to stomatal closure (Allen et al., 2000). However, ABA-induced cytosolic alkalinization was visible by 10 min and ABA-induced H2O2 and NO production were detected by ∼15 min (Suhita et al., 2004; Gonugunta et al., 2008). These results regarding time are almost consistent with ABA-induced initial Ca2+i transients at >10 min. It is well known that cytosolic alkalinization, and H2O2 and NO production are all required for ABA-induced stomatal closure and act upstream of long-term Ca2+i transients (Pei et al., 2000; Suhita et al., 2004; Bright et al, 2006; Gonugunta et al., 2008). Similar to ABA-induced H2O2 and NO production, both of them are also needed to maintain Ca2+i transients in response to Ca2+o, leading to stomatal closure.
Pharmacological analysis further supports the Ca2+o signalling transduction model
More evidence for the Ca2+o signalling model was provided by studying the effects of CAT and cPTIO on Ca2+o-induced H2O2 and NO as well as stomatal closure in various Arabidopsis genotypes. The use of these two compounds in CASas, atrbohD/F, and Atnoa1 plants reversed Ca2+o-induced stomatal closure and blocked Ca2+o-triggered H2O2 and NO production in guard cells. NOX is plasma membrane located; however, a report showed that ABA induced H2O2 synthesis not only via a plasma membrane NOX but also in the chloroplast, which occurred in chloroplasts earlier than within the other regions of guard cells (Zhang et al., 2001). In addition, a recent study revealed that chloroplasts played a critical role in CAS-mediated CICI and subsequent stomatal closure in Arabidopsis (Nomura et al., 2008). As shown in Table 1, compared with the wild type, atrbohD/F did respond to Ca2+o, but Ca2+o-induced stomatal closure was partially inhibited, while CAT, the H2O2 scavenger, showed more efficiency in this inhibition. Furthermore, compared with the wild type and atrbohD/F, Ca2+o could not induce stomatal closure in CASas, and Ca2+o-induced H2O2 production was largely impaired in guard cells and chloroplasts in CASas. The present data combined with those of other studies suggest that the CAS-mediated H2O2 synthesis in chloroplast is more critical for Ca2+o-induced stomatal closure than of plasma membrane NOX. The relationship between CAS, H2O2, and NO was further established by the observations that H2O2 and SNP caused stomatal closure in both wild-type and CASas plants. If CAS mediates Ca2+o-induced stomatal closure directly by Ca2+i transients and this does not require H2O2 and NO production, it could not be understood why Ca2+o-induced stomatal closure was also inhibited by the two compounds as well as in atrbohD/F and Atnoa1 plants. Further evidence suggests that H2O2 is required for NO generation because H2O2 could not induce stomatal closure in Atnoa1 plants. A similar conclusion has been drawn in a previous study (Bright et al., 2006). The finding that H2O2 and NO are involved in the CAS-mediated Ca2+o signalling in guard cells raises the questions of how the signals from CAS to H2O2 are transducted and what are the signalling components involved in this process.
The putative Ca2+o signal transduction pathway cross-talks with ABA and the antioxidant system
ABA was shown to promote stomatal closure in CASas plants (Nomura et al., 2008), suggesting that there is a signal converging site downstream of CAS during ABA and Ca2+o signalling. In addition, ABA signalling has been known to be involved in H2O2-dependent NO generation (Bright et al., 2006), which were proven to act as downstream signal molecules of CAS in this study. Furthermore, findings of both Ca2+o- and ABA-induced H2O2 generation in chloroplasts indicate that H2O2 may function as a common downstream component between ABA and CAS signalling (Fig. 6; Supplementary Fig. S5 at JXB online).
Interestingly, the antioxidant system seems to allow the plants to acclimatize to Ca2+o-induced H2O2 production. Antioxidant gene transcription was analysed after a short and long period of Ca2+o stimulation (Fig. 7). When exposed to Ca2+o, elevation of the CSD1 expression level would promote cytosolic H2O2 production, while biphasic responses of APX1 or ATGR1 expression could contribute to H2O2 production at the beginning and H2O2 scavenging after saturation. This ‘H2O2 buffer’ can protect the plant from oxidative damage due to a long period of Ca2+o signalling. A similar phenomenon was observed in ABA-treated maize plants, suggesting that the antioxidant system could also function as the convergence point between ABA and Ca2+o signalling.
