Abstract
The D-mannose/L-galactose pathway for the biosynthesis of vitamin C (L-ascorbic acid; AsA) has greatly improved the understanding of this indispensable compound in plants, where it plays multifunctional roles. However, it is yet to be proven whether the same pathway holds for all the different organs of plants, especially the fruit-bearing plants, at different stages of development. Micro-Tom was used here to elucidate the mechanisms of AsA accumulation and regulation in tomato fruits. The mRNA expression of the genes in the D-mannose/L-galactose pathway were inversely correlated with increasing AsA content of Micro-Tom fruits during ripening. Feeding L-[6-14C]AsA to Micro-Tom plants revealed that the bulk of the label from AsA accumulated in the source leaf was transported to the immature green fruits, and the rate of translocation decreased as ripening progressed. L-Galactose feeding, but neither D-galacturonate nor L-gulono-1,4-lactone, enhanced the content of AsA in immature green fruit. On the other hand, L-galactose and D-galacturonate, but not L-gulono-1,4-lactone, resulted in an increase in the AsA content of red ripened fruits. Crude extract prepared from insoluble fractions of green and red fruits showed D-galacturonate reductase- and aldonolactonase-specific activities, the antepenultimate and penultimate enzymes, respectively, in the D-galacturonate pathway, in both fruits. Taken together, the present findings demonstrated that tomato fruits could switch between different sources for AsA supply depending on their ripening stages. The translocation from source leaves and biosynthesis via the D-mannose/L-galactose pathway are dominant sources in immature fruits, while the alternative D-galacturonate pathway contributes to AsA accumulation in ripened Micro-Tom fruits.
Keywords: Ascorbate biosynthesis, D-galacturonate pathway, D-mannose/L-galactose pathway, translocation, tomato fruits
Introduction
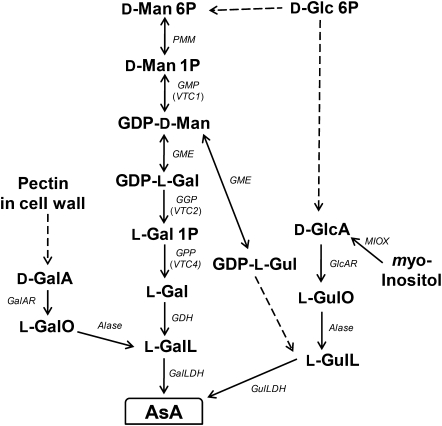
Ascorbate (AsA, vitamin C) is a multifunctional compound that plays roles in stress responses, plant defence, detoxification of reactive oxygen species, cell cycle regulation, cell wall synthesis, cell expansion, electron transfer, as the electron donor for some redox enzymes, in fruit ripening, and in production of other fruits acids (Smirnoff, 2000; Barth et al., 2004; Noctor, 2006; Debolt et al., 2007; Foyer and Shigeoka, 2011). It is an essential vitamin in the diet of humans who, due to a mutation, have lost the ability to synthesize the compound, and plant foods are the major source. The biosynthetic pathway of AsA in plants involves a complex network with D-mannose/L-galactose (D-Man/L-Gal) (Wheeler et al., 1998), L-gulose (Wolucka and Van Montagu, 2003), D-galacturonate (D-GalA) (Agius et al., 2003), and myo-inositol (Lorence et al., 2004) as key intermediates of the individual pathways (Fig. 1). The d-Man/L-Gal pathway, commonly called the Smirnoff–Wheeler pathway, was the first reported for the model plant Arabidopsis thaliana with all the genes involved in the pathway fully characterized (for reviews, see Ishikawa et al., 2006a; Ishikawa and Shigeoka, 2008; Linster and Clarke, 2008). The inability of other pathways to compensate for the low AsA content of A. thaliana vtc mutants and the analysis of a double knockout mutant for VTC2 and VTC5, encoding GDP-L-Gal phosphorylase, led to the conclusion that, at least in Arabidopsis, the D-Man/L-Gal pathway is pivotal to AsA biosynthesis while other pathways contribute only minute amounts to the overall AsA pool (Conklin et al., 1999; Dowdle et al., 2007). However, there is convincing evidence that the D-GalA pathway is functional in the green alga Euglena gracilis (Ishikawa et al., 2006b, 2008). Although the D-GalA pathway has also supported biosynthesis of AsA in strawberry fruits through the isolation of a key gene encoding D-GalA reductase (Agius et al., 2003), there have been no reports detailing the function of the enzyme in other plant species. Moreover, information on aldonolactonase, an important enzyme in the D-GalA pathway which converts L-galactonic acid to L-galactono-1,4-lactone, is still missing in higher plants.
Fig. 1.
A network for the biosynthesis of AsA. L-GaL, L-galactose; L-GalO, L-galactonic acid; D-GalA, D-galacturonic acid; L-GalL, L-galactono-1,4-lactone; D-Glc, D-glucose; D-GlcA, D-glucuronate; L-Gul, L-gulose; L-GulO, L-gulonate; L-GulL, L-gulono-1,4- lactone; D-Man, D-mannose. Enzymes catalysing the reactions are: Alase, aldonolactonase; GalLDH, L-galactono-1,4-lactone dehydrogenase; GalAR, D-galacturonate reductase; GDH, L-galactose dehydrogenase; GlcAR, D-glucuronate reductase; GME, GDP-D-mannose -3',5'-epimerase; GulLDH, L-gulono-1,4-lactone dehydrogenase; MIOX, myo-inositol oxygenase; PMM, phosphomannomutase; GMP, GDP-D-mannose pyrophosphorylase; GGP, GDP-L-galactose phosphorylase; GPP, L-galactose 1-P phosphatase. Broken arrows show more than one enzymatic reaction step.
Fruits generally contain a large amount of AsA and are an excellent source of the vitamin in foods consumed by humans (Davey et al., 2000). Therefore, a complete understanding of the biosynthesis pathway is of fundamental importance. Though the D-Man/L-Gal pathway has been reported for many fruit-bearing plants such as kiwifruits, acerola, peach, and tomato (Badejo et al., 2009; Bulley et al., 2009; Imai et al., 2009; Ioannidi, et al., 2009), there is still an outstanding issue concerning AsA pool size control during fruit maturation, especially one involving the contribution of the alternative AsA biosynthesis pathway which is currently under debate.
