Abstract
Arabidopsis CBF genes (CBF1–CBF3) encode transcription factors having a major role in cold acclimation, the adaptive process whereby certain plants increase their freezing tolerance in response to low non-freezing temperatures. Under these conditions, the CBF genes are induced and their corresponding proteins stimulate the expression of target genes configuring low-temperature transcriptome and conditioning Arabidopsis freezing tolerance. CBF2 seems to be the most determinant of the CBFs since it also regulates CBF1 and CBF3 expression. Despite the relevance of CBF genes in cold acclimation, little is known about the molecular components that control their expression. To uncover factors acting upstream of CBF2, mutagenized Arabidopsis containing the luciferase reporter gene under the control of the CBF2 promoter were screened for plants with de-regulated CBF2 expression. Here, the identification and characterization of five of these mutants, named acex (altered CBF2 expression), is presented. Three mutants show increased levels of cold-induced CBF2 transcripts compared with wild-type plants, the other two exhibiting reduced levels. Some mutants are also affected in cold induction of CBF1 and CBF3. Furthermore, the mutants characterized display unique phenotypes for tolerance to abiotic stresses, including freezing, dehydration, and high salt. These results demonstrate that cold induction of CBF2 is subjected to both positive and negative regulation through different signal transduction pathways, some of them also mediating the expression of other CBF genes as well as Arabidopsis responses to abiotic stresses.
Keywords: Abiotic stress, Arabidopsis mutants, CBFs, cold acclimation, dehydration, freezing tolerance, low temperature, salt stress, signal transduction
Introduction
Freezing temperature is a major environmental factor that affects growth and development of plants, and limits their geographical distribution and crop yield. Plants from temperate regions have evolved an adaptive process to increase their freezing tolerance after being exposed to low, non-freezing temperatures. This process, called cold acclimation (Guy, 1990), involves several physiological and biochemical changes, most of them controlled by low temperature through changes in gene expression (Salinas, 2002). Recent global expression analyses in Arabidopsis have shown that >1500 genes are induced or repressed in response to low temperature (Matsui et al., 2008; Zeller et al., 2009), suggesting that cold acclimation is mediated by different signal transduction pathways. Interestingly, a number of these genes are also regulated by other abiotic stresses such as drought and high salt (Matsui et al., 2008; Zeller et al., 2009), which indicates that plant responses to abiotic stresses are related and share common signalling pathways.
A significant step toward the understanding of how gene expression is regulated during cold acclimation was the identification of the Arabidopsis C-repeat-binding factors (CBF1–CBF3) (Gilmour et al., 1998; Medina et al., 1999), also termed dehydration-responsive element-binding factors (DREB1B, 1C, and 1A, respectively) (Liu et al., 1998). These factors bind to the low temperature-responsive DNA regulatory elements designated as C-repeat (CRT)/dehydration response element (DRE) (Stockinger et al., 1997; Gilmour et al., 1998; Liu et al., 1998). CRT/DRE motifs contain the conserved CCGAC core sequence, which is sufficient to activate gene transcription under cold stress (Baker et al., 1994; Yamaguchi-Shinozaki and Shinoaki, 1994) and is present in the promoters of many cold-inducible genes (Thomashow, 1999). The CBF genes do not contain the CCGAC sequence in their promoters but are also induced by low temperature. This induction is transient, rapid, and not caused by dehydration and salt stress (Gilmour et al., 1998; Liu et al., 1998; Medina et al., 1999). The CBFs regulate the expression of ∼12% of the Arabidopsis cold-inducible genes (Fowler and Thomashow, 2002), suggesting that they have an important role in cold acclimation. In fact, constitutive overexpression of CBF genes activates the expression of genes containing the CRT/DRE element in their promoters at control temperature, which results in constitutive freezing tolerance and enhanced tolerance to dehydration and high salt (Jaglo-Ottosen et al., 1998; Kasuga et al., 1999; Gilmour et al., 2004). Transgenic Arabidopsis overexpressing the CBF genes also display dwarf phenotypes as well as late flowering, low number of seeds, and leaf senescence (Kasuga et al., 1999; Gilmour et al., 2004; Sharabi-Schwager et al., 2010), which indicates that the expression of these genes must be subjected to a tight regulation. According to this presumption, different transcription factors have been reported to interact directly with the promoters of the CBF genes and regulate their induction. ICE1, a basic helix–loop–helix (bHLH) transcription factor, has been described to bind to the CBF3 promoter and activate the expression of CBF3 in response to low temperature (Chinnusamy et al., 2003). ICE2, an ICE1 homologue, is involved in regulating the cold induction of CBF1 (Fursova et al., 2009). An R2R3-MYB transcription factor, MYB15, was shown to interact with ICE1 and to negatively regulate the cold induction of the three CBF genes through the MYB elements located in their promoters (Agarwal et al., 2006). Vogel et al. (2005) reported a zinc finger, ZAT12, that also negatively regulates the expression of the CBF genes. Recently, different members of calmodulin-binding transcription activators (CAMTAs) have been uncovered that bind to the CBF2 promoter inducing the expression of CBF2 (Doherty et al., 2009). Finally, the bHLH factor PIF7 has been found to bind to the G-box element present in the CBF2 promoter and to function as a repressor in transient assays (Kidokoro et al., 2009). Furthermore, in addition to these transcription factors, during the last years other proteins that are also implicated in controlling CBF expression have been identified (for reviews see Chinnusamy et al., 2007; Medina et al., 2011).