In summary, by using pharmacological, biochemical, and genetic approaches, this study provided comprehensive supporting evidence for a putative signalling cascade during Ca2+o-induced stomatal closure. Briefly, CAS is probably activated in chloroplast stroma by a Ca2+o-induced early period of Ca2+ transients, which initiates an intracellular signalling cascade that involves H2O2 and NO production, Ca2+i transients, and subsequent stomatal closure in Arabidopsis guard cells. Ca2+o-induced H2O2 and NO production and Ca2+i transients as well as antioxidant enzymes transcriptional changes were also found in ABA-induced stomatal closure, which suggest a signalling interaction between Ca2+o, ABA, and the antioxidant enzymes system in stomatal movement.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Verifying the mutants using PCR and western blot.
Figure S2. The integrity of isolated chloroplasts was estimated by H2DCF fluorescent dye.
Figure S3. The effects of CAT or cPTIO on H2O2 or NO current detected by a H2O2- or NO-selective electrochemical sensor.
Figure S4. Localization of H2O2 and NO on Arabidopsis epidermal peels in response to Ca2+o.
Figure S5. ABA-induced H2O2 accumulation in Arabidopsis guard cell chloroplasts from the wild type with CeCl3 staining viewed using TEM.
Figure S6. Effect of Ca2+ on NO generation in isolated Arabidopsis chloroplasts.
Figure S7. TEM imgages of plasma membrane and cytoplasm in Arabidopsis guard cell with CeCl3 staining in response to Ca2+o.
Table S1. Primer sequences used for PCR analysis.
Table S2. Primer sequences used for RT-PCR analysis.
Acknowledgments
We are grateful to Xiang Tang, Lei Chen, Chun-Yan You, and Rui-Xue Duan for assistance in experiments. This study was financially supported by the Natural Science Foundation of China (NSFC nos 30930076, 30770192, 30670317, 30271065 and 39970438), the Foundation of the Chinese Ministry of Education (20070384033), the Program for New Century Excellent Talents in Xiamen University (NCETXMU X071l5), a Changjiang Scholarship (X09111), and grants from the Research Council of Hong Kong SAR (465009, 465410) and the Chinese University of Hong Kong.
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. The Plant Journal. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. doi: 10.1038/35082575. [DOI] [PubMed] [Google Scholar]
- Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F. An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. Journal of Biological Chemistry. 2006;281:23579–23588. doi: 10.1074/jbc.M602135200. [DOI] [PubMed] [Google Scholar]
- Aronsson H, Jarvis P. A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Letters. 2002;529:215–220. doi: 10.1016/s0014-5793(02)03342-2. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond BJ, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 227–287. [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv. phaseolicola. The Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard J, Yamasaki H. Heat stress stimulates nitric oxide production in Symbiodinium microadriaticum: a possible linkage between nitric oxide and the coral bleaching phenomenon. Plant and Cell Physiology. 2008;49:641–652. doi: 10.1093/pcp/pcn037. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Chen YL, Huang R, Xiao YM, Lü P, Chen J, Wang XC. Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiology. 2004;136:4096–4103. doi: 10.1104/pp.104.047837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino DP, Olson JE, Dean JB. Acute hyperoxia increases lipid peroxidation and induces plasma membrane blebbing in human U87 glioblastoma cells. Neuroscience. 2009;159:1011–1022. doi: 10.1016/j.neuroscience.2009.01.062. [DOI] [PubMed] [Google Scholar]
- de Oliveira HC, Wulff A, Saviani EE, Salgado I. Nitric oxide degradation by potato tuber mitochondria: evidence for the involvement of external NAD(P)H dehydrogenases. Biochimica et Biophysica Acta. 2008;1777:470–476. doi: 10.1016/j.bbabio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock JT, Neill S. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in. Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. The Plant Journal. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia—is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proceedings of the National Academy of Sciences, USA. 2003;100:11116–11121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiology. 2001;126:1196–1204. doi: 10.1104/pp.126.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiology. 2002;128:790–792. doi: 10.1104/pp.011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide. 2007;17:143–151. doi: 10.1016/j.niox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Gonugunta VK, Srivastava N, Puli MR, Raghavendra AS. Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant, Cell and Environment. 2008;31:1717–1724. doi: 10.1111/j.1365-3040.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- Gould KS, Lamotte O, Klinger A, Pugin A, Wendehenne D. Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant, Cell and Environment. 2003;26:1851–1862. [Google Scholar]
