Abstract
Leaf senescence is a highly regulated developmental process that is coordinated by several factors. Many senescence-associated genes (SAGs) have been identified, but their roles during senescence remain unclear. A sweet potato (Ipomoea batatas) SAG, named SPA15, whose function was unknown, was identified previously. To understand the role of SPA15 in leaf senescence further, the orthologue of SPA15 in Arabidopsis thaliana was identified and characterized, and it was named ARABIDOPSIS A-FIFTEEN (AAF). AAF was expressed in early senescent leaves and in tissues with highly proliferative activities. AAF was localized to the chloroplasts by transient expression in Arabidopsis mesophyll protoplasts. Overexpression of AAF (AAF-OX) in Arabidopsis promoted, but the T-DNA insertion mutant (aaf-KO), delayed age-dependent leaf senescence. Furthermore, stress-induced leaf senescence caused by continuous darkness was enhanced in AAF-OX but suppressed in aaf-KO. Transcriptome analysis of expression profiles revealed up-regulated genes related to pathogen defence, senescence, and oxidative stress in 3-week-old AAF-OX plants. Indeed, elevated levels of reactive oxygen species (ROS) and enhanced sensitivity to oxidative and dark stress were apparent in AAF-OX but reduced in aaf-KO. ETHYLENE INSENSITIVE2 (EIN2) was required for the dark- and ROS-induced senescence phenotypes in AAF-OX and the induction of AAF expression by treatment with the immediate precursor of ethylene, 1-aminocyclopropane-1-carboxylic acid. The results indicate the functional role of AAF is an involvement in redox homeostasis to regulate leaf senescence mediated by age and stress factors during Arabidopsis development.
Keywords: Arabidopsis thaliana, chloroplast, ethylene, leaf senescence, oxidative stress, reactive oxygen species, senescence-associated gene (SAG)
Introduction
Leaf senescence is a complex and highly regulated process in the final stage of plant development. One of the visible symptoms of senescence is leaf yellowing because of loss of chlorophyll (Smart, 1994). Environmental stresses, including heat, cold, drought, shedding, pathogen infection, and irradiation, induce leaf senescence (Lim et al., 2007). In addition, the regulation of leaf senescence by developmental signals mainly depends on phytohormones and age (reviewed in Buchanan-Wollaston, 1997; Lim et al., 2007). A number of senescence-associated genes (SAGs) have been identified to study the regulatory mechanism of leaf senescence (He et al., 2001; Buchanan-Wollaston et al., 2003; Gepstein et al., 2003). However, the physiological role of many SAGs has yet to be defined.
Free radicals are proposed to play an essential role in leaf senescence (Strother, 1988). Reactive oxygen species (ROS) are toxic by-products of aerobic metabolism and include singlet oxygen (1O2), superoxide radical (O2·–), hydroperoxyl radical (HO2·–), hydrogen peroxide (H2O2), and hydroxyl radical (OH·). Many biological activities in plants lead to accumulation of ROS (reviewed in Mittler et al., 2004; Foyer and Noctor, 2009). Several biotic and abiotic stresses induce abrupt elevated ROS levels in plant cells. The hypersensitive response is a well-documented example of the rapid accumulation of H2O2 (oxidative burst) leading to cell death to prevent the spread of pathogens (Lamb and Dixon, 1997). The elevated level of H2O2 acts as the signal molecule eliciting plant responses to various stresses (Orozco-Cardenas et al., 2001). Therefore, ROS are destructive by-products and also play a role in the activation of gene expression in response to pathogens and various abiotic stresses (Miller et al., 2008, 2010). However, the mechanistic role of ROS in modulating leaf senescence remains unknown.
Plants react to oxidative stress by activating a series of antioxidative enzymes such as catalases, superoxide dismutases (SODs), and components of the ascorbate–glutathione cycle to maintain cellular redox homeostasis (Mittler, 2002). Perturbation of these antioxidants results in oxidative damage that may lead to cell death. Excessive ROS are present in chloroplasts of ageing plants (Munné and Alegre, 2002). The coordinated increase in membrane permeability, lipid peroxidation, and reduced activities of SODs and catalases occurs during leaf senescence (Dhindsa et al., 1981). The connection between oxidative stress and leaf senescence is further supported by observations of the induction of several representative SAGs in response to oxidative stress (Miller et al., 1999; John et al., 2001; Navabpour et al., 2003) and mutants showing delayed leaf senescence, such as ore1, ore3/ein2, and ore9, being more resistant to oxidative stress (Woo et al., 2004; Cao et al., 2006). Therefore, disruption of cellular redox homeostasis can lead to leaf senescence in plants. Only a few reports have described the regulatory role of ROS homeostasis in leaf senescence. Arabidopsis cpr5/old1 shows accelerated leaf senescence during vegetative stages by induction of stress response leading to cell death due to a perturbed redox balance (Jing et al., 2008). Arabidopsis XDH1 encodes xanthine dehydrogenase involved in purine catabolism, and the xdh1 mutant showed premature senescence symptoms, elevated ROS levels, and a higher mortality rate than the wild type when stressed plants recovered from dark treatment (Brychkova et al., 2008).
A SAG was previously identified in sweet potato (Ipomoea batatas) by a differential display approach and it was named SWEET POTATO A15 (SPA15) (Yap et al., 2003). However, the physiological function of SPA15 was not clear. Here the characterization of a SPA15 orthologue in Arabidopsis thaliana, named ARABIDOPSIS A FIFTEEN (AAF), is reported. AAF has a predicted chloroplast transit peptide and was shown to be a plastid protein by transient expression assay in Arabidopsis mesophyll protoplasts. Overexpression of AAF resulted in an elevated level of cellular ROS, enhanced sensitivity to oxidative stress, and promoted leaf senescence induced by continuous darkness and by age-dependent signalling in plants. In addition, T-DNA insertion mutants of AAF were more resistant to oxidative stress and showed delayed leaf senescence mediated by developmental signalling or induced by darkness. Finally, it was shown that leaf senescence regulated by AAF depended on a functional EIN2. The results support AAF as a novel component involved in modulation of redox homeostasis that responds to developmental and stress-induced signals to regulate leaf senescence.
Materials and methods
Materials and plant growth conditions
All of the transgenic lines and mutants in this study were derived from the wild-type A. thaliana Columbia (Col-0) ecotype and cultivated in growth chambers under long days (LDs; 16 h light/8 h dark) or short days (SDs; 12 h light/12 h dark) at 22 °C under fluorescence illumination (100–150 μE m−2 s−1). Seeds were sterilized by 10% bleach for 20 min, and then rinsed with distilled water. The sterilized seeds were stratified in the dark at 4 °C for 3 d and germinated on half-strength Murashige and Skoog (0.5× MS) medium (pH 5.7) supplemented with 1% sucrose and 0.8% (w/v) agar. Two T-DNA insertion mutants of AAF (At1g66330) were obtained from the Arabidopsis Biological Resource Center (WiscDsLox453-456F9 and GABI-Kat 762H05) and homozygosity was verified by genotyping with gene-specific primers (Supplementary Table S2 available at JXB online). To generate AAF-OX/ein2-5, ein2-5 was crossed to AAF-OX (AAF overexpression), and F2 plants were scored for ethylene insensitivity to identify homozygous ein2-5 followed by genotyping of 35S::AAF. For rapid PCR screening of mutants and transgenic plants, genomic DNA was extracted for analysis as described (Murray and Thompson, 1980).
Flowering time was scored by days after germination until the inflorescence reached 1 cm in height. The length of primary roots and density of root hairs of 5-day-old seedlings were measured and analysed by National Institutes of Health ImageJ software (http://imagej.nih.gov/ij/). For dark treatment, 3-week-old plants or detached leaves were incubated in a dark controlled environment (70% relative humidity at 22 °C) until samples were collected at the indicated times. For hormone treatments, 7-day-old light-grown seedlings were transferred to 0.5× MS liquid medium for 24 h before addition of various hormones, and samples were collected at the indicated times for extraction of total RNA. 1-Aminocyclopropane-1-carboxylic acid (ACC) was purchased from Calbiochem (Merck), and other chemicals, including abscisic acid (ABA), methyl jasmonate (MeJA), and salicylic acid (SA), were from Sigma, unless otherwise indicated.
Plasmid construction and plant transformation
To generate the overexpression construct of AAF under the control of a Cauliflower mosaic virus (CaMV) 35S promoter, the full-length coding sequence of AAF was amplified by PCR with the primers ATA15SpeIF1 and ATA15PmlIR2, and digested by SpeI and PmlI. The SpeI/PmlI fragment was subcloned into SpeI and PmlI sites of pCAMBIA1302z for 35S::AAF. To construct the promoter fusion of AAF with β-glucuronidase (GUS) for analysis of tissue-specific expression profiles, a DNA fragment containing 2020 bp from 5′ upstream of AAF including 220 bp of 5′-untranslated region (UTR) was amplified by PCR with the primers ATA15-GFP-f1 and ATA15-GFP-r1, and digested with EcoRI and BamHI. The EcoRI/BamHI fragment was subcloned to pCAMBIA1391z Xb for AAFpro::GUS. These constructs were introduced into the Agrobacterium tumefaciens strain LBA4404 or GV3101 for subsequent transformation to Arabidopsis by the floral-dip method (Clough and Bent, 1998). Transgenic plants were selected by hygromycin (25 μg ml−1) resistance and propagated to homozygous lines. The coding sequence of AAF or SPA15 was amplified by PCR with the following primers: ATA15F8 and ATA15R9 for full-length cDNA of AAF (1–417); ATA15-41F and ATA15R9 for AAF41–417; ATA15FL-attB1F and ATA1540-attB2R for AAF1–41; and SPA15-attB1F and SPA15-attB1R for the full-length cDNA of SPA15 (1–426). The Gateway system (Invitrogen) was used to generate various AAF and SPA15 constructs for use in the transient expression assay with Arabidopsis mesophyll protoplasts. The DNA fragments were subcloned to pCR8/GW/TOPO or pDONR221 by BP reactions as the entry clones, which were subsequently cloned to a pPZP-based destination vector by LR reactions for the three 35S::AAF–GFP (green fluorescent protein) constructs used in Fig. 2. All of the primers described above are listed in Supplementary Table S2 at JXB online.