In this study, the focus was on a tomato variety called Micro-Tom, which is a miniature cultivar of tomato that has been used as a model system in tomato genomics (Aoki et al., 2010). Attempts were made to understand the regulation of AsA pool size in ripening Micro-Tom fruits by monitoring the translocation of labelled AsA within the Micro-Tom plant and at the same time to clarify whether the de novo AsA content of fruit is entirely biosynthesized or imported from the source leaf, or both. Finally, it was possible to confirm the existence of a functional alternative D-GalA pathway in ripened Micro-Tom fruits.
Materials and methods
Plant material
Micro-Tom seeds were washed with Milli Q water and planted in a pre-sterilized soil mixture. The plants were grown in a growth chamber at 25 °C under a 12 h light (80 μmol photons m−2 s−1) and 12 h dark cycle. The plants were watered once in 3 d with Hyponex solution diluted 1000 times with water. The flowers were hand pollinated and the fruits were harvested from the plant at different ripening stages for analyses.
Feeding experiment
Micro-Tom fruits were excised from the tree with the stalk in place and this was dipped in 5% sucrose, 5 mM L-GaL, 5 mM D-GalA, 5 mM L-gulono-1,4-lactone, and H2O, and incubated in the light (100 μmol photons m−2 s−1) for 24 h. At the end of the incubation period, the stalk was detached from the fruit, and the fruit was washed with Milli Q water twice and mopped gently. Samples were taken from the fruit for various analyses.
Feeding with inhibitors of photosynthetic electron transport
Micro-Tom fruits were treated with 10 μM of either 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) or 2,5-dibromo-3-methyl-6-isopropylp-benzoquinone (DBMIB) first for 1 h in the dark and then for 3 h under light (100 μmol photons m−2 s−1). The fruits were then fed with 5% sucrose for 48 h under the same light condition. The attached stalk was later detached and the fruits rinsed with Milli Q water before further analyses.
RNA isolation and real-time PCR
Total RNA was isolated from Micro-Tom fruit using RNAiso (Takara, Japan). Briefly, Micro-Tom fruit (∼100 mg) was pulverized in liquid N2 and homogenized in 1 ml of RNAiso followed by chloroform extraction at 13 000 rpm for 10 min. The RNA in the supernatant was precipitated with an equal volume of isopropanol and washed with 70% ethanol. To eliminate possible DNA contamination, the RNA was treated with 10 U of DNase I followed by purification with a FastPure RNA kit (Takara, Japan) according to the manufacturer’s instruction and quantified with Nanodrop 1000 (Thermo Scientific, USA). A 200 ng aliquot of the purified RNA was used for cDNA synthesis using PrimeScript RT Master Mix (Takara, Japan) according to the manufacturer’s instruction. The synthesized cDNA was used in a real-time PCR with the forward and reverse primers specific for each of the genes analysed: PMM (forward) 5′-ATTGAGTTCAGAAGTGGCATGC-3′, (reverse) 5′-GTTTCCCGTATCTTTTGTGCC-3′; GMP (forward) 5′-GGTCCTTCCT GGGTTTTGG-3′, (reverse) 5′-ATGCCAGTTTTGGTGAAGACC-3′; GME (forward) 5′-GAAGCTT-CGGGTCTCTATTACAGG-3′, (reverse) 5′-GACATGTGCTCATTCTT CTTCCAG-3′; GGP (forward) 5′-GAGTTTCGAGTTGATAAGGTTCTGC-3′ (reverse) 5′-CATCTGGATAGAGCTG-GACTTCATC-3′; VTC4 (forward) 5′-CTTCTTGCTACAGAGGCTGGAAC-3′, (reverse) 5′-CACACATACGAAGGGACCTAACC-3′; GDH (forward) 5′-AATTTGGGTCCCTCGATCAG-3′, (reverse) 5′-AATGAAACGGATCTTTCCAGC-3′; and L-galLDH (forward) 5′-CGAGTCAGTGGAGGAGCTTG-3′, (reverse) 5′-AATTCACCATCCCAGCTCG-3′. The real-time PCR was performed with the SYBR Premix Ex Taq (Takara, Japan) on a Thermal Cycler Dice Real Time System TP850 (Takara, Japan). Expression of three reference genes, phosphoglycerate kinase, tomato elongation factor 1α (EF1α), and glyceraldehyde-3-phosphate dehydrogenase, was compared in tomato fruits, and EF1α (forward) 5′-TGATCAAGCCTGGTATGGTTGT-3′ (reverse) 5′-CTGGGTCATCC-TTGGAGTT-3′ was found to be the most stable among the three and was used as internal standard.
Labelling of Micro-Tom leaves with L-[6-14C]AsA
In order to determine if the progressive increase in the AsA content of Micro-Tom during ripening is due to transport from the source leaves to the fruits, the ‘flap’ method described by Franceschi and Tarlyn (2002) was used to feed the whole plant with L-[6-14C]AsA (PerkinElmer, MA, USA) through the leaves. The flaps of the leaves were dipped in 5 mM AsA containing 1.0 μCi of L-[6-14C]AsA for 180 min in the light. Labelled plants were then incubated under continuous light conditions for 24 h. Five independent Micro-Tom plants were used to confirm the reproducibility of the observed phenomenon.
Autoradiography of Micro-Tom plants and fruits
After the 24 h light treatment, the tomato plant was fixed onto a sheet of chromatography paper and dried in the oven at 55 °C for ∼8 h. The dried labelled tomato plants and fruits were exposed to an Imaging Plate (FujiFilm, Japan) for 24 h and the image was scanned onto the computer via Fuji Imagine Plate Type BAS-IIIs (FujiFilm, Japan).
Measurement of radioactivity in the Micro-Tom fruits
The dried labelled tomato fruits were weighed and placed in a glass tube compatible with the scintillation counter, and 3–5 ml of scintillation fluid (Scintisol 500, Wako Japan) was added. The amount of radioactivity in the tomato was measured as disintegration per minute (dpm) using the Beckman LS6000SE scintillation counter.