Consistent with the fact that the expression of CBF genes is tightly regulated, the physiological and molecular characterization of an Arabidopsis cbf2 null mutant revealed that the absence of CBF2 provokes an increase in the accumulation of CBF1 and CBF3 transcripts under both control and low temperature conditions, indicating that CBF2 negatively modulates the expression of CBF1 and CBF3 (Novillo et al., 2004). This increase correlates with higher levels of transcripts corresponding to CBF target genes and an enhancement of Arabidopsis tolerance to freezing temperature, before and after cold acclimation, as well as to dehydration and high salt (Novillo et al., 2004). On the other hand, the characterization of Arabidopsis plants with reduced induction of CBF1 and/or CBF3 in response to low temperature revealed that CBF1 and CBF3 function additively in cold acclimation and differently from CBF2 (Novillo et al., 2007). Indeed, low levels of CBF1 or CBF3 cause a decrease in the capacity of Arabidopsis to cold acclimate, though to a lesser extent than the absence of CBF1 and CBF3 simultaneously. As expected, these effects on cold acclimation correlate with low levels of mRNAs corresponding to CBF target genes (Novillo et al., 2007). All these data strongly suggest that CBF2 represents a unique regulon for low temperature-regulated gene expression, different from those of CBF1 and CBF3.
Unfortunately, despite the relevance of CBF2 in cold acclimation, little is known about the molecular components that control its expression which should constitute crucial upstream intermediates in cold signalling. As mentioned above, some transcription factors, including MYB15, CAMTAs, and PIF7, have been shown to modulate CBF2 expression by binding to its promoter (Agarwal et al., 2006; Doherty et al. 2009; Kidokoro et al., 2009). Additional proteins involved in regulating CBF2 expression, however, remain to be found. In an attempt to identify new molecular components controlling the expression of CBF2, a population of ethyl methanesulphonate (EMS)-mutagenized Arabidopsis carrying the firefly luciferase (LUC) reporter gene under the control of the CBF2 promoter was screened for plants with altered CBF2 expression (acex). Here, the characterization of five acex mutants exhibiting different patterns of cold-induced CBF2 expression is reported. The results demonstrate that the induction of CBF2 in response to low temperature is subjected to both positive and negative regulation through several signal transduction pathways, some of them also mediating the expression of other CBF genes as well as Arabidopsis response to abiotic stress.
Materials and methods
Plant materials, growth conditions, and treatments
Arabidopsis thaliana (L.) Heynh, ecotype Columbia (Col-0), was used in this study. Plants were grown at 20 °C under a long-day photoperiod (16 h of cool-white fluorescent light, photon flux of 90 μmol m−2 s−1) in pots containing a mixture of organic substrate and vermiculite (3:1, v/v) or in Petri dishes containing MS medium (Murashige and Skoog, 1962) or GM medium (MS medium supplemented with 1% sucrose) solidified with 0.8% (w/v) agar.
LUC analysis was performed with 2-week-old plants grown in Petri dishes with MS medium. LUC activity in response to low temperature was detected after exposing plants to 4 °C in a growth chamber for 24 h. Low temperature treatments for expression analysis were performed by transferring 4-week-old pot-growing plants to a growth chamber set to 4 °C for different periods of time, under the photoperiodic conditions described above and a light intensity of 45 μmol m−2 s−1. After treatments, plants were immediately frozen in liquid N2, and stored at –80 °C until their use. Freezing assays were carried out in a temperature-programmable freezer. Non-acclimated or cold-acclimated (7 d at 4 °C) 3-week-old pot-grown plants were exposed to 4 °C for 30 min in darkness and subsequently the temperature was lowered by 1 °C h−1. The final desired freezing temperature was maintained for 6 h and then the temperature was increased again to 4 °C at the same rate. After thawing at 4 °C for 24 h in the dark, plants were returned to their original growth conditions (see above). Tolerance to freezing was determined as the capacity of plants to resume growth after 10 d of recovery under control conditions. Dehydration tolerance was analysed on 2-week-old plants grown in Petri dishes containing GM medium. Tolerance was determined, after removing plants from the medium, placing them on a dry filter paper, and allowing them to develop for 1 d without watering, as the percentage of initial fresh weight (FW) that remained following the treatment. Salt stress was accomplished by transferring 2-week-old plants vertically grown in Petri dishes containing GM medium to new dishes supplemented with 125 mM NaCl. Tolerance was estimated by determining the root elongation and the FW of plants after 7 d of treatment.
Generation of CBF2::LUC transgenic plants
To generate the CBF2 promoter::LUC fusion, a CBF2 promoter region (–870 to –10) was placed in front of the Tobacco mosaic virus 5'-untranslated Ω leader sequence (Gallie et al., 1987) fused to the firefly LUC gene coding sequence (Millar et al., 1992) and the Cauliflower mosaic virus (CaMV) 35S polyadenylation sequence (Topfer et al., 1988). The cassette was subsequently cloned into the plant transformation vector pBIN19 (Bevan, 1984) to yield the CBF2::LUC construct, which was transferred to Agrobacterium tumefaciens C58C1 (Deblaere et al., 1985). Transformation of Arabidopsis was performed by vacuum infiltration (Clough and Bent, 1998) and plants homozygous for one copy of the CBF2::LUC transgene were selected by segregation analysis.
Mutagenesis, LUC imaging screening, and genetic analysis
EMS mutagenesis was performed on 60 000 seeds from a selected transgenic line containing a single copy of the CBF2::LUC fusion in homozygosis. Seeds were incubated in 100 ml of 0.3% EMS, 0.5% Triton X-100 for 12 h on a rotary shaker, and then washed 15 times with 250 ml of sterile water. Mutagenized (M1) seeds were divided into 48 pools, sown in pots, and the resulting plants allowed to self-pollinate. M2 seeds from each pool were collected independently, sterilized, and plated in Petri dishes containing MS medium.
For luminescence imaging, 2-week-old M2 plants grown at 20 °C or exposed for 24 h at 4 °C were sprayed with 1 mM luciferin and then kept in the dark for 5 min to avoid fluorescence interference. Luminescence images were then collected to identify de-regulated CBF2 expression mutants. All images were acquired with 10 min exposure time, using an intensified CCD camera 3200 LN/C system (Astromed Ltd, Cambridge, UK) (Kost et al., 1995). Putative mutants were transferred to soil and allowed to self-pollinate. The M3 progeny were re-examined for altered LUC activity as described above to discard false positives.