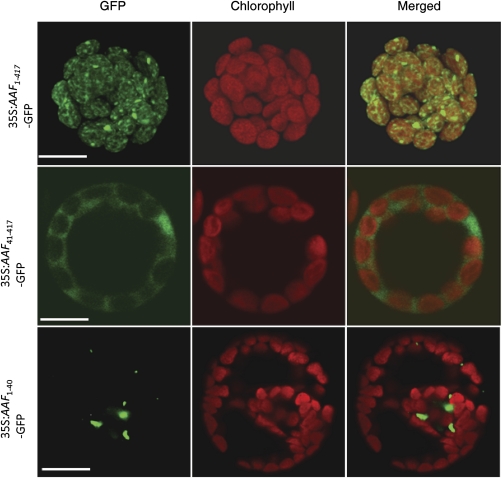
Fig. 2.
Localization of AAF in chloroplasts. Arabidopsis mesophyll protoplasts were transfected with the following constructs: 35S::AAF1–417–GFP (top); 35S::AAF41–417–GFP (middle); and 35S::AAF1–40–GFP (bottom). First column: fluorescence images with pseudocolouir for GFP (green channel). Second column: autofluorescence of chloroplasts (red channel). Third column: merged micrographs of red and green images. Experiments were repeated three times and representative data are shown. Bars=10 μm.
β-Glucuronidase assay and protoplast transient assay for protein localization
Seedlings and plant organs collected at different developmental stages were incubated with 0.5 mg ml−1 5-bromo-4-chloro-indolyl-β-D-glucuronide (X-Glu) in 100 mM phosphate buffer (pH 7.0) at 37 °C overnight. Chlorophyll was removed from leaves by clarification with 70% ethanol before observation. Preparation of Arabidopsis mesophyll protoplasts and subsequent transfection by polyethylene glycol (PEG)-4000 for transient expression assay were performed as described (Sheen, 2001). Fluorescence images of GFP fusions were acquired by confocal laser scanning microscopy (Zeiss LSM 510 Meta).
Assays for age- and dark-induced leaf senescence
For age-dependent leaf senescence, the third and fourth rosette leaves of individual plants were used for analyses of chlorophyll content, photochemical efficiency, and membrane ion leakage. Leaves were collected and incubated in 96% (v/v) ethanol (3 mg of tissue in 1 ml of ethanol) at room temperature in the dark for 30 min. After centrifugation, the supernatant was used for quantification of chlorophyll levels by spectrophotometry (U-2001, Hitachi) as described (Wintermans and de Mots, 1965). The photochemical efficiency of photosystem II (PSII) activity was measured by a hand-held chlorophyll fluorimeter (Pocket PEA, Hansatech Instruments). Membrane ion leakage was analysed by electrolytes released from leaves by using a bench-top conductivity meter (CON500, CLEAN Instruments). Four leaf discs were removed from designated leaves of experimental plants, and the third and fourth leaves were used in the age-dependent senescence assay in Fig. 3 and the fifth and sixth leaves in the dark-induced senescence assay in Fig. 5. These discs were thoroughly washed by deionized water three times, followed by immersion in 25 ml of 400 mM mannitol at 22 °C for 3 h with gentle shaking before measuring the initial conductivity. Total conductivity was measured after five freeze–thaw cycles using liquid nitrogen and a water bath set at 25 °C for maximum membrane disruption. The conductivity resulting from membrane ion leakage is presented as the percentage of initial conductivity versus total conductivity. These experiments were repeated three times with consistent results, and a representative set of data is shown with standard errors. For dark-induced leaf senescence, the fifth and sixth leaves of 4-week-old plants were collected and incubated in 3 mM MES buffer (pH 5.8) for continuous darkness treatment for 5 d. Analyses for leaf senescence by measuring chlorophyll levels, photochemical activity, and membrane ion leakage were performed as described above.
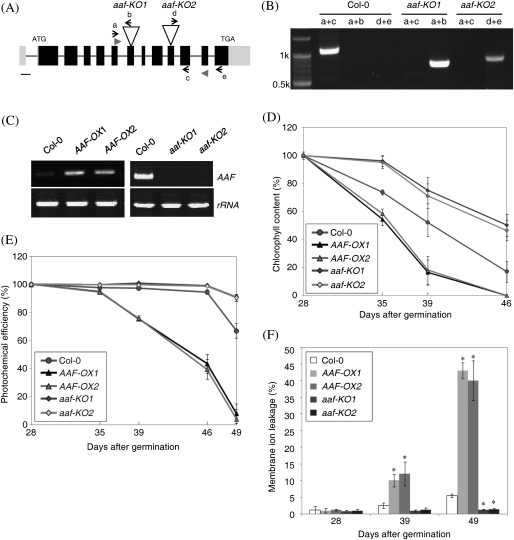
Fig. 3.
Age-dependent senescence is promoted by overexpression of AAF but delayed in aaf-KO mutants. (A) The genomic structure of AAF and the T-DNA insertion sites for the aaf-KO1 and aaf-KO2 mutants. Filled boxes, exons; full lines, introns; grey boxes at both ends, the 5′- and 3′-untranslated regions. Bar=100 bp. (B) Genotyping of aaf-KO1 and aaf-KO2 with the primers a, b, c, d, and e indicated in A. (C) RT-PCR analysis to verify the presence of AAF transcript in AAF-OX (mature green leaves) and aaf-KO (flower buds). AAF transcript was amplified by a pair of gene-specific primers, AAF-F and AAF-R, represented by arrowheads in A. The third and fourth leaves were collected from plants at the number of days after germination as indicated to analyse age-dependent leaf senescence by measuring the levels of chlorophyll content (D), photochemical efficiency of PSII (E), and membrane ion leakage (F). *P <0.01 versus the wild type, Col-0 (n=4–6). The measurement of chlorophyll content (D) and photochemical activity (E) was set as 100% at 28 days after germination for comparison with samples collected thereafter.
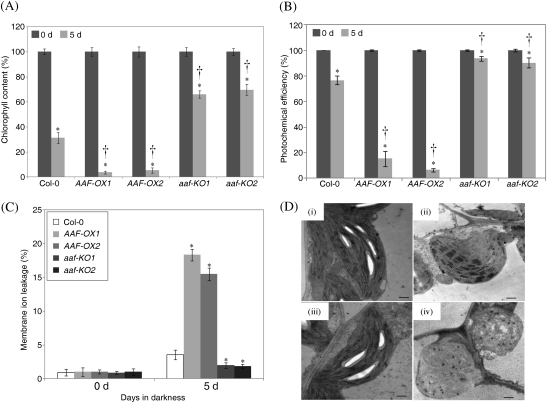
Fig. 5.
Leaf senescence induced by continuous darkness is enhanced in AAF-OX. (A–C) Dark-induced senescence was analysed by using detached leaves from 4-week-old plants. The fifth and sixth leaves were excised and incubated in darkness for 5 d followed by measuring the chlorophyll content (A), photochemical efficiency (B), and membrane ion leakage (C). *P <0.01, 5 d versus 0 d (n=6); †P <0.01, AAF-OX or aaf-KO versus the wild type (Col-0) by the percentage of chlorophyll reduction after dark treatment (n=6). Data represent means ±SE. (D) Electroscopic analysis of 3-week-old plants treated with continuous darkness for 5 d (ii and iv; wild type and AAF-OX, respectively) or without dark treatment (i and iii; wild type and AAF-OX). Bars=0.5 μm.
Electron microscopy
The fifth or sixth leaves of 3-week-old plants were fixed in 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.0, at room temperature for 4 h. After three 20 min rinses, the samples were fixed in 1% OsO4 in the same buffer for 4 h, then rinsed three times with buffer. Samples were dehydrated in an acetone series, embedded in Spurr's resin, and sectioned with use of a Leica Reichert Ultracut S or Leica EM UC6 ultramicrotome (Leica Microsystems GmbH). The ultrathin sections (70–90 nm) were stained with uranyl acetate and lead citrate. A Philips CM 100 TEM (FEI Company) at 80 kV was used for viewing.
Chemical treatments with oxidative stress-inducing agents and analysis of cellular levels of ROS
All the experiments on detached leaves were performed with the fifth and sixth rosette leaves of 3-week-old plants (counting from the true leaves). Detached leaves were incubated in 3 mM MES buffer (pH 5.8) in the presence of oxidative stress-inducing reagents, H2O2 and paraquat, then treated leaves were ground in liquid nitrogen, and the chlorophyll content was determined by spectrophotometry.
To visualize H2O2 in situ, DAB (3,3′-diaminobenzidine) was infiltrated into detached leaves and stained leaves as described (Rea et al., 2004). For the detection of ROS by fluorescence, 7-day-old seedlings were transferred to 0.5× MS liquid medium with 50 μM 2′,7′-dichlorofluorescein diacetate (DCFDA; Invitrogen) for 30 min in the dark. After being briefly washed with distilled water, the roots were examined for fluorescence by confocal laser scanning microscopy (Zeiss LSM 510 Meta) with excitation at 488 nm and emission at 500–530 nm.