Determination of AsA content
A Micro-Tom sample (∼200 mg) was pulverized in liquid N2, and 2 ml of a solution containing 0.1 M HCl and 1 mM EDTA was added for isolation. The sample was kept chilled and protected from light while allowed to thaw. This was followed by centrifugation at 13 000 rpm for 10 min at 4 °C. The supernatant was then transferred to a new tube and used immediately for total AsA content determination as described by Kampfenkel et al. (1995). The method employs the ability of AsA to reduce Fe3+ to Fe2+, forming a red compound under acidic condition with bipyridyl. A linear standard is run in parallel with the sample and the absorbance at 520 nm was measured with a microplate reader (Bio-Rad, model 680).
Enzyme extraction and assay
About 15 g of Micro-Tom fruits were squashed in their own juice with a pestle, and the liquid fraction (containing the cytoplasmic proteins) was separated by centrifugation at 5000 g for 10 min at 4 °C. The pellet which contained the membrane fraction was washed in 1 M KCl and centrifuged as above before grinding in liquid nitrogen with a pestle and mortar at 4 °C, and suspended in 20 ml of 50 mM acetate buffer, pH 6.0, containing 1 M CaCl2 and 0.5% (v/v) Tween-20. The paste was then subjected to brief sonication followed by centrifugation at 15 000 g for 30 min at 4 °C. (NH4)2SO4 was added to the supernatant containing the enzyme solution to ∼80% saturation with continuous stirring on ice for 1 h at 4 °C and then centrifugation at 15 000 g for 30 min. The pellet containing the concentrated enzyme was reconstituted in 2 ml of 50 mM acetate buffer containing 1 M CaCl2 and then desalted in a Sephadex G-10 column prepared with the same buffer to obtain the concentrated enzyme solution. The protein content of desalted enzyme was determined using the Bio-Rad protein quantication reagent (Bio-Rad, Hercules, CA, USA) following the manufacturer’s directions for the microassay.
The D-galacturonate reductase (D-GalAR) enzyme activity was measured with the ability to utilize D-GalA and D-glucuronic acid as a substrate, by the change in absorbance at 340 nm following the oxidation of NADPH. The assay contained 50 mM TRIS-HCl (pH 7.2), 0.25 mM NADPH, 10 mM D-GalA or D-glucuronic acid, and the enzyme in a total volume of 1 ml. Aldonolactonase activity was also measured by the change in absorbance of p-nitrophenol through acidification at 405 nm essentially as described recently (Ishikawa et al., 2008).
For L-Gal dehydrogenase (GDH) activity, samples were prepared as described by Mieda et al. (2004) and assayed at 340 nm by measuring the reduction of NAD+ in a reaction mixture containing 0.5 mM NAD+, 1 mM L-Gal, and the enzyme extract. L-Galactono-1,4-lactone dehydrogenase (L-GalLDH) activity was assayed by the reduction of cytochrome c resulting in an increase in absorbance at 550 nm in a reaction mixture containing 50 mM TRIS-HCl, pH 8.5, 1 mM sodium azide, 42 mM L-Gal, 0.1% Triton X-100, 1.05 mg−1 ml cytochrome c, and the extract in a final volume of 1 ml as described by Yabuta et al. (2000).
Histochemical staining of AsA with acidic AgNO3
Longitudinal and transverse sections of Micro-Tom immature green or red fruits cut with a surgical razor were stained in a solution containing 5% AgNO3, 70% methanol, and 5% acetic acid, and fixed in a solution containing 70% methanol and 5% ammonia as described by Chinoy (1969). Samples pre-treated in a solution containing 5% CuSO4, 70% methanol, and 5% acetic acid to oxidize the AsA content of the Micro-Tom fruits were used as control. The images were captured with a Nikon digital camera DXM 1200F fixed to an Olympus microscope SZX16.
Protein extraction and immunoblot analysis
Proteins were extracted from Micro-Tom fruits with 50 mM potassium phosphate buffer containing 1 mM EDTA by grinding to a fine paste in a mortar. The slurry was clarified by centrifugation at 5000 rpm for 5 min. The protein concentration of the supernatant was assayed by the Bradford protein reagent (Bio-Rad, Hercules, CA, USA). Proteins (10 mg) were fractionated on a 12.5% SDS–polyacrylamide gel and then electroblotted onto a polyvinylidene diuoride membrane. The membrane was blocked with Odyssey Blocking Buffer (Li-Cor Bioscience, Lincoln, NE, USA) and then incubated with polyclonal antibody raised against His-VTC2 recombinant protein. The membrane was later washed in phosphate-buffered saline (PBS) containing 0.1% Tween-20 and then incubated with peroxidase-conjugated anti-rabbit secondary antibody. Luminescent Image Analyzer, ImageQuant LAS 4000 (GE lifescience, USA), was used for densitometry analysis of the immunoblot.
Results
Total AsA content of Micro-Tom is inversely correlated to the expression of D-Man/l-Gal pathway genes
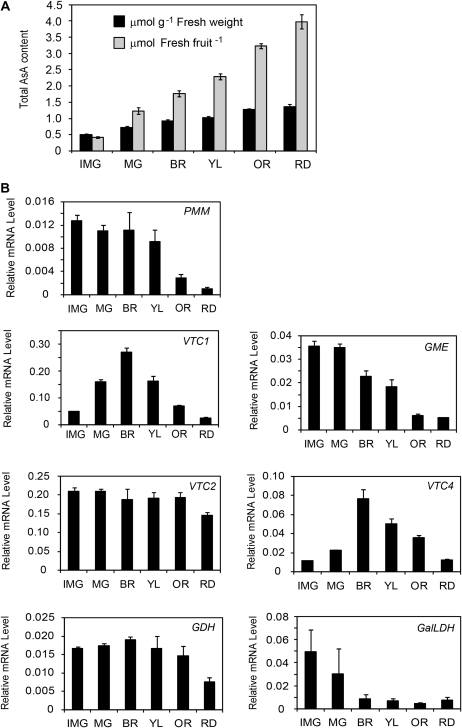
The fruits of Micro-Tom were harvested at different stages of ripening between 5 h and 7 h into the light photoperiod. The immature green fruits contained the lowest amount of AsA of ∼0.5 μmol per g fresh weight and 0.4 μmol per fresh fruit, while the red ripened mature fruits contained the highest amount of AsA of ∼1.5 μmol g−1 fresh weight and 4.0 μmol per fresh fruit, indicating that the AsA content in the fruits was positively correlated with the ripening stages (Fig. 2A). On the other hand, the transcript levels of most genes in the D-Man/l-Gal pathway were inversely correlated with the ripening stages of Micro-Tom fruits (Fig. 2B). The expression of phosphomannomutase (PMM), GDP-D-Man-3′,5′-epimerase (GME), and L-GalLDH was decreased with fruit ripening, while the expression of the GDP-L-gal phosphorylase (VTC2) gene remained almost constant throughout the ripening process. The expression of GDP-D-Man pyrophosphorylase (VTC1) and L-Gal 1-P phosphatase (VTC4) genes peaked at the breaker stage of ripening before decreasing all the way through to the red stage of ripening (Fig. 2B). The activity of the penultimate enzyme, GDH, in the D-Man/l-Gal pathway tended to show higher values in the immature green compared with the red fruits, while the reverse was the case for the terminal L-GalLDH activity (Table 1), suggesting that the D-Man/l-Gal pathway is still functional even in red ripened fruits.