The character of selected mutations was determined by crossing the mutant lines with wild-type plants. In all crosses, wild-type plants were used as recipients and mutant lines as pollinators. The resulting F1 plants and their corresponding F2 families were analysed for LUC activity in response to low temperature as described above. For allelism tests, the selected mutants were crossed reciprocally and the F1 progeny analysed for their cold-induced luminescence.
Molecular biology methods
Total RNA was isolated from 4-week-old wild-type and mutant plants according to the method described by Logeman et al. (1987). Restriction digestions, cloning, and RNA-blot hybridizations were performed following standard protocols (Sambrook et al., 1989). Specific probes for CBF1, CBF2, CBF3, COR15A, COR47, KIN1, LTI78, and RCI2A have been described before (Novillo et al., 2004). Similar RNA loading in the experiments was monitored by rRNA staining with ethidium bromide. In some cases, the intensity of hybridization bands was quantified by densitometry with the ImageJ image processing program and corrected for the differences detected in RNA loading. RNA samples from each experiment were analysed in at least two independent blots, and each experiment was repeated at least twice.
Results
Isolation of Arabidopsis mutants de-regulated in CBF2 expression
To identify mutants de-regulated in CBF2 expression, Arabidopsis transgenic lines containing a single copy of a fusion between the CBF2 promoter and the LUC reporter gene were generated (Fig. 1A). The CBF2 promoter used (–870 to –10) contains all regulatory elements necessary to confer expression in response to low temperature (Novillo et al., 2007; Doherty et al., 2009; Kidokoro et al., 2009). A line homozygous for the insertion [hereafter referred to as the wild type (WT)], which showed a clear and homogeneous induction of LUC activity under cold conditions, was selected for EMS mutagenesis. Morphologically, this line was identical to the Col-0 parental ecotype.
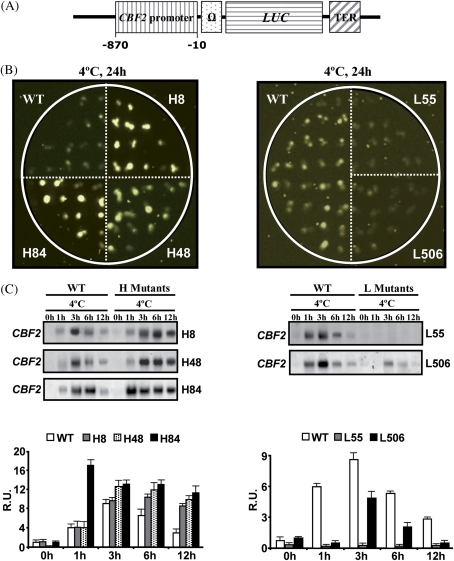
Fig. 1.
Selected mutants showing altered CBF2 gene expression. (A) Schematic representation of the CBF2::LUC fusion. The LUC gene was placed under the control of a CBF2 promoter fragment (–870 to –10). The position of the translational enhancer (Ω) and termination (TER) sequences is indicated. (B) Luciferase activity in mutants with high (H, left panel) or low (L, right panel) luminescence compared with the WT line. Thirteen-day-old plants grown in MS medium at 20 °C and exposed for an additional 1 d at 4 °C. (C) CBF2 expression patterns in H (left panel) and L (right panel) mutants. Total RNA was prepared from 4-week-old WT and mutant plants exposed to 4 °C for the indicated times, and hybridized with a CBF2-specific probe. Histograms represent the relative quantification of the hybridization signals as obtained by densitometric analysis after correction for the RNA loading differences detected by rRNA staining. In the histogram corresponding to H mutants, data are expressed as means of nine or three independent quantifications (from independent RNA-blot hybridizations) for the WT and mutants, respectively. In the histogram corresponding to L mutants, data are expressed as means of six or three independent quantifications (from independent RNA-blot hybridizations) for the WT and mutants, respectively. Bars indicate the SE. R.U., relative units. (This figure is available in colour at JXB online.)
A primary screening was conducted with 25 000 2-week-old M2 seedlings for mutants with altered LUC activity after low temperature treatment. Seedlings displaying constitutive luminescence (seven), or lower (34) or higher (54) LUC activity than the WT line were selected and allowed to self-pollinate. From them, only two, 17, and 29 plants belonging to the different classes mentioned above, respectively, survived and set seeds. The progeny of those plants that set enough seeds were subjected to a secondary screening to eliminate false positives. At the end, five mutants having a clear altered LUC activity in response to cold were selected. Three of them (lines 8, 48, and 84) exhibited higher activity than the WT line, while the other two (lines 55 and 506) disclosed lower activity (Fig. 1B).
To establish whether the five mutants selected on the basis of their LUC activity phenotypes were actually affected in the expression of the endogeneous CBF2 gene, RNA-blot hybridizations were performed with WT plants and the mutant lines grown under control conditions or exposed to 4 °C for different times. The results revealed that in the five mutants the induction of CBF2 was de-regulated and correlated with the LUC activity (Fig. 1C). The induction of CBF2 in mutants with increased LUC activity was significantly higher and more sustained than in WT plants, particularly in the mutant H84. In the case of mutants having reduced LUC activity, the induction of CBF2 was significantly lower than in the WT line, especially in the mutant L55 (Fig. 1C). All these mutants with altered CBF2 expression were designated as acex. Compared with the WT line, acex mutants did not present any obvious morphological or developmental abnormality (data not shown).
Genetic characterization of acex mutants
In all cases, the F1 plants resulting from crosses between the acex mutants and the WT line displayed wild-type LUC activity in response to low temperature (Table 1). Furthermore, the progeny of these heterozygous F1 plants always exhibited a segregation of their LUC activity phenotypes of ∼3:1 between WT and mutant (Table 1). These data indicated that each one of the five selected acex mutants was caused by a recesive mutation in a single nuclear gene.