Quantification of H2O2 and MDA
The Amplex Red Hydrogen Peroxide/Peroxidase Assay kit (Molecular Probes) was used to quantitate H2O2 levels. Plant leaves were ground to powder with liquid nitrogen, and 30 mg of tissues were mixed with 200 μl of phosphate buffer (20 mM K2HPO4, pH 6.5). After centrifugation, 50 μl of the supernatant was incubated with 50 μl of working solution consisting of 100 μM Amplex Red reagent and 0.2 U ml−1 horseradish peroxidase at room temperature for 30 min under dark conditions. The fluorescence was quantified by use of a plate reader (Plate CHAMELEON, Hidex Oy) with excitation at 545 nm and emission at 580 nm.
For quantification of MDA, 100 mg of leaf tissue was ground to powder with liquid nitrogen and extracted by 1 ml of 20 mM phosphate buffer (pH 7.4). Butylated hydroxytoluene (10 μl, 0.5 M) in acetonitrile was added to 1 ml of tissue homogenate to prevent sample oxidation, and 200 μl of supernatant was used for assay after centrifugation. A colorimetric assay for lipid peroxidation was used to quantitate MDA levels following the manufacturer's protocol (Oxford Biomedical Research).
Microarray experiments, statistical analysis and RT-PCR
Microarray experiments were performed by use of an Arabidopsis ATH1 GeneChip (Affymetrix). Rosette leaves of 3-week-old plants were used to prepare RNA samples. At this stage, no senescent phenotype was visible. RNA preparation, labelling, and hybridization followed the instructions of an in-house core facility (http://ipmb.sinica.edu.tw/affy/). Data from two independent biological replicates were imported to Genespring GX10 software and analysed by the RMA method. Genes were selected by at least a 1.5-fold change of signals compared with the wild type and if present in two biological replicates. The distribution of Gene Ontology (GO) annotations of genes up-regulated in AAF-OX was processed by the web-based program at TAIR (http://www.arabidopsis.org). GO-specific terms were investigated by use of AmiGO (http://amigo.geneontology.org) and the GO database released in October 2009 (Ashburner et al., 2000). P-values were calculated by Fisher's test (http://www.langsrud.com/fisher.htm) (Agresti, 1992). Statistical analysis involved Student's t-test. A significant difference between the control and experimental groups was considered with P <0.05.
Total RNA was extracted by TRIzol reagent, and reverse transcription was performed by using 1 μg of RNA at 50 °C by MMLV (TOYOBO) reverse transcriptase according to the manufacturer's protocol. RT-PCR was performed with gene-specific primers (Supplementary Table S2 at JXB online). Real-time PCR analyses were performed with a qPCR machine (MX3000P, Stratagene) in a volume of 20 μl, including qPCR master mix (Kapa Biosystems), 0.2 μM of gene-specific primers (Supplementary Table S3), and 50 nM ROX™ as a passive reference dye for factor calibration. Data were normalized to the expression of ACTIN2 (At3g46520).
Results
Expression patterns of AAF in Arabidopsis
SPA15 was classified as a SAG isolated from senescent leaves of sweet potato with unknown functions (Huang et al., 2001). The expression of SPA15 and its orthologue in rice was induced in senescing leaves, which suggests a physiological role for SPA15 in leaf senescence. To study further the function of SPA15 in a reference plant, AAF was identified as the A. thaliana orthologue of SPA15. AAF is encoded by a single locus based on the latest release of the Arabidopsis genome database (TAIR10). AAF is a plant-specific gene present in both monocots and dicots, and encodes a novel protein without any defined domain of known function. Protein alignment revealed that two-thirds of the C-terminal region of AAF (amino acids 118–417) was highly conserved in orthologues from different species with sequence identity >70%, except for the moss (50% identity), Physcomitrella patens, which has extended sequences at both its N- and C-termini (Supplementary Fig. S1 at JXB online). Interestingly, phylogenetic analysis indicated that AAF was related more to monocots and P. patens than to dicots (Supplementary Fig. S2). However, AAF and its dicotyledon orthologues seem to have a chloroplast-targeted signal peptide as predicted by TargetP1.1 (http://www.cbs.dtu.dk/) (Supplementary Table S1).
To determine whether AAF is regulated by senescence factors, the expression pattern of AAF was analysed in four stages during leaf development. The expression of AAF was induced in early senescent leaves but not in mature green (MG) or late senescent leaves (Fig. 1A). SAG12 was used as a senescence marker (Lohman et al., 1994). Therefore, it was confirmed that AAF is also a SAG in Arabidopsis, similar to its orthologues in rice and sweet potato (Yap et al., 2003). However, AAF was also expressed in young leaves (Fig. 1A, Y), which suggests that an additional function of AAF may be present in developmental stages other than organ senescence. To understand further the physiological role of AAF in non-senescent tissues, expression of AAF was analysed in different tissues. AAF was found to be expressed in flower buds, cauline leaves, rosette leaves, roots, and seedlings, but not in flowers, siliques, or stems, by RT-PCR analysis (Fig. 1B). Furthermore, histochemical staining of transgenic plants containing an AAF promoter fusion with the uidA gene encoding the enzyme GUS, AAFpro::GUS, was used to examine the expression of AAF in planta. AAF was expressed in the emerging true leaves (Fig. 1C, i and ii) and the elongation zone of primary and lateral roots in 7-day-old seedlings (i and iii). Moreover, AAF was expressed in cauline leaves (iv), young leaves (v), and senescent leaves (vii), but not in cotyledons (i), MG leaves (vi), or siliques (ix), which was fairly consistent with the expression profiles in Fig. 1B. Interestingly, AAF was expressed in the immature anthers of flower buds (Fig. 1C, viii; Fig. 1B, FB), but not in open flowers (Fig. 1B, F). Histochemical results indicated that AAF is expressed in various organs and leaves during different development stages and is not restricted to senescent leaves.
Fig. 1.
Expression patterns of AAF in different developmental stages. (A) RT-PCR analysis of the AAF transcript in different stages of leaf development. Y, young; MG, mature green; ES, early senescence; LS, late senescence. (B) RT-PCR analysis of AAF in different organs. F, flowers; FB, flower buds; SL, siliques; CL, cauline leaves; ST, stems; RL, rosette leaves; R, roots; D7S, 7-day-old seedlings. Rosette leaves were taken from the aerial part of 5-week-old plants including Y and MG leaves. 18S rRNA was used as a loading control in A and B. (C) GUS staining of transgenic Arabidopsis with the AAFpro::GUS construct. (i) Seven-day-old seedlings. (ii) First true leaves. (iii) Lateral roots. (iv) Cauline leaves. (v) Young leaves. (vi) Mature green leaves. (vii) Early senescent leaves. (viii) Immature flower buds. (ix) Siliques. Bars = 1 mm (i, iv, v, vi, vii, and ix) or 0.1 mm (ii, iii, and viii).
AAF is a plastid protein and the N-terminal signal peptide sequence is required for plastid targeting
Computational analysis with TargetP1.1 and ChloroP1.1 to predict the potential subcellular localization of AAF and its orthologues showed that all except for those in maize, rice, and moss have an N-terminal putative transit peptide for chloroplast localization (Supplementary Table S1 at JXB online) (Nielsen et al., 1997; Emanuelsson et al., 2000). AAF contains a 36-residue peptide at the N-terminus predicted as a chloroplast localization signal. To validate the prediction of AAF subcellular localization, AAF was fused with GFP under the control of the 35S promoter from CaMV, CaMV35S::AAF:GFP, and transient expression assays were performed with Arabidopsis mesophyll protoplasts. AAF:GFP was localized in chloroplasts (Fig. 2, top panel). Deletion of 40 residues at the N-terminus (AAF41–417:GFP) to remove the predicted transit peptide resulted in loss of chloroplast localization (Fig. 2, middle panel). However, the N-terminal 40 amino acids were not sufficient to direct the AAF1–40:GFP fusion protein to chloroplasts (Fig. 2, bottom panel). Thus, AAF is a chloroplast protein in Arabidopsis, and the predicted transit peptide is essential but not sufficient for targeting to chloroplasts.
A 68-residue signal peptide for chloroplast targeting was predicted in SPA15 (Supplementary Table S1 at JXB online), suggesting the subcellular localization of SPA15 in plant cells. However, SPA15 was detected to associate specifically with the cell wall of various cell types, predominantly in senescent sweet potato leaves, by immunoelectron microscopic analysis (Yap et al., 2003). To test the functionality of the predicted transit peptide in SPA15, a GFP fusion of SPA15 under the control of the CaMV 35S promoter, CaMV35S::SPA15:GFP, was generated for a transient expression assay in Arabidopsis mesophyll protoplasts. The result indicated that the SPA15:GFP fusion protein was targeted to chloroplasts (Supplementary Fig. S3). Therefore, it is concluded that the predicted transit peptides in both AAF and SPA15 are functional chloroplast targeting signals.