Fig. 2.
The AsA content and expression of AsA biosynthesis genes in Micro-Tom fruits during ripening. (A) Fruits were harvested at six different stages of ripening: immature green (IMG), mature green (MG), breaker (BR), yellow (YL), orange (OR), and red (RD), and the total AsA content measured as μmol per fresh fruit and μmol per gram fresh weight. (B) The expression levels of the genes in the D-Man/l-Gal pathway for AsA biosynthesis. Total RNAs were isolated from the fruits above and transcribed into cDNA for use as template in the real-time PCR; the results were normalized to the expression of EF1α. Values are the mean ±SE (n=3).
Table 1.
Activities of D-mannose/L-galactose enzymes in extracts from Micro-Tom immature green (IMG) and red (RD) fruitsOne unit of enzyme activity is defined as the number of micromoles of NAD+ reduced per minute for L-galactose dehydrogenase activity and cytochrome c reduced per minute for L-galactono-1,4-lactone dehydrogenase activity. Values are mean ±SE of three independent determinations.
| IMG | RD | |
| L-Galactose dehydrogenase | 0.34±0.09 a | 0.26±0.05 a |
| L-Galactono-1,4-lactone dehydrogenase | 1.14±0.09 a | 1.89±0.15 b |
Values with different letters are significantly different according to t-test (P <0.05).
AsA is concentrated in the mesocarp of Micro-Tom fruits
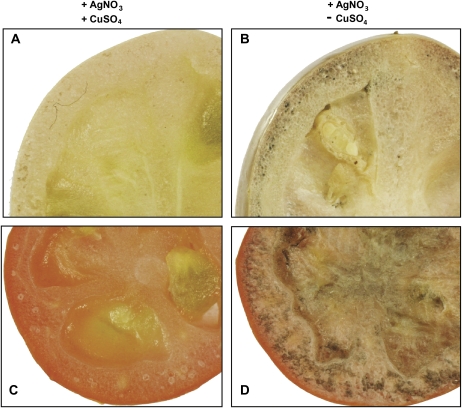
Histochemical staining was used to clarify the localization of the bulk of AsA in the green and red tomato fruits. The endogenous AsA in the control fruit pre-treated in CuSO4 solution was oxidized before treating it with AgNO3. The fruits treated directly with AgNO3 showed completely different images from those pre-treated with CuSO4 (Fig. 3). The fruits showed accumulation of AsA in the mesocarp, septum, and loculi of both the green and red fruits (Fig. 3B, D). The concentration of the dark patches seen in the red fruits stained with AgNO3 was higher compared with that of the scanty dark patches on the green fruit (Fig. 3B, D), which was in agreement with the fact that AsA content in the red fruits was higher in comparison with that in the green fruit (Fig. 2A).
Fig. 3.
Localization of AsA within Micro-Tom fruits. The control images of green (A) and red (C) fruits pre-treated with 5% CuSO4·5H2O and the methanolic AgNO3 stains showing dark patches around the membrane of the green (B) and red (D) fruits. The pictures shown are representative of three repetitions.
The effect of photosynthesis electron transport inhibitors on the accumulation of AsA and expression of the VTC2 gene in Micro-Tom fruits
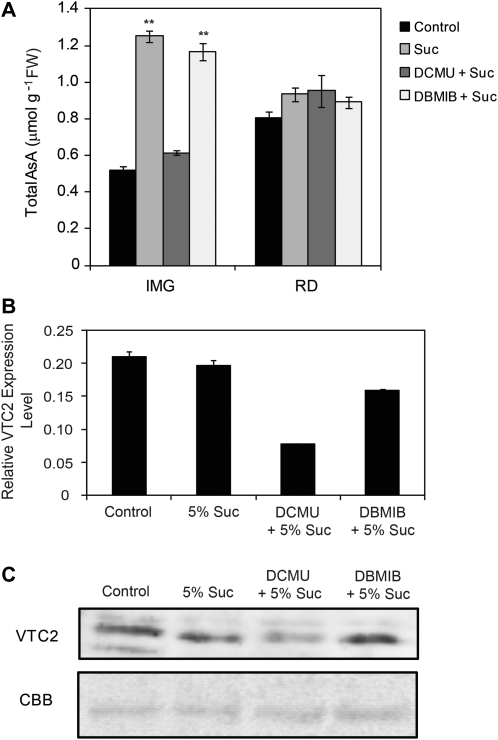
First the fruits were fed with 0.5% sucrose, but there was no significant difference between the AsA content of these fruits and those fed with water (Supplementary Fig. S1 available at JXB online). When the concentration of the sucrose was increased to 5%, the AsA contents of the fruits were more than 2-fold greater than those of the control fruits fed with water at the green and breaker stages and there was a >30% increase at the orange stage of ripening (Supplementary Fig. S1). The fruits were then treated with photosynthesis electron transport inhibitors, DCMU or DBMIB before sucrose feeding. The DCMU blocks the electron transfer from electron carriers QA to QB of photosystem II (PSII) into the plastoquinone (PQ) pool and then inhibits the water oxidation, thereby preventing reduction of the PQ pool. DBMIB, however, acts as an antagonist to PQ by occupying the QO site of the cytochrome b6f complex and thus inhibits the plastoquinol (reduced PQ; PQH2) oxidation and also blocking electron flow from PQH2 to PSI (Pfannschmidt et al., 1999). The capacity to accumulate AsA was reduced in the fruits treated with DCMU before feeding with sucrose. DCMU had no effect upon red ripened fruit, but the green fruit was particularly affected to such a level that the AsA content was ∼150% lower when compared with the fruit fed with only sucrose (Fig. 4A). On the other hand, treating the fruits with DBMIB before feeding with sucrose did not impair the capacity for AsA synthesis and accumulation when compared with the fruits that were fed with only sucrose (Fig. 4A). Since the expression of VTC2 was fairly constant in high or low AsA-containing fruits (Fig. 2B), and it is vital to AsA biosynthesis (Dowdle et al., 2007), the gene and protein expression of VTC2 was measured in the green fruits upon treatment with sucrose, DCMU, or DBMIB. Fruit treated with DCMU had VTC2 gene expression that was more than 50% lower than the untreated control fruit (Fig. 4B). Western blot analysis also showed lower expression of the VTC2 protein in the DCMU-treated fruit compared with the control (Fig. 4C).