Table 1.
Genetic characterization of acex mutations
| Crosses | F1 (WT:MUT)a | F2 (WT:MUT) | χ2 |
| WT×acex8 | 9:0 | 127:35 | 0.99 |
| WT×acex48 | 8:0 | 162:48 | 0.51 |
| WT×acex84 | 13:0 | 128:34 | 1.39 |
| WT×acex55 | 12:0 | 147:39 | 1.61 |
| WT×cex506 | 15:0 | 145:41 | 0.86 |
WT, wild-type LUC activity; MUT, mutant LUC activity.
acex8, acex48, and acex84 are mutants showing higher LUC activity than the WT line. acex55 and acex506 are mutants showing lower LUC activity than the WT line.
Values of χ2 <3.84 correspond to a 3:1 segregation.
Allelism analyses revealed that the five acex mutants belonged to five different complementation groups. In fact, all F1 plants obtained from crosses between mutants disclosed wild-type LUC activity in response to low temperature (Table 2), demonstrating that the selected acex mutations were not allelic.
Table 2.
Allelism analysis of acex mutations
| Crosses | F1 (WT:MUT) |
| acex8×acex84 | 9:0 |
| acex8×acex48 | 11:0 |
| acex48×acex84 | 11:0 |
| acex55×acex506 | 9:0 |
WT, wild-type LUC activity; MUT, mutant LUC activity.
acex8, acex48, and acex84 are mutants showing higher LUC activity than the WT line. acex55 and acex506 are mutants showing lower LUC activity than the WT line.
Physiological characterization of acex mutants
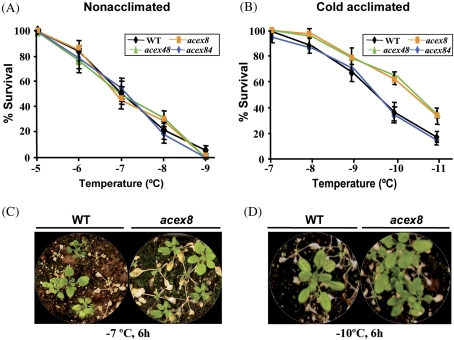
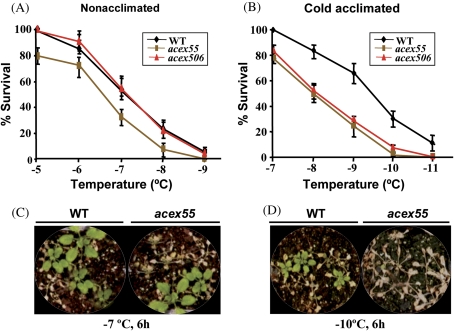
The physiological characterization of the acex mutants was carried out by analysing their sensitivity to freezing and other related abiotic stresses such as dehydration and high salt. Freezing tolerance was determined in non-acclimated and cold-acclimated (7 d at 4 °C) plants as their capacity to resume growth after being exposed for 6 h to different freezing temperatures when returned to control conditions. Figure 2A shows that the three mutants with high CBF2 induction (acex8, acex48, and acex84) had similar levels of freezing tolerance to the WT plants when non-acclimated, the temperature that causes 50% lethality (LT50) being around –7.0 °C in all cases. Nevertheless, mutants acex8 and acex48 were significantly more freezing tolerant than the WT line after cold acclimation (Fig. 2B). The LT50 values of the mutants were very similar (–10.3 °C) and lower than that of WT plants (–9.4 °C). Mutant acex84 did not present any difference from the WT line regarding its capacity to cold acclimate (Fig. 2B). The freezing tolerance phenotypes of non-acclimated and cold-acclimated WT and acex8 plants are displayed in Fig. 2C and D, respectively, as a representative example. As for the mutants having low induction of CBF2, while the mutant acex55 exhibited a significant decreased freezing tolerance when non-acclimated, the LT50 of mutant and WT plants being –6.5 °C and –7.2 °C, respectively, the mutant acex506 behaved like the WT line (Fig. 3A). After being cold acclimated, however, both mutants were significantly impaired in their capacity to tolerate freezing. The LT50 values of acex55 and acex506 mutants were –8.0 °C and –8.1 °C, respectively, while that of WT plants was –9.5 °C (Fig. 3B). As a representative example, Fig. 3C and D shows the freezing tolerance phenotypes of non-acclimated and cold-acclimated WT and acex55 plants, respectively.
Fig. 2.
Freezing tolerance of acex mutants showing increased induction of CBF2 in response to low temperature. Non-acclimated and cold-acclimated (7 d, 4 °C) 3-week-old WT and mutant (acex8, acex 48, and acex 84) plants were exposed to different freezing temperatures for 6 h. Freezing tolerance was estimated as the percentage of plants surviving each specific temperature after 10 d of recovery under control conditions. (A) Freezing tolerance of non-acclimated plants. (B) Freezing tolerance of cold-acclimated plants. (C) Representative non-acclimated WT and acex8 plants 10 d after being exposed to –7 °C for 6 h. (D) Representative cold-acclimated WT and acex8 plants 10 d after being exposed to –10 °C for 6 h. In A and B, data are expressed as the means of three independent experiments with at least 50 plants each. Bars indicate the SE.
Fig. 3.
Freezing tolerance of acex mutants showing reduced induction of CBF2 in response to low temperature. Non-acclimated and cold-acclimated (7 d, 4 °C) 3-week-old WT and mutant (acex55 and acex506) plants were exposed to different freezing temperatures for 6 h. Freezing tolerance was estimated as the percentage of plants surviving each specific temperature after 10 d of recovery under control conditions. (A) Freezing tolerance of non-acclimated plants. (B) Freezing tolerance of cold-acclimated plants. (C) Representative non-acclimated WT and acex55 plants 10 d after being exposed to –7 °C for 6 h. (D) Representative cold-acclimated WT and acex55 plants 10 d after being exposed to –10 °C for 6 h. In A and B, data are expressed as the means of three independent experiments with at least 50 plants each. Bars indicate the SE.