Overexpression of AAF promotes leaf senescence, early flowering, and elongation of roots and root hairs
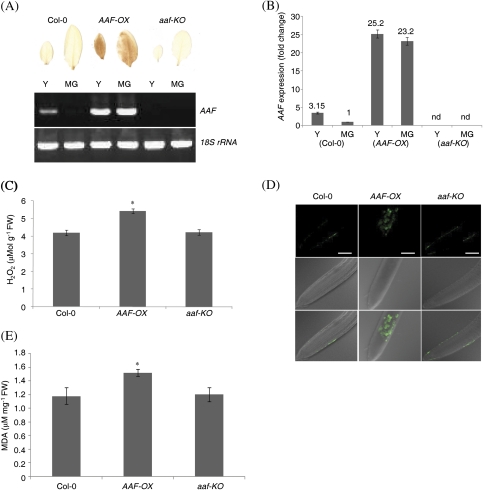
To investigate the functional role of AAF in Arabidopsis, multiple transgenic lines containing CaMV35S::AAF were generated and are referred to hereafter as AAF overexpression (AAF-OX) plants. Two independent transgenic lines, AAF-OX1 and AAF-OX2, were selected that both showed a comparable level of ectopic expression of AAF (Fig. 3C). In addition, two independent T-DNA insertional mutants to homozygosity were identified and they were named aaf-KO1 and aaf-KO2 (Fig. 3A, B); both are null mutants by RT-PCR analysis (Fig. 3C).
Age-dependent senescence was analysed by using the third and fourth rosette leaves of individual plants in three assays, namely chlorophyll content, photochemical activity of PSII, and membrane ion leakage, from DAG28 (days after germination) to DAG49. The results revealed that age-dependent leaf senescence was promoted in AAF-OX but delayed in aaf-KO plants (Fig. 3D, E). Leaf yellowing and photosynthetic efficiency were first analysed by quantitative measurement of chlorophyll content and photochemical activity, respectively. The chlorophyll content of rosette leaves was reduced to 54–58% in AAF-OX, 74% in the wild type (Col-0), and 95–96% in aaf-KO at DAG35 when compared with those in 4-week-old plants (100% at DAG28) (Fig. 3D). It was observed that the chlorophyll content was gradually reduced to a complete loss in AAF-OX1 and AAF-OX2 at DAG46, while 17% and 46–50% of chlorophyll levels remained in wild-type and aaf-KO plants, respectively (Fig. 3D). In addition, the photochemical activity of PSII began to show a reduction in AAF-OX at DAG39 and reached barely detectable levels at DAG49, while it was retained at 67% and 90% in the wild type and aaf-KO, respectively, at DAG49 (Fig. 3E). Next the electrolyte leakage was measured to analyse the membrane integrity of cells in senescent leaves. It was found that AAF-OX transgenics showed a significant increase in membrane ion leakage by 10–12% and 40–43% at DAG39 and DAG49, respectively, when compared with a mere 5% increase in the wild type at DAG49 (Fig. 3F). The levels of electrolyte leakage in aaf-KO were barely detectable at the same growth stages. Moreover, the expression of SAG12 and SAG13 was up-regulated in AAF-OX and not expressed in aaf-KO when compared with 7-week-old wild-type plants by RT-PCR analysis (Supplementary Fig. S4C at JXB online). By using three different assays to evaluate the role of AAF involved in age-dependent leaf senescence, it was revealed that overexpression of AAF promoted, and deletion of AAF delayed, leaf senescence, and AAF was required for the expression of SAG12 and SAG13 in 7-week-old plants.
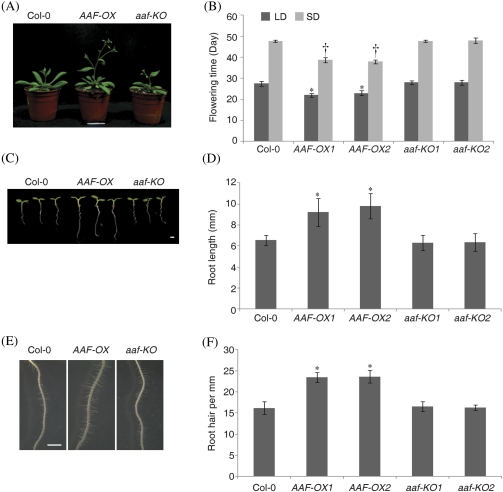
In addition to promoting early senescence in leaves, AAF-OX showed an early flowering phenotype (Fig. 4A). The flowering time of AAF-OX was approximately at 22 d and 39 d as compared with 28 d and 48 d for the wild-type under LD and SD conditions, respectively (Fig. 4B). The wild type and aaf-KO did not significantly differ in flowering time. In addition, the roots of 5-day-old AAF-OX seedlings were ∼40% longer than those in the wild type and aaf-KO (Fig. 4C, D). AAF-OX seedlings also showed an enhanced development of root hairs by ∼40% more than in the wild type (Fig. 4E, F). However, wild-type and aaf-KO seedlings did not differ in root length and root hair development.
Fig. 4.
Overexpression of AAF promotes early flowering and root growth. (A) Analysis of the flowering phenotype of 33-day-old plants cultivated under long-day conditions. (B) Flowering time under long-day (LD, black) or short-day (SD, grey) conditions. *P <0.01 (LD) and †P <0.01 (SD) versus the wild type, Col-0. Data represent means ±SE (n=6). (C) Root phenotype of 5-day-old seedlings grown under white light in long-day conditions. (D) Length of primary roots of 5-day-old seedlings. (E) Images of root hairs in a region 1.5 mm from the root tip in D. (F) Root hair density of 5-day-old seedlings. Data represent means ±SE (n=23–29) in D and F. *P <0.01 versus the wild type, Col-0. Bars=1 mm.
Accelerated leaf yellowing and degradation of chloroplasts in AAF-OX by continuous darkness
Next, it was decided to determine whether AAF is also involved in leaf senescence induced by stress. Continuous darkness acts as an inducer of leaf senescence in whole plants and detached leaves (Weaver and Amasino, 2001; Lin and Wu, 2004). AAF-OX showed enhanced leaf yellowing after continuous darkness for 5 d, whereas aaf-KO showed delayed leaf yellowing as compared with the wild type in whole plants (data not shown). To quantify the leaf senescence induced by continuous darkness, the fifth and sixth leaves detached from 4-week-old plants were used for the three senescence assays described above. The chlorophyll content of AAF-OX1 and AAF-OX2 was reduced dramatically to 4% and 5% of that at day 0 (100% at day 0), respectively, after a 5 d dark treatment, as compared with 31% in the wild type, whereas that in aaf-KO1 and aaf-KO2 was reduced to 66% and 69%, respectively (Fig. 5A). Analyses of the photosynthetic efficiency indicated that overexpression of AAF resulted in reduction in photochemical activity to 85–90% after a 5 d dark treatment, whereas only an ∼23% and a <10% reduction was observed in wild-type and aaf-KO plants, respectively (Fig. 5B). In addition, the electrolyte leakage was increased to 18% and 15% in AAF-OX1 and AAF-OX2, respectively, and <4% in the wild type and 2% in aaf-KO plants after dark treatment (Fig. 5C). Thus, overexpression of AAF promoted, but loss of AAF function in aaf-KO delayed, leaf senescence induced by continuous darkness.
Transmission electron microscopy was then used to examine whether the ultrastructure of chloroplasts is affected by darkness. The wild type and AAF-OX did not differ in chloroplast ultrastructure before dark treatment; both showed intact grana and thylakoids, comparable size and/or number of starch grains, and a few plastoglobuli (Fig. 5D, i and iii). After a 5 d dark treatment, wild-type chloroplasts became spherical and lacked starch grains, but the granal stacking remained (Fig. 5D, ii). However, the chloroplasts of AAF-OX showed complete loss of starch grains and grana, and a deformed shape with rupture of membranes, which led to hollow stroma in severe cases and increased accumulation of plastoglobuli (Fig. 5D, iv). Therefore, darkness-induced chloroplast degradation was enhanced in AAF-OX.
Global gene expression analysis reveals that AAF is involved in redox homeostasis
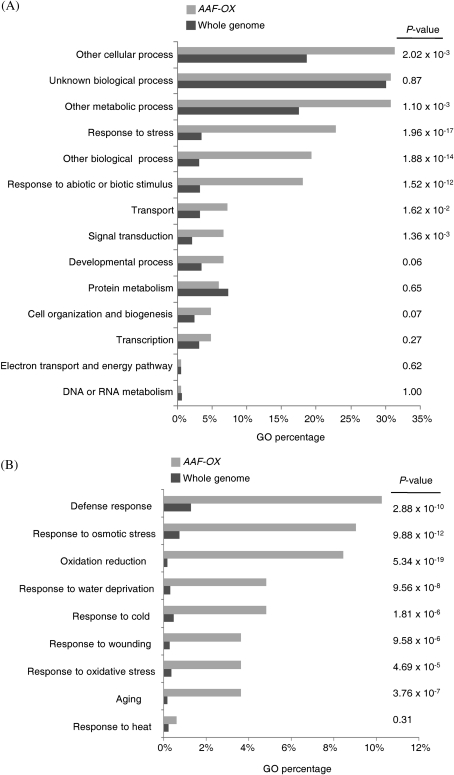
To understand the function of AAF at the gene expression level, transcriptome profiles were analysed in leaves of 3-week-old AAF-OX by microarray experiments. Genes with differential expression in the wild-type and AAF-OX plants with a cut-off at 1.5-fold were selected for GO analysis. Fisher's test was used to calculate the significance of the percentage distribution of GO annotations for comparison with those in the whole genome. Genes regulated by AAF-OX in the distribution of GO annotation are highly related to ‘response to stress’ and ‘response to abiotic or biotic stimulus’, with a P-value of 1.96×10−17 and 1.52×10−12, respectively (Fig. 6A), which suggests that genes responsive to stress are overrepresented in AAF-OX. Moreover, genes regulated by AAF-OX were highly related to ‘oxidation reduction’ and ‘oxidative stress’, with a P-value of 5.34×10−19 and 4.69×10−5, respectively (Fig. 6B). Results from transcriptome analysis suggested that AAF is involved in regulation of redox status responding to various stress conditions in Arabidopsis. Further analysis indicated that several groups of genes involved in ‘defence response’, ‘response to osmotic stress’, ‘response to water deprivation’, ‘response to cold’, ‘response to wounding’, and ‘ageing’ were significantly overrepresented in AAF-OX as compared with the whole genome (Fig. 6B). However, AAF may not be involved in all types of stress response, such as heat stress (Fig. 6B).