Fig. 4.
Effect of inhibitors of photosynthetic electron transport, DCMU and DBMIB, and sucrose feeding on AsA levels. Fruits were treated with 10 μM of either DCMU or DBMIB, first in the dark for 30 min and then in the light for 3 h followed by subsequent transfer of the fruits to 5% sucrose solution for the next 48 h under continuous light. (A) The total AsA content of the immature green (IMG) and ripened red (RD) treated fruits. Values are the mean ±SE (n=3). The statistical significance of AsA was compared with the control (fed with water) fruits using the t-test, **P <0.01. (B) The transcript level of the VTC2 gene in the IMG fruits subjected to different treatments. The transcript levels were normalized to EF1α. (C) VTC2 protein content determined by immunoblot of 1 mg of protein from immature green fruits treated as described above. The Coomassie Brilliant Blue R250 (CBB) stain is shown as a loading control.
Translocation of exogenously applied L-[6-14C]AsA in Micro-Tom fruits
Since it was unclear if the AsA accumulated in the tomato fruits was derived from the source leaf or not, the plants containing tomato fruits were labelled with L-[6-14C]AsA at the leaf tips and incubated under light (Fig. 5A, B). It was anticipated that the strongest signal would be from the red ripened fruits since it had the highest AsA content in comparison with the green fruits (Fig. 2A). Counter to expectation, the bulk of the label from AsA was transported to the immature green fruits. The ripened fruits had the least signal of all the fruit contained on the labelled plants (Fig. 5C, D). The 14C from AsA was also transported through the stem to the flower buds and roots, but not to the leaves (Fig. 5B). Leaves showing signals were those closest to the site of labelling. When the amount of radioactivity in each of the fruits was determined it was discovered that the immature green fruit had the most while the red tomato had the lowest, ∼400 times lower than the dpm observed in the immature green fruit (Supplementary Fig. S2 at JXB online).
Fig. 5.
AsA translocation in a fruit-bearing Micro-Tom plant. The whole plant (A) and its autoradiograph (B) showing the distribution of labelled L-[6-14C]AsA after 24 h. The fruits were cut off before autoradiography of the whole plant so that the distribution could be viewed without distortion. Label from L-[6-14C]AsA was transported to the fruits, developing flowers, and roots, but not the source leaves. Tomato fruits (C) were dried in the oven at 55 °C for 8 h to minimize the flow of juice out of the fruits, before being exposed to Fuji film and autoradiographed (D) after 24 h of labelling with L-[6-14C]AsA. 1, Immature green fruit; 2–5, mature green fruits; and 6–9, mature red fruits. The pictures shown are representative of three repetitions.
l-Galactose and D-galacturonate, but not L-gulono-1,4-lactone, contributed to the total AsA content of Micro-Tom fruits
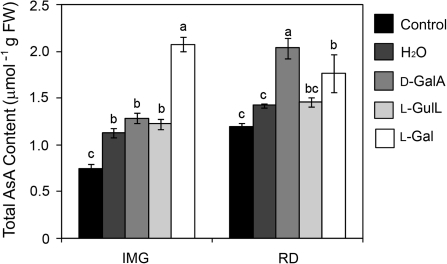
As neither the transcription levels of genes from the D-Man/l-Gal pathway nor the translocation of AsA from source to sink effectively account for the increase in the AsA content of ripened tomato fruits (Fig. 2), the possibility was considered of a contribution from alternative pathways and thus the fruits were fed with substrates from alternative pathways as well as the D-Man/l-Gal pathway. The green and red fruits were fed with 5 mM L-Gal, D-GalA, or L-gulono-1,4-lactone and were incubated under continuous light for 24 h. No significant difference was observed in the AsA content of the fruits fed with D-GalA, L-gulono-1,4-lactone, or H2O at the immature green stage; however, the AsA content of the green fruit fed with L-gal increased by >150% and 70% compared with the control fruits and those fed with H2O, respectively (Fig. 6). On the other hand, while there was no significant difference between the AsA contents of the red fruits fed with L-gulono-1,4-lactone or H2O, the AsA content of the red fruits fed with L-gal was ∼20% higher than that of those fed with H2O. Interestingly, the AsA content of the red fruit fed with D-GalA was ∼88% and 45% more than that of the control and H2O-fed fruits, respectively (Fig. 6). These results suggest that ripened tomato fruits, but not the immature ones, possess enzymes able to convert D-GalA effectively to AsA. D-GalA reductase, which catalyses the conversion of D-GalA to L-galactonic acid, is a soluble enzyme in non-climacteric strawberry fruits (Agius et al., 2003) and Euglena (Ishikawa et al., 2006b). On the other hand, though there is no report on aldonolactonase, which reversibly converts L-galactonic acid to L-galactono-1,4-lactone, in higher plants, similar enzymes exist in soluble fraction of rat (Kondo et al., 2006) and Euglena (Ishikawa et al., 2008), and in the membrane fraction of Gluconobacter (Shinagawa, et al., 2009). Therefore, experiments were carried out to examine whether climacteric tomato fruit possesses D-GalA reductase and aldonolactonase activities in the soluble or insoluble fractions. D-GalA reductase activity was detected in the insoluble fraction, but not the soluble fraction, of both the green and red fruits, with higher activity in the red fruits (Table 2). From the membrane fraction aldonolactonase activity was also found in both the green and red fruits, with the ability to utilize D-glucono-1,5-lactone, L-galactono-1,4-lactone, L-gulono-1,4-lactone, and L-galactonic acid as substrates (Table 2). The activities recorded when L-galactono-1,4-lactone and L-galactonic acid were used as substrates were more than doubled in the red ripened fruits compared with the immature green fruits. These results suggest that an alternative pathway different from the D-Man/l-Gal pathway, but not the D-glucuronic acid pathway, also contributes to AsA biosynthesis especially in the red ripened tomato fruits.