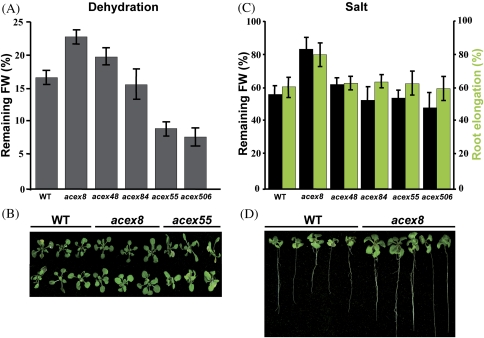
Dehydration was induced by maintaining plants on a dry filter paper for 1 d without watering. The rate of dehydration was determined as the percentage of initial FW that remained following the treatment. Wild-type and acex plants did not present significant differences in their initial FW values (data not shown). After dehydration, acex8 and acex48 plants maintained an average of 22.5% and 20% of their initial FW, respectively, whereas WT plants maintained only 17% (Fig. 4A). Mutant acex84 did not show a significant difference in its remaining FW with respect to WT plants (Fig. 4A). Mutants acex55 and acex506, in turn, were significantly more sensitive to dehydration than the WT line, only maintaining an average of 9% and 7.5% of their initial FW, respectively, after treatment (Fig. 4A). Figure 4B displays the dehydration phenotypes of mutants acex8 and acex55 as representative examples of tolerant and sensitive mutants, respectively, compared with WT plants.
Fig. 4.
Tolerance to dehydration and salt stress of acex mutants. (A) Dehydration tolerance of WT and acex plants. Tolerance was estimated as the percentage of initial FW that remains after transferring 2-week-old plants to a dry filter paper and allowing them to develop for 1 d without watering. (B) Representative WT, acex8, and acex55 mutant plants after dehydration treatment. (C) Salt tolerance of WT and acex plants. Tolerance was estimated by determining the root elongation (green bars) and remaining FW (black bars) of 2-week-old plants transferred to a medium containing 125 mM NaCl for 7 d. (D) Representative WT and acex8 mutant plants after salt treatment. In A and C, data are expressed as means of three independent experiments with at least 20 plants each. Bars indicate the SE. In A, values obtained from the WT and acex8, acex48, acex55, and acex506 mutants were significantly different (P <0.05), as determined by Student’s test, except in the case of mutant acex84. In C, values obtained from the WT and the acex8 mutant were significantly different (P <0.05).
The tolerance to salt stress was estimated by determining the root elongation in acex and WT plants after growing for 7 d in a medium containing 125 mM NaCl. The FW of the plants after treatment also proved to be an estimate of their salt tolerance. WT and acex plants had similar root elongation and FW values under control conditions (data not shown). All mutants, except acex8, exhibited the same levels of salt tolerance as the WT line. acex8 plants subjected to salt stress, however, showed increased root elongation (20%) and remaining FW (23%) compared with WT plants (Fig. 4C). These significant differences among acex8 and WT plants were clearly apparent at the phenotypical level (Fig. 4D).
Molecular characterization of acex mutants
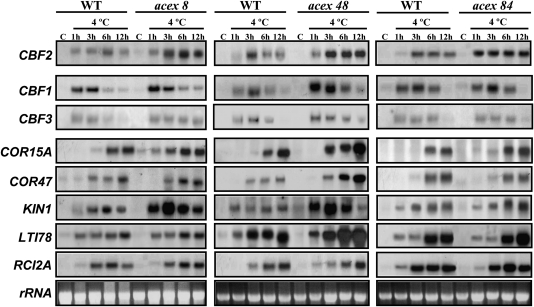
The characterization of the acex mutants was completed by analysing the expression of CBF1 and CBF3, as well as of different genes whose transcripts accumulate in response to low temperature through CBF-dependent (COR15A, COR47, KIN1, and LTI78) and CBF-independent (RCI2A) pathways (Novillo et al., 2004, 2007). In mutants acex8, acex48, and acex84, the expression levels of all genes analysed under control conditions were the same as in the WT plants (Fig. 5). However, when exposed to 4 °C, several differences in the expression patterns of some genes were observed between mutants acex8 and acex48, and the WT line. Thus, in the mutant acex8 the induction of CBF1 and CBF3 was slightly higher and more sustained than in the WT. In addition, COR15A and KIN1 also showed higher induction than in WT plants (Fig. 5). The mutant acex48 displayed increased induction levels of all genes except RCI2A. Furthermore, as in the case of mutant acex8, the induction of CBF1 and CBF3 was a little more sustained than in the WT line (Fig. 5). In the case of mutant acex84, the induction levels of all genes analysed were unaffected (Fig. 5).
Fig. 5.
Transcript levels of CBF1, CBF3, and different cold-inducible genes in acex8, acex48, and acex84 mutants. RNA-blot hybridizations were performed with total RNA isolated from 4-week-old Col (WT) and mutant plants grown under control conditions (C) or exposed to 4 °C for the indicated times. Specific probes for CBF1 and CBF3, as well as for CBF target genes COR15A, COR47, KIN1, and LTI78, and the non-CBF-target gene RCI2A were used for hybridizations. The transcript levels of CBF2 in the mutants are shown as internal controls. Similar amounts of RNA were present in each sample as confirmed by ethidium bromide staining of rRNA.