Fig. 6.
Global analysis of gene expression profiles in AAF-OX. (A) Expression profiles of genes up-regulated in AAF-OX by analysis based on the Gene Ontology (GO) annotation (biological process) in TAIR. (B) Analysis of genes induced in AAF-OX by specific GO terms. P-values in A and B were calculated by Fisher's test to compare the percentage distribution of GO annotation from genes induced by AAF-OX and the whole genome.
To verify whether the differentially expressed genes identified from microarray data were up-regulated in AAF-OX, the expression levels of selected genes were examined by real-time PCR analysis (Table 1). Defence genes such as LTP4, PDF1.2b, and PDF1.2a were ∼19.73-, 73.9-, and 48.22-fold more highly expressed, respectively, in AAF-OX than in the wild type (Table 1). AtERF1 and HEL, classified as defence genes and also regulated by ethylene, were induced by 8.87- and 4.19-fold, respectively, in AAF-OX. In addition, the expression of several senescence-associated genes, such as SAG12, SAG13, CHITINASE, and SEN1, and oxidative stress-related genes, such as those encoding glutathione S-transferase and peroxidase, were up-regulated in AAF-OX (Table 1). Thus, overexpression of AAF may interfere with cellular redox homeostasis to induce stress- and senescence-related genes at the developmental stage before senescence.
Table 1.
Defence-, senescence-, and oxidative stress-related genes are up-regulated in 3-week-old AAF-OX
| Function | AGI number | Description | Fold changea | RERb |
| Defence | At5g59319 | Lipid transfer protein (LTP4) | 17.42 | 19.73±1.36 |
| At2g26020 | Plant defensin (PDF1.2b) | 14.7 | 73.9±11.10 | |
| At5g44020 | Plant defensin (PDF1.2a) | 11.17 | 48.22±7.20 | |
| At3g04720 | Hevein-like protein (HEL) | 3.03 | 8.87±1.27 | |
| At4g17500 | ATERF1 | 1.74 | 4.19±0.46 | |
| Senescence | At5g45890 | Cysteine proteinase (SAG12) | 32.31 | 65.01±4.77 |
| At2g43570 | Chitinase, putative | 5.99 | 17.82±1.87 | |
| At2g29350 | Alcohol dehydrogenase (SAG13) | 2.06 | 29.16±9.61 | |
| At3g45590 | SEN1 | 1.67 | 2.04±0.33 | |
| Oxidative stress | At5g37940 | NADP-dependent oxidoreductase, putative | 4.28 | 2.59±0.30 |
| At1g02920 | Glutathione S-transferase | 2.14 | 12.3±0.39 | |
| At1g06290 | Acyl-CoA oxidase (ACX3) | 1.85 | 1.85±0.99 | |
| At4g37520 | Peroxidase | 1.62 | 9.8±2.40 | |
| At5g58390 | Peroxidase | 1.56 | 3.41±0.51 |
Fold change is the mean of two independent experiments with the ratio of raw signal values in AAF-OX and the wild type. The raw data are available in the GEO database with the accession no. GSE18336.
Gene expression levels were quantified by real-time PCR and the relative expression rate (RER) was compared between AAF-OX and the wild type. Data represent means ±SE (n=3).
Overexpression of AAF in Arabidopsis leads to accumulation of ROS
Because of the similar phenotypes and expression levels (Figs 3–5) of ectopically expressed AAF in two independent transgenic lines, AAF-OX1 was used as a representative transgenic for further characterization in subsequent experiments. Similarly, one of the two null mutants of AAF, aaf-KO2, was selected for phenotypic analyses hereafter. Since oxidative stress-related genes were significantly overrepresented in those up-regulated in AAF-OX, it was reasoned that overexpression of AAF may result in changes in cellular redox poise and/or altered response to ROS levels. First, the H2O2 level was analysed in young and MG leaves by using DAB for histochemical staining. It was previously shown that AAF is expressed in young but not MG leaves in the wild type (Fig. 1A) and the slightly expressed AAF does not lead to accumulation of sufficient H2O2 to be detected by DAB staining (Fig. 7A). However, the young and MG leaves in AAF-OX showed a dense DAB staining, which indicates that an elevated level of H2O2 was induced by overexpression of AAF (Fig. 7A). aaf-KO plants revealed neither gene expression of AAF nor DAB staining of H2O2. With real-time PCR analysis used to quantify the expression levels of AAF, a 25.2- and 23.2-fold increase in AAF expression was found in young and MG leaves of AAF-OX, respectively, as compared with MG leaves of the wild type (Fig. 7B). Thus, the 3.15-fold elevated AAF expression in young leaves of the wild type was not sufficient to induce H2O2 accumulation, as visualized by DAB staining. Quantification of the H2O2 level indicated ∼29% more H2O2 in AAF-OX plants than in the wild-type and aaf-KO plants (Fig. 7C). A fluorescence probe, DCFDA, was next used to detect ROS in roots of seedlings. The roots of AAF-OX showed more fluorescence than did those of the wild type or aaf-KO, which suggests a significant accumulation of ROS in the roots of AAF-OX (Fig. 7D). Because only non-pigmented plastids (leucoplasts) are present in roots, elevated levels of ROS by overexpression of AAF can be induced independently of photosynthesis. Lipid peroxidation resulting from oxidative degradation of polyunsaturated fatty acids in lipids by ROS was next analysed by measuring the levels of MDA, an end-product of oxidized lipids. Approximately 30% more MDA was generated in the leaves of AAF-OX as compared with those of the wild type and aaf-KO (Fig. 7E). The use of four different assays to analyse ROS levels demonstrated that overexpression of AAF promoted ROS accumulation in rosette leaves and roots.
Fig. 7.
ROS are accumulated in AAF-OX. (A) Histochemical staining by DAB of H2O2 in young (Y) and mature green leaves (MG) from 3-week-old plants. RT-PCR analysis of AAF expression levels from plants of the same developmental stages. (B) Real-time PCR analysis of AAF expression levels in 3-week-old plants. All transcripts were normalized to ACTIN2 expression as a reference gene, and MG of the wild type (Col-0) was set as 1. Data represent means ±SE (n=6). nd, not detected, in aaf-KO plants. (C) Analysis of H2O2 levels in rosette leaves from 3-week-old plants using the Amplex Red kit. (D) Analysis of ROS levels in the roots of 7-day-old seedlings by DCFDA. Panels at the top, images of fluorescence in pseudocolour; middle, DIC images; bottom, merged images. Bars=50 μm. (E) Analysis of lipid peroxidation in 3-week-old plants by quantification of malondialdehyde levels. *P <0.01 versus the wild type, Col-0 in C and E; data represent means ±SE (n=6).
AAF is involved in response to oxidative stress
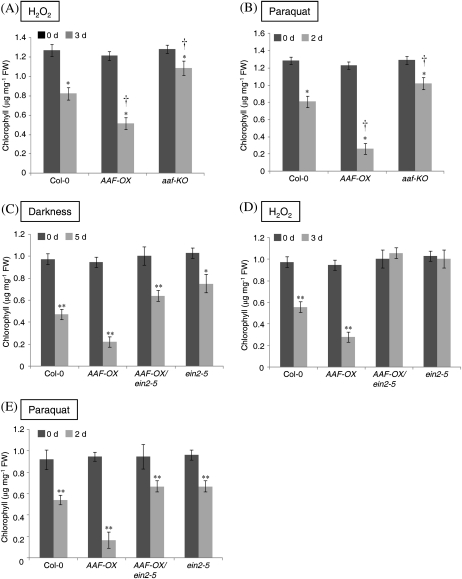
Overexpression of AAF promotes ROS accumulation and induces leaf senescence during development (Fig. 3D–F) and on exposure to darkness (Fig. 5). It was surmised that the elevated levels of ROS in AAF-OX might result in hypersensitivity to oxidative stress and therefore lead to leaf senescence. Thus, the chlorophyll content was measured in detached leaves as an indicator of senescence in the presence of reagents to induce oxidative stress. Detached leaves were floated in MES buffer supplemented with 10 mM H2O2 for 3 d before the chlorophyll content was measured. AAF-OX showed a significant loss of chlorophyll (42% of day 0) as compared with the wild type (64% of day 0) and aaf-KO (84% of day 0) (Fig. 8A), which indicated that AAF-OX was hypersensitive and aaf-KO was hyposensitive to exogenous H2O2. It was next asked whether the overexpression of AAF becomes more sensitive to ROS generated endogenously by treatment with paraquat (methyl viologen), which perturbs cyclic electron flow in PSI to generate superoxide, a major ROS, during photosynthesis. Detached leaves from AAF-OX showed severe chlorophyll loss in the presence of 12.5 μM paraquat for 2 d. Quantification of the chlorophyll content revealed a marked reduction, to 22% of that on day 0, in AAF-OX treated with paraquat as compared with 63% and 79% in the wild type and aaf-KO, respectively (Fig. 8B). These results suggest that AAF is involved in the response to cellular redox status. Therefore, ectopic expression of AAF in AAF-OX plants results in hypersensitivity and a null mutation in aaf-KO hyposensitivity to oxidative stress induced by H2O2 and paraquat. The hypersensitivity to ROS-inducing agents in AAF-OX may be due to an elevated endogenous H2O2 level and lipid peroxidation even in the absence of any oxidative stress (Fig. 7C, E).