Fig. 6.
Effect of feeding D-Man/l-Gal and alternative pathway precursors to Micro-Tom fruits. The immature green fruits (IMG) and red fruits (RD) were fed with 5 mM L-GaL, D-GalA, or L-GulL and incubated under light (100 μmol photons m−2 s−1) for 24 h. The AsA content of the fruits at the start of the feeding experiment was used as control. Values are the mean ±SE (n=3). Different letters are used to show means that differ significantly (P <0.05).
Table 2.
Enzyme activities of extracts from membrane-bound fractions of immature green (IMG) and red (RD) Micro-Tom fruits. One unit of enzyme activity is defined as the number of micromoles of NADPH oxidized [for D-galacturate reductase (D-GalAR) activity] or p-nitrophenol decolourized by L-galactono-1,4- lactone, L-galactonic acid, l-gulono-1,4-lactone, and D-glucono-1,5-lactone [for aldonolactonase (Alase) activity]. Values are the mean ±SE of three independent determinations.
| Substrate | IMG | RD |
| D-GaIAR activity | ||
| D-Galacturonic acid | 22.02±0.86 a | 26.18±0.88 b |
| Alase activity | ||
| L-Galactono-1,4-lactone | 5.55±0.51 a | 12.38±1.38 b |
| L-Galactonic acid | 10.76±1.96 a | 21.39±2.97 b |
| L-Gulono-1,4-lactone | 16.19±0.89 a | 15.87±1.65 a |
| D-Glucono-1,5-lactone | 45.13±3.07 a | 45.36±3.78 a |
Values with different letters are significantly different according to t-test (P <0.05).
Discussion
Relationship between the D-Man/L-Gal pathway and AsA accumulation during Micro-Tom fruit ripening
The increases in the AsA pool size during the ripening of Micro-Tom and the AsA content in the mature ripened fruits are almost compatible with those seen in other tomato cultivar species reported so far (Jimenez et al., 2002; Ioannidi et al., 2009), indicating that Micro-Tom is also suitable as a model fruit for AsA pool size regulation in tomato (Fig. 2A). Histochemical staining of AsA in the green and red fruits showed AsA around the membrane, which is understandable as the green cells are rapidly undergoing development (Fig. 3). Dumville and Fry (2003) have reported that hydroxyl radicals generated by AsA resulted in non-enzymatic degradation of polysaccharides, leading to tomato fruit softening. Thus, the elevated level of AsA and the distribution around the mesocarp observed in the red ripened fruits is reasonable from the view point described by Dumville and Fry (2003).
Although the D-Man/l-Gal pathway is recognized as a major source of AsA supply in tomato as well as Arabidopsis, there is still no convincing evidence for the contribution from alternative pathways in either plant (Ishikawa et al., 2006a; Ishikawa and Shigeoka, 2008). The mRNA expression profile of the genes related to the D-Man/l-Gal pathway was down-regulated during fruit ripening and thus was not correlated with elevated AsA levels (Fig. 2B), suggesting a negative correlation of the pathway to increasing AsA levels in ripening fruits. A similar observation has been reported earlier by Ioannidi et al. (2009), with the exception of the mRNA expression level of the VTC4 gene that was elevated throughout the fruit ripening stages. The discrepancy in VTC4 gene expression could be attributed to the difference of tomato variety chosen for the analyses.
The possible relationship and impact of light and photosynthesis on the AsA content in tomato fruits have been reported (Davies and Hobson, 1981; Gautier et al., 2009). In Arabidopsis leaves, AsA contents change with light conditions and the VTC2 gene has been identified as key for light regulation of the D-Man/l-Gal pathway (Dowdle et al., 2007), and the gene expression changes depending on the redox status of the PQ pool in photosynthetic electron transport (Yabuta et al., 2007). It was demonstrated here that the availability of light as well as the redox status of PQ is vital to mediating photosynthetic control of AsA content in Micro-Tom, especially in the immature green fruits. It was found that the inhibition of water oxidation and prevention of PQ reduction by DCMU drastically affected the accumulation of AsA in Micro-Tom fruits, whereas inhibition of plastoquinol (reduced PQ) oxidation by DBMIB had little effect on AsA accumulation. The immature green fruits treated with DCMU before feeding with sucrose showed a decreased transcript level of the VTC2 gene as well as the protein level, thus leading to the observed decrease in AsA content. This showed that the VTC2 gene is indispensable in the biosynthesis of AsA in Micro-Tom fruits.
The contribution of sucrose to AsA biosynthesis
Sucrose can readily be hydrolysed to the hexoses, fructose and glucose, which may serve as substrate for AsA biosynthesis (Wheeler et al., 1998). As much as 10% (w/v) sucrose has been reported in the phloem of plants (Hayashi and Chino, 1990), and the presence of AsA in the phloem sap was confirmed by radiolabelling (Franceschi and Tarlyn, 2002). Feeding 10% (w/v) sucrose to cut broccoli halted the loss of AsA (Nishikawa et al., 2005), while feeding detached leaves with sucrose increased AsA content, but the mechanism is yet to be unravelled. The 5% sucrose fed to the tomato fruits may increase the respiration rate within the mitochondria, thus increasing the AsA content of the fruits. The contribution of sugar to the AsA content has been found to be genotype specific (Stevens et al., 2007; Massot et al., 2010). The presence of sugar within the plant acts as a potent signal that promotes gene expression (Hanson and Smeekens, 2009). It was reported that the expression of AsA biosynthetic genes in broccoli was enhanced upon feeding with sucrose (Nishikawa et al., 2005). Positive correlations between sucrose feeding and the expression level in some genes of the D-Man/l-Gal pathway, such as VTC1, VTC2, and L-GalLDH, can be observed in Micro-Tom fruits (Supplementary Fig. S1 at JXB online), also supporting the view that transportation of sugars from source tissues could affect the AsA content in sink tissues through the up-regulation of AsA biosynthesis pathway genes.