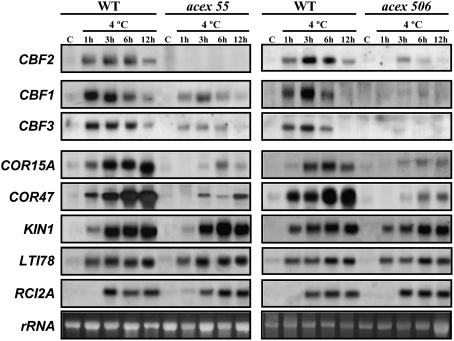
The molecular characterization of the acex mutants with reduced cold induction of CBF2, acex55 and acex506, revealed that they had similar expression patterns of the genes analysed both under control conditions and in response to low temperature (Fig. 6). Mutants acex55 and acex506 did not present increased expression of CBF1, CBF3, and CBF target genes as did the cbf2 null T-DNA mutant (Novillo et al., 2004), in all likelihood because they are caused by trans-acting mutations. Instead, at 20 °C, the transcript levels of all genes were very much alike in the mutants and WT plants (Fig. 6). Furthermore, when exposed to cold, acex55 and acex506 exhibited a decreased induction of CBF1 and CBF3, as well as of the CBF target genes COR15A and COR47. The other genes had the same induction levels as in the WT line (Fig. 6).
Fig. 6.
Transcript levels of CBF1, CBF3, and different cold-inducible genes in acex55 and acex506 mutants. RNA-blot hybridizations were performed with total RNA isolated from 4-week-old Col (WT) and mutant plants grown under control conditions (C) or exposed to 4 °C for the indicated times. Specific probes for CBF1 and CBF3, as well as for CBF target genes COR15A, COR47, KIN1, and LTI78, and the non-CBF-target gene RCI2A were used for hybridizations. The transcript levels of CBF2 in the mutants are shown as internal controls. Similar amounts of RNA were present in each sample as confirmed by ethidium bromide staining of rRNA.
Discussion
In an attempt to uncover molecular components acting upstream of CBF2, an essential gene in cold acclimation, Arabidopsis mutants in which its expression is de-regulated have been isolated. The screening procedure was based on transgenic Arabidopsis plants containing the LUC reporter gene under the control of a CBF2 promoter fragment that includes all the elements needed to confer CBF2 cold expression (Novillo et al., 2007; Doherty et al., 2009; Kidokoro et al., 2009). This experimental strategy, namely using promoter::LUC constructs to screen for Arabidopsis mutants affected in stress-regulated gene expression, has been previously carried out by several laboratories (Ishitani et al., 1997; Foster and Chua, 1999; Chinnusamy et al., 2003; Medina et al., 2005; Dong et al., 2009) and has been crucial to reveal signalling intermediates underlying plant responses to different adverse environmental situations. In this work, the characterization of five mutants with altered CBF2 expression is reported. These mutants, named acex, account for five different loci. Three of them, acex8, acex48, and acex84, show higher induction of CBF2 than WT plants in response to low temperature. The other two, acex55 and acex506, display lower induction. Under control conditions, the expression of CBF2 in all mutants is as in the WT line.
The identification of Arabidopsis mutants with increased or reduced cold induction of CBF2 indicates the existence of, at least, one signal transduction pathway that negatively modulates the expression of CBF2 in response to low temperature and another pathway that promotes it. The fact that the selected acex mutations are not allelic indicates that each of them defines a different component in the signalling cascades that mediate CBF2 induction under cold conditions, confirming that the expression of CBF2 is highly regulated. Mutants with constitutive expression of CBF2 were not isolated. Similar studies conducted to identify mutants de-regulated in cold induction of CBF3 did not allow the isolation mutants having constitutive expression of this gene (Chinnusamy et al., 2003; Dong et al., 2009). Since the constitutive expression of CBF genes originates dwarf phenotypes and a low number of seeds (Kasuga et al., 1999; Gilmour et al., 2004), most probably mutants with constitutive CBF2 expression are difficult to detect or do not set enough seeds for the screening.
To characterize the acex mutants molecularly, the impact of the corresponding mutations on the expression of CBF1 and CBF3, as well as on the expression of different cold-regulated genes whose induction is mediated (COR15A, COR47, KIN1, and LTI78) or not (RCI2A) by the CBFs (Novillo et al., 2004, 2007) was analysed. Under control conditions, the expression of CBF1 and CBF3 was not detected in any mutant and, consequently, not the expression of CBF target genes either. Accordingly, the WT phenotype that is present in the acex mutants is consistent with the absence of CBF gene expression under unstressed conditions. Interestingly, however, in response to low temperature, the induction patterns of CBF1 and CBF3 are altered in all mutants except in acex84. In fact, paralleling the levels of CBF2 transcripts, the induction of CBF1 and CBF3 is increased in mutants acex8 and acex48 and reduced in mutants acex55 and acex506, indicating that the proteins identified by these mutations, in addition to modulating the cold induction of CBF2, are also involved in regulating the expression of CBF1 and CBF3 under cold conditions. In mutant acex84, however, only the levels of CBF2 transcripts are higher than in the WT when exposed to low temperature, which indicates that the corresponding protein specifically regulates the cold induction of CBF2. Taken together, all these data suggest that, as already described for CBF3 regulation (Chinnusamy et al., 2003; Agarwal et al., 2006), the expression of CBF2 is also mediated through both specific and non-specific signalling pathways.