Fig. 8.
Enhanced sensitivity to oxidative stress in AAF-OX is dependent on a functional EIN2. Chlorophyll content of detached leaves was quantitated in the absence and presence of (A) 10 mM H2O2 for 3 d; and (B) 12.5 μM paraquat for 2 d. *P <0.01 versus 0 d; †P <0.01, AAF-OX or aaf-KO versus the wild type, Col-0. Data represent means ±SE (n=6). Chlorophyll content of detached leaves treated with (C) continuous darkness for 5 d; (D) 20 mM H2O2 for 3 d; (E) 50 μM paraquat for 2 d. *P <0.05 or **P <0.01 versus the wild type, Col-0. Data represent means ±SE (n=6).
A functional EIN2 is required for the enhanced senescence phenotype in AAF-OX
The role of ethylene in promoting leaf senescence in plant species is well known (Grbi'c and Bleecker, 1995). The EIN2 gene encodes a master positive regulator in the ethylene signalling pathway and is involved in response to oxidative stress (Alonso et al., 1999; Jing et al., 2002; Cao et al., 2006). Mutations in EIN2 delayed leaf senescence (Grbi'c and Bleecker, 1995) and resulted in constitutive activation of several antioxidant enzymes that conferred enhanced resistance to oxidative stress (Alonso et al., 1999; Cao et al., 2006). Microarray analysis from this study also revealed that several ethylene-responsive genes, such as PDF1.2a, AtERF1, and HEL1, were up-regulated in AAF-OX (Table 1). To investigate whether EIN2 is involved in the function of AAF in response to oxidative stress to accelerate leaf senescence, ein2-5 was introduced into AAF-OX by a genetic cross to generate AAF-OX/ein2-5. Detached leaves from 3-week-old plants were floated in MES buffer and treated with continuous darkness for 5 d to analyse chlorophyll levels. AAF-OX was more sensitive to dark treatment, with the chlorophyll content reduced to 24% of that on day 0, but the content was 49% and 73% in the wild type and ein2-5, respectively (Fig. 8C). However, the level of chlorophyll was reduced to only 64% in AAF-OX/ein2-5, which indicates that ein2-5 recovered the chlorophyll loss in AAF-OX induced by darkness (Fig. 8C). In the presence of 20 mM H2O2, the chlorophyll content was reduced to 29% of that on day 0 in AAF-OX and 57% in the wild type (Fig. 8D). Surprisingly, neither ein2-5 nor AAF-OX/ein2-5 was affected by exogenous H2O2 (Fig. 8D). Finally, flotation of detached leaves on 50 μM paraquat for 2 d resulted in significant chlorophyll loss in AAF-OX, with a reduction to 17% of that on day 0 and 59% in the wild type (Fig. 8E). Both ein2-5 and AAF-OX/ein2-5 showed a similar reduction in chlorophyll content, to 70% (Fig. 8E). Thus, ein2-5 apparently suppresses the chlorophyll loss in AAF-OX induced by darkness and oxidative stress. Therefore, a functional EIN2 is required for AAF to respond to oxidative stress and continuous darkness to promote the subsequent senescence in leaves.
Expression of AAF is induced by ACC in Arabidopsis seedlings
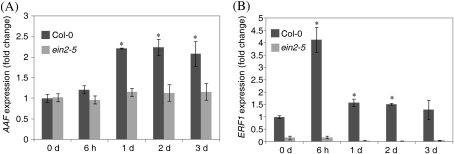
Several stress hormones have been studied for their roles in age-dependent and/or stress-induced leaf senescence by an extensive and comparative microarray analysis (Buchanan-Wollaston et al., 2005). Among the stress-related hormones, ethylene, JAs, SA, and ABA have been shown to promote leaf senescence responding to developmental, pathogen-, or dark-induced signals (Grbi'c and Bleecker, 1995; Morris et al., 2000; He et al., 2002; Cho et al., 2009). Overexpression of AAF in Arabidopsis is sensitive to oxidative stress that requires a functional EIN2 (Fig. 8C–E). Yap et al. (2003) showed that expression of SPA15 was significantly induced by ethylene in detached sweet potato leaves. It is possible that the expression of AAF is induced by ethylene in Arabidopsis. To test this possibility, 7-day-old light-grown seedlings treated with ACC (100 μM) were used and samples were collected at the designated time points for preparation of total RNA. Expression of AAF was induced 2-fold by ACC at 1 d after treatment, and the induced level continued for 3 d in the presence of ACC. AAF expression was not induced in ein2-5, suggesting that a functional ethylene response is required for the induction of the AAF gene by ACC (Fig. 9A). ERF1, an early ethylene-responsive gene, was used as a control, and its expression was induced ∼4-fold at 6 h by ACC in the wild type, but not in ein2-5 (Fig. 9B). These data support that expression of AAF can be induced by ethylene and suggest a potential positive feedback regulation of AAF function by the ethylene signalling pathway.
Fig. 9.
Expression of AAF is induced by ACC. Expression levels of AAF (A) and ERF1 (B) were quantified by real-time PCR in 7-day-old light-grown seedlings of Col-0 and ein2-5 in the absence and presence of 100 μM ACC. Samples of total RNA were prepared from seedlings collected at the indicated times. Data were normalized to the expression of ACTIN2 (At3g46520). Fold change of Col-0 at 0 day was set as 1. *P <0.01 versus the wild type, Col-0 at 0 d. Data represent means ±SE (n=3).
Discussion
Leaf senescence is regulated by different factors resulting from developmental and environmental signals. Here a study of the physiological roles of AAF in age-dependent, dark-induced, and oxidative stress-promoted leaf senescence in Arabidopsis is reported. The data support that overexpression of AAF promotes leaf senescence involving response to ROS that is dependent on a functional EIN2, and a null mutation in aaf-KO delays leaf senescence and becomes more resistant to oxidative stress. The expression of AAF is regulated by age-dependent senescence factors and is probably involved in stress response and pathogen defence based on transcriptome analysis. The interplay of the endogenous and exogenous factors may regulate the expression of AAF to modulate ROS homeostasis and/or modify sensitivity to oxidative stress, thus leading to leaf senescence dependent on EIN2. Furthermore, because the expression of AAF is not limited to senescent leaves, AAF may also be involved in regulating plant growth and development prior to organ senescence by modulating redox homeostasis. The current work demonstrates that a functional EIN2 or ethylene pathway is required for AAF to respond to darkness and oxidative stresses to induce leaf senescence. Whether additional signals from environmental stresses or phytohormones are relayed to AAF to regulate cellular redox homeostasis remains to be investigated.
The gradual loss of antioxidant capacity is one of the pivotal factors resulting in ageing in animals and plants. ROS production and accumulation has been shown to be associated with leaf senescence in plants (Procházková and Wilhelmová, 2007). Results from the current work suggest that there are two possible roles of AAF that may be linked to ROS to regulate leaf senescence; one is that AAF directly modulates ROS levels to regulate cellular redox homeostasis (Fig. 7) and the other is the differential sensitivities to oxidative stress or elevated ROS levels conferred by different expression levels of AAF (Fig. 8). Analysis of the ROS levels in aaf-KO and the wild type at the senescent stage will provide important information to delineate the role of AAF during leaf senescence. Previous reports indicated that mutants with increased tolerance to oxidative stress had extended leaf longevity or showed a late flowering phenotype (Kurepa et al., 1998; Woo et al., 2004). While it did not show the late flowering phenotype (Fig. 4A), aaf-KO exhibited increased tolerance to oxidative stress and delayed leaf senescence (Figs 3D–F, 5A–C, 8A, B). The Arabidopsis ore1, ore3, and ore9 mutants characterized by Woo et al. (2004) showed increased leaf longevity and enhanced tolerance to several ROS-inducing agents. The regulatory mechanism in the ore mutants was suggested to be the altered response to oxidative stress instead of modulated activity of antioxidant enzymes. The nature of AAF function in leaf senescence involving redox homeostasis requires further research and it may also have a similar role to the ore mutants in plant physiology. Interestingly, ore3 is a mutation allele in EIN2, which is required for the action of AAF in modulation of leaf senescence (Fig. 8C–E). On the other hand, studies on xdh1 (Brychkova et al., 2008) and cpr5/old1 (Jing et al., 2008) revealed that elevated cellular ROS levels resulted in accelerated leaf senescence.
The present results (Figs 3, 5) indicated that AAF-OX and aaf-KO promoted and delayed leaf senescence, respectively, in both age-dependent (4- to 7-week-old plants, Fig. 3) and darkness-induced (3-week-old plants, Fig. 5) conditions. On the basis of GO analysis from microarray data (Fig. 6), it was hypothesized that AAF was also involved in response to or modulation of ROS levels in stress-induced senescence in leaves. It was found that AAF-OX, but not aaf-KO plants, showed visible phenotypes in flowering time (Fig. 4A, B, 5-week-old plants), elongation of primary roots, and development of root hairs (Fig. 4C–F, 5-day-old seedlings). When the ROS levels were measured, only AAF-OX showed elevated levels of H2O2 and MDA in the rosette leaves of 3-week-old plants (Fig. 7C, E) and in the roots of 7-day-old seedlings (Fig. 7D). Results from phenotypic analyses (Fig. 4) and measurements of ROS levels (Fig. 7) were obtained by using transgenic plants prior to their showing developmental senescence in leaves. It was argued that ROS levels were tightly regulated and maintained homeostasis in the young and/or unstressed plants and ROS homeostasis would be disrupted by loss of antioxidant capacity or increased ROS production in senescent and stressed plants. Therefore, AAF-OX can promote ROS accumulation and/or increase response to ROS levels in both young and senescent plants. However, aaf-KO will not show visible phenotypes unless plants are stressed (Fig. 8A, B) or enter the senescent stage at a later time (Fig. 3D–F), which may be because aaf-KO is more tolerant to oxidative stress than wild type.