The rate of AsA translocation from the source leaves to sink fruits decreases with ripening
Long-distance transport of AsA from leaves of A. thaliana and Medicago sativa to the shoot, floral organs. and root tips has been reported (Franceschi and Tarlyn, 2002). The same transport of AsA from the phloem to the sink tissue has been reported in potato root tuber (Tedone et al., 2004). Therefore, the transport of AsA from the source leaves to the sink fruits leading to the elevated AsA level in ripened red Micro-Tom fruits was anticipated. The result was different however. More than 90% of the label from AsA was transported to the young developing immature green fruits (Fig. 5C, D). The autoradiograph showed labels in the stem, flower buds, and roots. Although the red ripened fruit had the highest AsA content (Fig. 2B), the bulk of it was not from the source leaf, as shown in the labelling result (Fig. 5) in which the red ripened fruits contained >1% of the label found in the immature green fruits (Supplementary Fig. S2 at JXB online). This showed that AsA is accumulated in abundance in the ripened tomato fruits and is independent of the source leaf. Translocation of label from AsA to immature green fruits might help boost the AsA pool size required for development together with in situ biosynthesis.
Contribution of the alternative D-GalA pathway to AsA biosynthesis in Micro-Tom fruits
Although the D-GalA pathway has been proposed in plants (Loewus and Kelly, 1961a, b; Agius et al., 2003), there is still no convincing evidence showing authentic activities for D-GalA reductase and aldonolactonase in fruit tissues. The present study showed that the ability to convert exogenously fed D-GalA to AsA was elevated in red ripened tomato fruits, and the two enzyme activities were detectable in the insoluble fraction of the red fruits (Table 1), suggesting strongly that the D-GalA pathway is induced depending on the fruit developmental stage. As ripening progresses, the amount of AsA found around the mesocarp increased greatly (Fig. 3) which may be partly due to pectin breakdown. D-GalA is an abundant component of the cell wall that results from the turnover of pectin in senescencing plant cells. Actually, recent metabolome analysis of tomato fruits showed an increasing accumulation of D-GalA accompanied by ripening (Carrari et al., 2006). It is also worth noting that an introgression line of tomato (Solanum pennellii) with higher AsA compared with the Solanum lycopersicum parent showed increased transcript levels of two polygalacturonase and pectinesterase genes, which might be correlated with D-GalA accumulation in tomato (Di Matteo et al., 2010). Perhaps the AsA flux through the D-GalA pathway is as a result of the availability of the substrate within the fruit cells. The possible contribution of D-GalA to AsA biosynthesis in ripe grape berries has also been reported (Melino et al., 2009) as well as light-dependent regulation of a putative GalAR gene in grapes (Cruz-Rus et al., 2010). While it is clear that photosynthesizing protists use the D-GalA pathway for AsA biosynthesis (Ishikawa et al., 2008), a contribution from the D-GalA pathway in some higher plants is still not convincing perhaps due to the lack of a report on aldonolactonase, a crucial enzyme in the pathway. Blast search with the Euglena aldonolactonase on the JGI website (http://genome.jgi-psf.org/) showed very high similarity to a gene from higher plants; castor oil (Ricinus communis) 49%, foxtail millet (Setaria italica) 47%, and Sorghum (Sorghum bicolor) 53%, confirming the presence of related genes within higher plants. Moreover, quantitative trait locus analysis has been used to identify major regions affecting AsA contents in tomato fruits (Stevens et al., 2007). These regions are distributed across chromosomes 2, 8, 9, 10, and 12, with strong candidates for controlling AsA contents. Since two of them included genes annotated by the known D-Man/l-Gal and AsA/glutathione pathway, it is conceivable that the rest of the candidate loci might include the unidentified enzymes for the D-GalA pathway.
Conclusion
The present study addressed the accumulation of AsA in tomato fruits during ripening. Novel conclusions were drawn relevant to understanding the regulation of the AsA pool size in tomato fruits. The translocation of label from AsA from the source leaf to the fruit contributed to the total AsA content of Micro-Tom fruits, especially in the young developing immature green fruits. Evidence is provided for the functionality of both the D-Man/l-Gal and D-GalA pathways in AsA biosynthesis of Micro-Tom fruits. Considering the journey so far, the community is closer now to establishing and associating different pathways with specific plant organs and/or developmental stages. Further investigation of the control of AsA translocation and the expression of dual pathways should reveal the switching mechanisms that determine the AsA pool size and thereby modulate the operational biosynthetic routes of AsA in tomato fruits which will ultimately enhance resistance to oxidative stress and aid in manipulating the organoleptic characteristics of fruits for the benefit of man through biotechnology.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Effect of sucrose feeding on AsA content and AsA biosynthesis gene expression of Micro-Tom fruits.
Figure S2. The distribution of L-[6-14C]AsA in tomato fruits (shown in Fig. 5C and D) at the immature green (IMG), mature green (MG), and red (RD) stages of ripening expressed as disintegration per minute per gram dry weight.
Acknowledgments
This work was partly supported by a Grant-in-aid for Scientific Research (A) (22248042 to SS) and (B) (21380207 to TI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the Japan Society for the Promotion of Science, Mishima Kaiun Memorial Foundation (grant to T.I.), and Postdoctoral Fellowships for Foreign Researchers by Japan Society for the Promotion of Science (P09342 to AAB).