Remarkably, mutants acex8 and acex48, both of them having high induction levels of all CBF genes, exhibit different expression patterns of CBF target genes in response to low temperature. In the mutant acex8, only COR15A and KIN1 are more induced than in the WT line. However, in the mutant acex48 the induction of all CBF targets analysed is increased. This difference may be due to the variation that exists between acex8 and acex48 in the levels of CBF1 and CBF3 transcripts. In this regard, it has been proposed that the induction of CBF target genes depends on the amount of total CBFs (Novillo et al., 2007). In contrast to acex8 and acex48, the mutant acex84, which shows WT induction levels of CBF1 and CBF3 transcripts when exposed to cold, also has the same cold induction levels of the CBF target genes as the WT plants. Probably, the amount of total CBF transcripts in this mutant would not be sufficient to promote an increase in the induction of the CBF targets. As for the mutants acex55 and acex506, although they have low induction levels of all CBF genes when exposed to cold, they are only affected in the induction of COR15A and COR47. Again, this expression pattern might be determined by the amount of CBFs. Nonetheless, it cannot be excluded that, in addition to regulating the levels of CBF transcripts in response to low temperature, the signalling intermediates defined by the acex mutations could also function in controlling the cold induction of CBF target genes through CBF-independent pathways. Indeed, various studies have described that genes belonging to the CBF regulon are also induced by cold in a CBF-independent way (Baker et al., 1994; Wang and Cutler, 1995; Zhu et al., 2004; Vogel et al., 2005; Yoo et al., 2007). All acex mutants show induction levels of RCI2A in response to low temperature identical to those of the WT plants, confirming that this gene does not belong to the CBF regulon and demonstrating that the acex mutations do not affect any intermediate step involved in regulating the cold induction of RCI2A.
Mutants acex8, acex48, acex84, and acex506 are not altered in their constitutive capacity to tolerate freezing, which is consistent with their WT gene expression profiles under control conditions. In contrast, mutant acex55, which also has WT gene expression patterns, is impaired in its constitutive freezing tolerance. The corresponding mutation, therefore, should uncover a positive regulator of the Arabidopsis constitutive freezing tolerance that would function through a CBF-independent signalling pathway. Regarding the freezing tolerance of acex mutants after cold acclimation, acex8 and acex48 plants display a higher capacity to cold acclimate than the WT line, whereas acex55 and acex506 plants are impaired in their cold-induced freezing tolerance. These tolerance phenotypes are consequent on the induction levels of CBF genes and CBF target genes in these mutants in response to low temperature. The mutant acex84, in turn, is not affected in its freezing tolerance after cold acclimation. Considering that acex84 has increased induction levels of CBF2 when exposed to cold, but WT induction levels of CBF1, CBF3, and CBF target genes, the results reported here suggest that the amount of CBFs in acex84 in response to low temperature should be insufficient to promote an increase in the induction levels of CBF targets, as already mentioned, and, therefore, in its cold-induced freezing tolerance.
In addition to causing an increase in the capacity of Arabidopsis to cold acclimate, the acex8 mutation also provokes higher tolerance to dehydration and salt stress compared with the WT line. However, the acex48 mutation that also increases freezing tolerance after cold acclimation gives rise to higher tolerance to dehydration but not to salt stress. The acex84 mutation, that does not affect the cold-induced freezing tolerance of Arabidopsis, does not alter the tolerance to dehydration and salt stress either. In the case of mutants acex55 and acex506, an increased sensitivity to dehydration but a tolerance to salt stress like the WT line was observed. From these results, it can be concluded that the factors defined by the identified mutations not only play an important role in the freezing tolerance of Arabidopsis but are also involved in the tolerance of Arabidopsis to other related abiotic stresses such as dehydration and high salt. This illustrates, once more, that the signalling pathways that mediate plant responses to low temperature, dehydration, and salt stress converge at different points like those defined by the acex mutations.
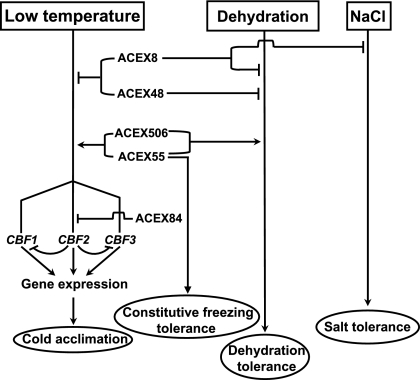
As already mentioned, some factors have been reported to regulate the expression of CBF2 (Agarwal et al., 2006; Chinnusamy et al., 2007; Doherty et al., 2009; Kidokoro et al., 2009; Medina et al., 2011). Although the possibility that some of the acex mutants correspond to genes already described as CBF2 regulators cannot be excluded, from the data available it is highly unlikely. In fact, when compared with the previously isolated mutants affected in CBF2 expression, the acex mutants have very different phenotypes. The acex mutants are morphologically, physiologically, and/or molecularly different from the mutants previously described as having altered CBF2 expression (Agarwal et al., 2006; Chinnusamy et al., 2007; Doherty et al., 2009; Kidokoro et al., 2009; Medina et al., 2011), which strongly indicates that they are not allelic and identify new molecular components controlling CBF2 expression. Based on the phenotypes displayed by the acex mutants, a working model is proposed for the function of the gene products identified in regulating CBF2 expression and Arabidopsis tolerance to abiotic stress (Fig. 7). According to this model, proteins ACEX8, ACEX48, and ACEX84 would act, directly or indirectly, as negative regulators of CBF2 induction in response to low temperature. Furthermore, ACEX8 and ACEX48 would also modulate, negatively, the cold induction of CBF1 and CBF3, and, most probably through the CBF targets, the capacity of Arabidopsis to cold acclimate. Since the mutant acex84 is not affected in its capacity to cold acclimate, the ACEX84 protein, that would specifically regulate the induction of CBF2 under cold conditions, would not play an apparent role in cold acclimation. ACEX8, moreover, would have a function, as a negative regulator, in Arabidopsis tolerance to dehydration and salt stress. ACEX48, however, would only be involved in the tolerance of Arabidopsis to dehydration. On the other hand, proteins ACEX55 and ACEX506 would positively regulate the induction of the three CBF genes and some target genes by low temperature and, therefore, the capacity of Arabidopsis to cold acclimate. ACEX55 would also control, in a positive way, the consitutive freezing tolerance of Arabidopsis through a CBF-independent signalling cascade. Additionally, both ACEX55 and ACEX506 proteins would act as positive modulators of Arabidopsis tolerance to dehydration. These results demonstrate the complexity of the molecular mechanisms plants have evolved to respond and adapt to their environment. The molecular identification of acex mutations and the subsequent functional characterization of the corresponding factors will contribute to further understanding of the role of CBF2 in cold acclimation and the intricate signalling networks that regulate CBF genes expression and Arabidopsis response to abiotic stresses.