Flowering time and development of root hairs influenced in AAF-OX
Ascorbic acid (AsA) is an important antioxidant that detoxifies H2O2 to water. AsA-deficient mutants (vtc1, vtc2, vtc3, and vtc4) have shown that the levels of AsA in Arabidopsis modulate the expression of genes in the flowering pathways to promote early flowering and enhance premature leaf senescence (Kotchoni et al., 2009). An early flowering phenotype was shown in AAF-OX (Fig. 4A, B), which may be due to elevated ROS levels and decreased levels of antioxidants such as AsA. Investigating whether genes in the flowering pathways are up-regulated in AAF-OX to contribute to the early flowering phenotype is of interest.
The expression of AAF is not restricted to senescent leaves but is also present in tissues where cells are highly proliferative (Fig. 1C, ii, iii, and viii); AAF may play a role in promoting growth by regulating local ROS levels. Several examples suggest ROS as signalling molecules instead of merely destructive by-products of cellular metabolism. For example, O2·– was found located in the apoplast of the cell elongation zone in roots, whereas H2O2 was accumulated in the differentiation zone and the cell wall of root hairs (Dunand et al., 2007). Chemical treatments to reduce the local ROS levels affected root elongation and suppressed the formation of root hairs, which implies that ROS may function as signalling molecules to regulate root development (Dunand et al., 2007). The Arabidopsis rhd2 mutant has shorter roots and fewer root hairs than does the wild type, and the phenotype can be suppressed in part by exogenous ROS or is mimicked by chemical treatment to suppress ROS generation in roots (Foreman et al., 2003). RHD2 encodes an NADPH oxidase that can transfer electrons from NADPH to an electron acceptor to generate increased levels of ROS, so local ROS in roots is involved in cell outgrowth. In addition, localization and accumulation of ROS in roots can result from nutritional deprivation by nitrogen, phosphorus, and potassium deficiency that consequently modulates development of root hairs (Shin et al., 2005). Levels of ROS were higher in the epidermis than in the cortex under potassium or nitrogen deprivation, whereas phosphorus deficiency resulted in ROS accumulation predominantly in the cortex of roots (Shin et al., 2005). The pattern of patches of fluorescence in the roots of AAF-OX was similar to that seen with mineral deprivation. Moreover, the histochemical staining of AAFpro::GUS in the roots of seedlings indicated the tissue-specific expression of AAF in the elongation zone of primary and lateral roots (Fig. 1C, i and iii). The elongation of primary roots (Fig. 4C, D) and the formation of root hairs were enhanced in AAF-OX (Fig. 4E, F). Thus, AAF may be involved in development of root elongation and root hairs by modulating local ROS levels. However, aaf-KO did not show a delayed flowering phenotype or restricted root elongation and outgrowth of root hairs, which suggests that excessive cellular ROS (in AAF-OX) but not an elevated antioxidant capacity (in aaf-KO) plays a major role in flowering time and root development. Whether AAF is involved in the stress response to mineral deprivation remains to be explored.
AAF encodes a plastid protein in Arabidopsis and may be involved in redox homeostasis in chloroplasts
AAF is targeted to chloroplasts by transient expression of a GFP fusion protein in Arabidopsis mesophyll cells, and the putative transit peptide is required for plastid localization (Fig. 2). Intriguingly, despite a higher score in predicted specificity than in AAF, SPA15 was localized to the cell wall in leaf tissues, as observed by immunogold electron microscopy (Supplementary Table S1 at JXB online) (Yap et al., 2003). Both SPA15 and AAF have predicted transit peptides for targeting to plastids, but the subcellular localization results are not identical by two different assays. To clarify this discrepancy and verify the function of the predicted transit peptide in SPA15, a transient expression analysis was performed in Arabidopsis mesophyll protoplasts and it was found that SPA15–GFP was targeted to plastids (Supplementary Fig. S3). The results strongly support that the transit peptides in both AAF and SPA15 are bona fide plastid targeting signals in the protoplast transient assay.
A search of the plant genome database revealed that all of the AAF orthologues in different species are present as single loci (Supplementary Table S1). Phylogenetic analysis indicated AAF in the same clade as orthologues in monocots and moss, and with those in dicots in a different clade (Supplementary Fig. S2 at JXB online). The discovery of orthologues in moss suggests that the function of AAF may be present in the early evolution of land plants. The transgenic approach used here to elucidate the function of AAF provides a system to analyse the functional conservation of AAF orthologues from different species.
Chloroplasts are the major source of generating ROS in plants because of photosynthesis in an aerobic environment, and the oxidant scavenging systems and antioxidant networks are essential to remove excessive ROS to maintain redox homeostasis (Mittler, 2002; Foyer and Noctor, 2009). Paraquat perturbs the electron flow in PSI during photosynthesis and consequently induces accumulation of ROS to interfere with the redox poise in chloroplasts. Treatment with paraquat greatly enhanced the senescence-like phenotype and chlorophyll loss in AAF-OX, which was suppressed in aaf-KO, thus implying that AAF is probably involved in the redox balance in chloroplasts. Elevation of ROS levels in chloroplasts needs the recycling of oxidized antioxidants by enzymes such as SOD, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) to remove excess ROS efficiently. In addition, NADPH generated by ferredoxin-NADP reductase (FNR) in PSI provides the reducing equivalents in the ascorbate–glutathione cycle, which produces the most abundant soluble antioxidants in plants. The increased levels of cellular ROS in AAF-OX may be due to lack of fully functional antioxidant enzymes for efficient reduction and recycling of oxidized antioxidants to remove ROS. It is possible that AAF negatively regulates the activity of one or several antioxidant enzymes to change the redox poise, whereas loss of AAF may contribute to an increased antioxidant capacity. Alternatively, overexpression of AAF may interfere with photosynthetic electron transport in chloroplasts to result in accumulation of ROS. Further analysis of the precise location of AAF in chloroplasts and identification of interacting proteins of AAF will provide more information to elucidate the physiological role of AAF in chloroplasts.
Despite the fact that the functional transit peptides at AAF and SPA15 for chloroplast localization were confirmed, the results did not exclude the possibility that both proteins may also localize to the cell wall in senescent leaves because of the nature of the transient protoplast experiment. ROS generated in apoplasts and chloroplasts result from biotic (pathogens) or abiotic (ozone, high light, and continuous darkness) stresses that may lead to leaf senescence and cell death. Because AAF is expressed in seedlings and in early senescent leaves and can be induced by ethylene and other stress hormones, it is possible that AAF is involved in response to developmental and stress signals to regulate redox homeostasis. On the basis of microarray data (Fig. 6, Table 1), it is possible that AAF is involved in response to oxidative stress, which may take place in chloroplasts as well as in apoplasts. Apoplastic ROS are involved in intra- and intercellular signalling and have a role in regulation of defence gene expression (Miller et al., 2009). Furthermore, apoplastic ROS are not only involved in oxidative burst during pathogen infection, but also regulate cell growth (Gapper and Dolan, 2006; Sagi and Fluhr, 2006). ROS as signals to respond to plant development and stress depend on the ROS species, ROS intensity, and the site of production (Gechev et al., 2006). Plasma membrane-bound NADPH oxidase and cell wall-associated peroxidase are the main enzymes to produce superoxide and H2O2 in the apoplast (Sagi and Fluhr, 2006). Localized superoxide generated by NADPH oxidase in the root hair tips triggers Ca2+ uptake essential for the root hair growth, which indicates that the site of ROS production is important (Foreman et al., 2003). In addition, H2O2 can migrate across the membrane to act as a signal in activating the mitogen-activated protein kinase (MAPK) cascade to regulate downstream genes (Apel and Hirt, 2004). Whether AAF is involved in ROS homeostasis and/or response in apoplasts requires further research.
Hypersensitivity to oxidative stress and enhanced leaf senescence by overexpression of AAF depends on a functional EIN2
The mutant ein2 shows enhanced resistance to oxidative stress as compared with the wild type (Alonso et al., 1999; Cao et al., 2006), which implies that the ethylene response is required to relay signals of induced ROS and the subsequent sensitivity to oxidative stress. By introducing the ein2-5 allele in AAF-OX, it was shown that sensitivity to paraquat and H2O2 and dark-induced chlorophyll loss in AAF-OX was suppressed by ein2-5, which strongly supports that EIN2 is epistatic to AAF and that a functional ethylene signalling pathway is required for AAF function. EIN2 has been shown to localize in the endoplamic reticulum (ER) membrane and interacts with the ethylene receptor ETR1 (Bisson et al., 2009). The link of signal transduction from AAF in plastids to the ER-localized EIN2 to respond to oxidative stress and regulate leaf senescence remains to be established.