References
- Agius F, Gonzalez-Lamonthe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by over-expression of a D-galacturonic acid reductase. Nature Biotechnology. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- Aoki K, Yano K, Suzuki A, et al. Large-scale analysis of full-length cDNAs from the tomato (Solanum lycopersicum) cultivar Micro-Tom, a reference system for the Solanaceae genomics. BMC Genomics. 2010;11:210. doi: 10.1186/1471-2164-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badejo AA, Fujikawa Y, Esaka M. Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff–Wheeler pathway in acerola (Malpighia glabra) Journal of Plant Physiology. 2009;166:652–660. doi: 10.1016/j.jplph.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin C-1. Plant Physiology. 2004;134:1784–1792. doi: 10.1104/pp.103.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley SM, Rassam M, Hoser D, Otto W, Schunemann N, Wright M, MacRae E, Gleave A, Laing W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. Journal of Experimental Botany. 2009;60:765–778. doi: 10.1093/jxb/ern327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, et al. Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiology. 2006;142:1380–1396. doi: 10.1104/pp.106.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy NJ. On the specificity of the alcoholic, acidic silver nitrate reagent for the histochemical localization of ascorbic acid. Histochemie. 1969;20:105–107. doi: 10.1007/BF00268703. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences, USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rus E, Botella MA, Valpuesta V, Gomez-Jimenez MC. Analysis of genes involved in L-ascorbic acid biosynthesis during growth and ripening of grape berries. Journal of Plant Physiology. 2010;167:739–748. doi: 10.1016/j.jplph.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Davies JN, Hobson GE. The constituents of tomato fruit—the influence of environment, nutrition, and genotype. Critical Reviews in Food Science and Nutrition. 1981;15:205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- Davey MD, Van Montagu M, Inze D, Sanmartin M, Kanellis AK, Smirnoff N, Benzie IJJ, Strain JJ, Favel D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 2000;80:825–860. [Google Scholar]
- Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Annals of Botany. 2007;99:3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo A, Sacco A, Anacleria M, Pezzotti M, Delledonne M, Ferrarini A, Frusciante L, Barone A. The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant Biology. 2010;10:163. doi: 10.1186/1471-2229-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Fry SC. Solubilisation of tomato fruit pectins by ascorbate: a possible non-enzymic mechanism of fruit softening. Planta. 2003;217:951–961. doi: 10.1007/s00425-003-1061-0. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H, Massot C, Stevens R, Serino S, Genard M. Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Annals of Botany. 2009;103:495–504. doi: 10.1093/aob/mcn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Smeekens S. Sugar perception and signaling—an update. Current Opinion in Plant Biology. 2009;12:562–567. doi: 10.1016/j.pbi.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Chino M. Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant and Cell Physiology. 1990;31:247–251. [Google Scholar]
- Imai T, Ban Y, Terakami S, Yamamoto T, Moriguchi T. L-Ascorbate biosynthesis in peach: cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiologia Plantarum. 2009;136:139–149. doi: 10.1111/j.1399-3054.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. Journal of Experimental Botany. 2009;60:663–678. doi: 10.1093/jxb/ern322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum. 2006a;126:343–355. [Google Scholar]
- Ishikawa T, Masumoto I, Iwasa N, Nishikawa H, Sawa Y, Shibata H, Nakamura A, Yabuta Y, Shigeoka S. Functional characterization of D-galacturonic acid reductase, a key enzyme of the ascorbate biosynthesis pathway. from Euglena gracilis. Bioscience, Biotechnology, and Biochemistry. 2006b;70:2720–2726. doi: 10.1271/bbb.60327. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nishikawa H, Gao Y, Sawa Y, Shibata H, Yabuta Y, Maruta T, Shigeoka S. The pathway via D-galacturonate/L-galactonate is significant for ascorbate biosynthesis in Euglena gracilis: identification and functional characterization of aldonolactonase. Journal of Biological Chemistry. 2008;283:31133–31141. doi: 10.1074/jbc.M803930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Bioscience, Biotechnology, and Biochemistry. 2008;72:1143–5114. doi: 10.1271/bbb.80062. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K, Van Montagu M, Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, Goto S, Nishikimi M, Maruyama N, Ishigami A. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proceedings of the National Academy of Sciences, USA. 2006;103:5723–8572. doi: 10.1073/pnas.0511225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Clarke SG. l-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends in Plant Science. 2008;13:567–573. doi: 10.1016/j.tplants.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus FA, Kelly S. Identity of l-ascorbic acid formed from d-glucose by the strawberry (Fragaria) Nature. 1961a;191:1059–1061. doi: 10.1038/1911059a0. [DOI] [PubMed] [Google Scholar]
- Loewus FA, Kelly S. The metabolism of D-galacturonic acid and its methyl ester in the detached ripening strawberry. Archives of Biochemistry and Biophysics. 1961b;95:483–493. doi: 10.1016/0003-9861(61)90180-1. [DOI] [PubMed] [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler C. myo-inositol oxygenase offers a possible entry point into plant AsA biosynthesis. Plant Physiology. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot C, Genard M, Stevens R, Gautier H. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiology and Biochemistry. 2010;48:751–757. doi: 10.1016/j.plaphy.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S. Feedback inhibition of spinach L-galactose dehydrogenase by L-ascorbate. Plant and Cell Physiology. 2004;45:1271–1279. doi: 10.1093/pcp/pch152. [DOI] [PubMed] [Google Scholar]
- Melino VJ, Soole KL, Ford CM. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biology. 2009;9:145. doi: 10.1186/1471-2229-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa F, Kato M, Hyodo H, Ikoma Y, Sugiura M, Yano M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. Journal of Experimental Botany. 2005;56:65–72. doi: 10.1093/jxb/eri007. [DOI] [PubMed] [Google Scholar]
- Noctor G. Metabolic signaling in defence and stress: the central roles of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF. Photosynthetic control of chloroplast gene expression. Nature. 1999;397:625–628. [Google Scholar]
- Shinagawa E, Ano Y, Yakushi T, Adachi O, Matsushita K. Solubilization, purification, and properties of membrane-bound D-glucono-δ-lactone hydrolase from. Gluconobacter oxydans. Bioscience, Biotechnology, and Biochemistry. 2009;73:241–244. doi: 10.1271/bbb.80554. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology. 2000;3:229–235. [PubMed] [Google Scholar]
- Stevens R, Buret M, Duffé P, Garchery C, Baldet P, Rothan C, Causse M. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiology. 2007;143:1943–1953. doi: 10.1104/pp.106.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R. Long-distance transport of L-ascorbic acid in potato. BMC Plant Biology. 2004;4:16. doi: 10.1186/1471-2229-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu M. GDP-mannose 3',5'-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. Journal of Biological Chemistry. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. Journal of Experimental Botany. 2007;58:2661–2671. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Yoshimura K, Takeda T, Shigeoka S. Molecular characterization of tobacco mitochondrial l-galactono-gamma-lactone dehydrogenase and its expression in. Escherichia coli. Plant and Cell Physiology. 2000;41:666–675. doi: 10.1093/pcp/41.6.666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.