Fig. 7.
Proposed model for the function of identified ACEX factors in regulating CBF2 expression and Arabidopsis tolerance to abiotic stresses. Gene products defined by the selected acex mutants are shown to positively (arrowheads) or negatively (end lines) regulate the expression of CBF genes in response to low temperature as well as the tolerance of Arabidopsis to freezing, before and after cold acclimation, dehydration, and high salt.
Acknowledgments
We thank Dr G. Salcedo for helpful discussion and reading of the manuscript. Work in the laboratory of JS is supported by grants BIO2010-17545, CSD2007-00057, and EUI2009-04074 from the Spanish Ministry of Science and Innovation. JM is currently supported by INIA RTA action 2009-0004-CO2-01.
References
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. Journal of Biological Chemistry. 2006;281:37636–37645. doi: 10.1074/jbc.M605895200. [DOI] [PubMed] [Google Scholar]
- Baker SS, Wilhelm KS, Thomashow MF. The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Molecular Biology. 1994;24:701–713. doi: 10.1007/BF00029852. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Research. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes and Development. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends in Plant Science. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Research. 1985;13:4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. The Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Zolman BK, Bartel B, Lee BH, Stevenson B, Agarwal M, Zhu JK. Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signalling. Molecular Plant. 2009;2:59–72. doi: 10.1093/mp/ssn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Chua NH. An Arabidopsis mutant with deregulated ABA gene expression: implications for negative regulator function. The Plant Journal. 1999;17:363–372. doi: 10.1046/j.1365-313x.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Fowler S, Thomasow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. The Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fursova OV, Pogorelko GV, Tarasov VA. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene. 2009;15:98–103. doi: 10.1016/j.gene.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TM. The 5'-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Research. 1987;15:3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF. Arabidopsis transcriptional activators CBF1, CBF2 and CBF3 have matching functional activities. Plant Molecular Biology. 2004;54:767–781. doi: 10.1023/B:PLAN.0000040902.06881.d4. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. The Plant Journal. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Guy CL. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:187–223. [Google Scholar]
- Ishitani M, Xion L, Stevenson B, Zhu JK. Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. The Plant Cell. 1997;9:1935–1949. doi: 10.1105/tpc.9.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kidokoro S, Maruyama K, Nakashima K, et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiology. 2009;151:2046–2057. doi: 10.1104/pp.109.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Schnorf M, Potrykus I, Neuhaus G. Non-destructive detection of firefly luciferase (LUC) activity in single plant cells using a cooled, slow-scan CCD camera and an optimized assay. The Plant Journal. 1995;8:155–166. [Google Scholar]
- Liu Q, Kasuga M, Kasuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeman J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Annual Review of Biochemistry. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Matsui A, Ishida J, Morosawa T, et al. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tilling array. Plant and Cell Physiology. 2008;49:1135–1149. doi: 10.1093/pcp/pcn101. [DOI] [PubMed] [Google Scholar]
- Medina J, Bargues M, Terol J, Pérez-Alonso M, Salinas J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiology. 1999;119:463–469. doi: 10.1104/pp.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Catalá R, Salinas J. The CBFs: three Arabidopsis transcription factors to cold acclimate. Plant Science. 2011;180:3–11. doi: 10.1016/j.plantsci.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Medina J, Rodriguez-Franco M, Peñalosa A, Carrascosa M.J, Neuhaus G, Salinas J. Arabidopsis mutants deregulated in RCI2A expression reveal new signalling pathways in abiotic stress response. The Plant Journal. 2005;42:586–597. doi: 10.1111/j.1365-313X.2005.02400.x. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Chua NH, Kay SA. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. The Plant Cell. 1992;4:1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige Y, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology. 1962;15:473–497. [Google Scholar]
- Novillo F, Alonso JM, Ecker JR, Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proceedings of the National Academy of Sciences, USA. 2007;104:21002–21007. doi: 10.1073/pnas.0705639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas J. Molecular mechanisms of signal transduction in cold acclimation. In: Hames BD, Glover DM, editors. Frontiers in molecular biology. Oxford: Oxford University Press; 2002. pp. 116–139. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sharabi-Schwager M, Lers A, Samach A, Guy CL, Porat R. Overexpression of the CBF2 transcriptional activator in Arabidopsis delays leaf senescence and extends plant longevity. Journal of Experimental Botany. 2010;61:261–273. doi: 10.1093/jxb/erp300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences, USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Topfer R, Schell J, Steinbiss HH. Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acid Research. 1988;16:8725. doi: 10.1093/nar/16.17.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. The Plant Journal. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Cutler AJ. Promoters from kin1 and cor6.6, two Arabidopsis thaliana low-temperature- and ABA-inducible genes, direct strong beta-glucuronidase expression in guard cells, pollen and young developing seeds. Plant Molecular Biology. 1995;28:619–634. doi: 10.1007/BF00021188. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. The Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SY, Kim Y, Kim SY, Lee JS, Ahn JH. Control of flowering time and cold response by a NAC-domain protein in Arabidopsis. PloS One. 2007;2:e642. doi: 10.1371/journal.pone.0000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller G, Henz SR, Widmer CK, Sachsenberg T, Rätsch G, Weigel D, Laubinger S. Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. The Plant Journal. 2009;58:1068–1082. doi: 10.1111/j.1365-313X.2009.03835.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shi H, Lee BH, Damsz B, Cheng S, Stirm V, Zhu JK, Hasegawa PM, Bressan RA. An Arabidopsis homeodomain transcription factor gene, HOS9, mediates cold tolerance through a CBF-independent pathway. Proceedings of the National Academy of Sciences, USA. 2004;101:9873–9878. doi: 10.1073/pnas.0403166101. [DOI] [PMC free article] [PubMed] [Google Scholar]