Phytohormones play differential roles in the onset, maintenance, and final stage of leaf senescence (Schippers et al., 2007). The ethylene level is elevated at the onset of developmental leaf senescence, followed by the levels of JA, ABA, and finally SA that are increased to sustain the process of leaf senescence. Ethylene-insensitive mutants etr1-1 and ein2-1 showed delayed senescence and increased leaf longevity in Arabidopsis (Grbi'c and Bleecker, 1995; Oh et al., 1997). Age-dependent leaf senescence was delayed in AAF-OX/ein2-5 as in ein2-5 (Chen and Wang, unpublished results). To understand the causal role of ethylene in AAF function, it was asked whether the expression of AAF is induced by ethylene. Indeed, it was found that ACC, the immediate precursor of ethylene, induced the expression of AAF by at least 2-fold and that the induction of AAF is dependent on a functional EIN2, suggesting that an ethylene response pathway is required to relay the signal (Fig. 9). However, AAF is not likely to be an immediate responsive gene, like ERF1 (Fig. 9) (Solano et al., 1998). Based on the essential role of a functional ethylene signalling pathway in AAF induction and in sensitivity to ROS, it is likely that there is a positive feedback regulation by ethylene to manifest AAF function during stress-induced and/or age-dependent leaf senescence. Yap et al. (2003) showed that SPA15 transcript and protein in the detached leaves of sweet potato and rice were induced by ethylene as well as phytohormones involved in stress response and developmental senescence such as ABA, MeJA, and SA. The induction of AAF in light-grown seedlings by the same group of hormones was analysed in the present study. It was found that AAF expression was also induced by ABA, MeJA, and SA with different magnitudes and patterns (Supplementary Fig. S5 at JXB online). Both MeJA and SA induced AAF expression by ∼2.5 fold, but the former showed an early induction at 6 h, and the latter at 2 d after treatment. Interestingly, AAF was induced by 4-fold at 6 h and gradually increased to 10-fold at 2 d after ABA treatment (Supplementary Fig. S5). These results indicate that the expression of AAF can be induced by all of the hormones tested, which is similar to previous observations for induction of SAP15 in the leaves of sweet potato and rice (Yap et al., 2003). Because ROS levels are affected by different stress responses and developmental senescence mediated by phytohormones such as ethylene, ABA, MeJA, and SA (Cho et al., 2009), it is possible that AAF is involved in a common regulatory circuit to modulate redox homeostasis in various stress conditions. Further investigation of the mechanistic role of AAF in regulating cellular redox homeostasis will provide insights into the role of ROS in promoting leaf senescence and stress responses mediated by hormones.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Protein alignment of AAF orthologues.
Figure S2. Phylogenetic analysis of AAF orthologues.
Figure S3. SPA15 is targeted to chloroplasts in Arabidopsis mesophyll protoplasts.
Figure S4. Age-dependent leaf senescence and expression of SAG12 and SAG13 in 7-week-old AAF-OX and aaf-KO plants.
Figure S5. ABA, MeJA, and SA can induce AAF expression in light-grown Arabidopsis seedlings.
Table S1. Prediction of chloroplast targeting peptides of AAF orthologues by TargetP1.1.
Table S2. Primers used for genotyping, RT-PCR, and cloning.
Table S3. Primers used for quantitative PCR.
Acknowledgments
This work is dedicated to the late Professor Shu-Chen Grace Chen (1948–2007). We thank Dr Wann-Neng Jane and Mei-Jane Fang for technical assistance with electron microscopy and confocal laser microscopy; and Shu-Jen Chou and Min-Yan Kuo for technical support in the microarray experiments. We thank the ABRC and the Salk Institute for seeds of aaf-KO lines, and three anonymous reviewers for valuable comments. This work was supported partly by grants from the National Science Foundation to LCW (grant nos 94-2311-B-001, 97-2311-B-001) and by the Institute of Plant and Microbial Biology, Academia Sinica.
References
- Agresti A. A survey of exact inference for contingency tables. Statistical Science. 1992;7:131–153. [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson MMA, Bleckmann A, Allekotte S, Groth G. EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochemical Journal. 2009;424:1–6. doi: 10.1042/BJ20091102. [DOI] [PubMed] [Google Scholar]
- Brychkova G, Alikulov Z, Fluhr R, Sagi M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. The Plant Journal. 2008;54:496–509. doi: 10.1111/j.1365-313X.2008.03440.x. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. The molecular biology of leaf senescence. Journal of Experimental Botany. 1997;48:181–199. [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. The Plant Journal. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- Cao S, Jiang S, Zhang R. Evidence for a role of Ethylene-Insensitive 2 gene in the regulation of the oxidative stress response in Arabidopsis. Acta Physiologiae Plantarum. 2006;28:417–425. [Google Scholar]
- Cho D, Shin D, Jeon B, Kwak J. ROS-mediated ABA signaling. Journal of Plant Biology. 2009;52:102–113. [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany. 1981;32:93–101. [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist. 2007;174:332–341. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants and Redox Signaling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiology. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Gepstein S, Sabehi G, Carp M-J, Hajouj T, Nesher MFO, Yariv I, Dor C, Bassani M. Large-scale identification of leaf senescence-associated genes. The Plant Journal. 2003;36:629–642. doi: 10.1046/j.1365-313x.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Grbi'c V, Bleecker AB. Ethylene regulates the timing of leaf senescence in Arabidopsis. The Plant Journal. 1995;8:595–602. [Google Scholar]
- He Y, Fukushige H, Hildebrand DF, Gan S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiology. 2002;128:876–884. doi: 10.1104/pp.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tang W, Swain JD, Green AL, Jack TP, Gan S. Networking senescence-regulating pathways by using Arabidopsis enhancer trap lines. Plant Physiology. 2001;126:707–716. doi: 10.1104/pp.126.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-J, To K-Y, Yap M-N, Chiang W-J, Suen D-F, Chen S- CG. Cloning and characterization of leaf senescence up-regulated genes in sweet potato. Physiologia Plantarum. 2001;113:384–391. doi: 10.1034/j.1399-3054.2001.1130312.x. [DOI] [PubMed] [Google Scholar]
- Jing HC, Hebeler R, Oeljeklaus S, Sitek B, Stühler K, Meyer HE, Sturre MJG, Hille J, Warscheid B, Dijkwel PP. Early leaf senescence is associated with an altered cellular redox balance in Arabidopsis cpr5/old1 mutants. Plant Biology. 2008;10:85–98. doi: 10.1111/j.1438-8677.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- Jing HC, Sturre MJG, Hille J, Dijkwel PP. Arabidopsis onset of leaf death mutants identify a regulatory pathway controlling leaf senescence. The Plant Journal. 2002;32:51–63. doi: 10.1046/j.1365-313x.2002.01400.x. [DOI] [PubMed] [Google Scholar]
- John CF, Morris K, Jordan BR, Thomas B, A-H-Mackerness S. Ultraviolet-B exposure leads to up-regulation of senescence-associated genes in Arabidopsis thaliana. Journal of Experimental Botany. 2001;52:1367–1373. [PubMed] [Google Scholar]
- Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiology. 2009;149:803–815. doi: 10.1104/pp.108.132324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Va M, Montagu N, Inzé D. Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. The Plant Journal. 1998;14:759–764. doi: 10.1046/j.1365-313x.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annual Review of Plant Biology. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- Lin J-F, Wu S- H. Molecular events in senescing Arabidopsis leaves. The Plant Journal. 2004;39:612–628. doi: 10.1111/j.1365-313X.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiologia Plantarum. 1994;92:322–328. [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling. 2009 doi: 10.1126/scisignal.2000448. 2, ra45. [DOI] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. Reactive oxygen signaling and abiotic stress. Physiologia Plantarum. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell and Environment. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Miller JD, Arteca RN, Pell EJ. Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiology. 1999;120:1015–1024. doi: 10.1104/pp.120.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Morris K, Mackerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V. Salicylic acid has a role in regulating gene expression during leaf senescence. The Plant Journal. 2000;23:677–685. doi: 10.1046/j.1365-313x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- Munné B, Alegre L. Plant aging increases oxidative stress in chloroplasts. Planta. 2002;214:608–615. doi: 10.1007/s004250100646. [DOI] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S, Morris K, Allen R, Harrison E, A-H-Mackerness S, Buchanan- Wollaston V. Expression of senescence-enhanced genes in response to oxidative stress. Journal of Experimental Botany. 2003;54:2285–2292. doi: 10.1093/jxb/erg267. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Engineering. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Oh SA, Park J-H, Lee GI, Paek KH, Park SK, Nam HG. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. The Plant Journal. 1997;12:527–535. doi: 10.1046/j.1365-313x.1997.00527.x. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Procházková D, Wilhelmová N. Leaf senescence and activities of the antioxidant enzymes. Biologia Plantarum. 2007;51:401–406. [Google Scholar]
- Rea G, de Pinto MC, Tavazza R, Biondi S, Gobbi V, Ferrante P, De Gara L, Federico R, Angelini R, Tavladoraki P. Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants. Plant Physiology. 2004;134:1414–1426. doi: 10.1104/pp.103.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JHM, Jing H-C, Hille J, Dijkwel PP. Developmental and hormonal control of leaf senescence. In: Gan S, editor. Senescence processes in plants. Oxford: Blackwell Publishing; 2007. pp. 145–170. [Google Scholar]
- Sheen J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiology. 2001;127:1466–1475. [PMC free article] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant and Cell Physiology. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytologist. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother S. The role of free radicals in leaf senescence. Gerontology. 1988;34:151–156. doi: 10.1159/000212945. [DOI] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiology. 2001;127:876–886. [PMC free article] [PubMed] [Google Scholar]
- Wintermans JF, de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochimica et Biophysica Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Woo HR, Kim JH, Nam HG, Lim PO. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant and Cell Physiology. 2004;45:923–932. doi: 10.1093/pcp/pch110. [DOI] [PubMed] [Google Scholar]
- Yap M-N, Lee R-H, Huang Y-J, Liao C- J, Grace Chen S-C. Molecular characterization of a novel senescence-associated gene SPA15 induced during leaf senescence in sweet potato. Plant Molecular Biology. 2003;51:471–481. doi: 10.1023/a:1022334820332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.