- Gupta KJ, Kaiser WM. Production and scavenging of nitric oxide by barley root mitochondria. Plant and Cell Physiology. 2010;51:576–584. doi: 10.1093/pcp/pcq022. [DOI] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang A, Lu J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta. 2005;223:57–68. doi: 10.1007/s00425-005-0068-0. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang J, Zhang A, Lin F, Tan M. Calcium–calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytologist. 2007;173:27–38. doi: 10.1111/j.1469-8137.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- Jasid S, Simontacchi M, Bartoli CG, Puntarulo S. Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiology. 2006;142:1246–1255. doi: 10.1104/pp.106.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde RA, Last RL. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiology. 1998;118:637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom JL, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A. Reactive oxygen signaling: the latest news. Current Opinion in Plant Biology. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. Mechanisms of nitric oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radical Biology and Medicine. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, Pugin A, Wendehenne D. Analysis of nitric oxide signalling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Liu YQ, Lü P, Lin HF, Bai Y, Wang XC, Chen YL. A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiology. 2009;150:114–124. doi: 10.1104/pp.109.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lum HK, Butt YK, Lo SC. Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus) Nitric Oxide. 2002;6:205–213. doi: 10.1006/niox.2001.0395. [DOI] [PubMed] [Google Scholar]
- MacRobbie E. Calcium and ABA-induced stomatal closure. Philosophical Transactions of the Royal Society B: Biological Sciences. 1992;338:5–18. [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. The Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergemann H, Sauter M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiology. 2000;124:609–614. doi: 10.1104/pp.124.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signalling in stomatal guard cells. Plant Physiology. 2002a;128:13–16. [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock J. Hydrogen peroxide signalling. Current Opinion in Plant Biology. 2002b;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. The Plant Journal. 2008;53:988–998. doi: 10.1111/j.1365-313X.2007.03390.x. [DOI] [PubMed] [Google Scholar]
- Pandey S, Wang XQ, Coursol SA, Assmann SM. Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytologist. 2002;153:517–526. doi: 10.1046/j.0028-646X.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Pandolfi C, Pottosin I, Cuin T, Mancuso S, Shabala S. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiology. 2010;51:422–434. doi: 10.1093/pcp/pcq007. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvo J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. The Plant Journal. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T. Chloroplast redox signals: how photosynthesis controls its own genes. Trends in Plant Science. 2003;8:33–41. doi: 10.1016/s1360-1385(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai J, Johnson CH. Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. The Plant Cell. 2002;14:1279–1291. doi: 10.1105/tpc.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang J, Zhang A, Lin F, Tan M, Jiang M. Cross-talk between calcium–calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants. Cell Research. 2008;18:577–588. doi: 10.1038/cr.2008.39. [DOI] [PubMed] [Google Scholar]
- Schwartz A. Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiology. 1985;79:1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, Jackson RB, Pei ZM. Coupling diurnal cytosolic Ca2+ oscillations to the CAS–IP3 pathway in Arabidopsis. Science. 2007;315:1423–1426. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- Vandelle E, Poinssot B, Wendehenne D, Bentejac M, Pugin A. Integrated signalling network involving calcium, nitric oxide, active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses. Molecular Plant-Microbe Interactions. 2006;19:429–440. doi: 10.1094/MPMI-19-0429. [DOI] [PubMed] [Google Scholar]
- Wang P, Du Y, Li Y, Ren D, Song CP. Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. The Plant Cell. 2010;22:2981–2998. doi: 10.1105/tpc.109.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Song CP. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytologist. 2008;178:703–718. doi: 10.1111/j.1469-8137.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ries A, Wu K, Yang A, Crawford NM. The Arabidopsis prohibitin gene PHB3 functions in nitric oxide-mediated responses and in hydrogen peroxide-induced nitric oxide accumulation. The Plant Cell. 2010;22:249–259. doi: 10.1105/tpc.109.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytologist. 2008;179:675–686. doi: 10.1111/j.1469-8137.2008.02492.x. [DOI] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Tan M, Hu X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology. 2006;141:475–487. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in. Vicia faba. Plant Physiology. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.