Abstract
The response of mesophyll conductance to CO2 (gm) to environmental variation is a challenging parameter to measure with current methods. The ‘variable J’ technique, used in the majority of studies of gm, assumes a one-to-one relationship between photosystem II (PSII) fluorescence and photosynthesis under non-photorespiratory conditions. When calibrating this relationship for Populus trichocarpa, it was found that calibration relationships produced using variation in light and CO2 were not equivalent, and in all cases the relationships were non-linear—something not accounted for in previous studies. Detailed analyses were performed of whether different calibration procedures affect the observed gm response to CO2. Past linear and assumed calibration methods resulted in systematic biases in the fluorescence estimates of electron transport. A sensitivity analysis on modelled data (where gm was held constant) demonstrated that biases in the estimation of electron transport as small as 2% (∼0.5 μmol m−2 s−1) resulted in apparent changes in the relationship of gm to CO2 of similar shape and magnitude to those observed with past calibration techniques. This sensitivity to biases introduced during calibrations leads to results where gm artefactually decreases with CO2, assuming that gm is constant; if gm responds to CO2, then biases associated with past calibration methods would lead to overestimates of the slope of the relationship. Non-linear calibrations were evaluated; these removed the bias present in past calibrations, but the method remained sensitive to measurement errors. Thus measurement errors, calibration non-linearities leading to bias, and the sensitivity of variable J gm hinders its use under conditions of varying CO2 or light.
Keywords: Chlorophyll fluorescence, curve fitting, electron transport rate, gm, mesophyll conductance to CO2, Populus trichocarpa, variable J technique
Introduction
Mesophyll conductance (gm) is the conductance of CO2 from the intercellular airspaces to Rubisco, a largely liquid pathway through the cell wall and three membranes. Whether gm is a constitutive or dynamic characteristic of a leaf is fundamental to our understanding of plant responses to the environment. As gm may represent up to 40% of the CO2 diffusional limitation on photosynthesis (Warren, 2008), dynamic variation in gm would offer a major avenue for photosynthetic regulation, comparable with that of the stomata. The most commonly used technique to measure gm, the variable J method, consistently demonstrates a large reduction in gm with increasing CO2 (Flexas et al., 2007). However, the size and presence of the response of gm to CO2 varies between studies using a variety of methods (Flexas et al., 2007; Tazoe et al., 2009; Vrabl et al., 2009). For example, a less steep response of gm to CO2 was found for Nicotiana tabacum using the independent carbon isotope method relative to the variable J method (Flexas et al., 2007). In a separate experiment, Arabidopsis thaliana and N. tabacum were reported to reduce gm by ∼85% and 65%, respectively, when CO2 changed from 200 μmol mol−1 to 1000 μmol mol−1 at 21% O2 and measured using the variable J method (Flexas et al., 2007). In a second investigation, the carbon isotope method resulted in only a 10% reduction and a 5% increase for the same species, respectively, when measured across the same range of CO2 mole factions; measurements at 2% O2 showed reductions of 26% and 40% (Tazoe et al., 2011). The widely used curve-fitting techniques for estimating gm explicitly assume a constant gm across the range of CO2 used to generate CO2 response curves (Ethier et al., 2006; Warren, 2006; Sharkey et al., 2007; Gu et al., 2010). To date the underlying mechanisms determining gm and the reason for the different results between methods remain unresolved.
The ‘variable J’ technique encompasses a group of methods that estimate gm, chloroplastic CO2 concentration (Cc), and the rate of oxygenation or photorespiration (Vo) from combined fluorescence and gas exchange data. Mesophyll conductance to CO2 is calculated as the ratio of net photosynthetic CO2 flux (A) to the difference in CO2 concentration between the intercellular airspaces (Ci) and the chloroplast (Cc). Cc is related to the ratio of carboxylation to photorespiration at Rubisco, and photorespiration is then proportional to the difference between fluorescence-derived estimates of the total electron transport rate and the rate of electron use by carboxylation estimated from gas exchange. This derivation is described in detail in the Materials and methods and reviewed by Warren (2006) and by Pons et al. (2009).
Fluorescence estimates of total electron transport are derived from the work of Genty et al. (1989), who established that under non-photorespiratory conditions (low oxygen and high CO2), a linear relationship exists between the quantum yield of fluorescence (ΦPSII) and measured quantum efficiency of rates of CO2 fixation (ΦCO2). This proportionality has subsequently been used to provide an estimate of electron transport rates: in the absence of alternative electron sinks, the relationship between carboxylation estimates of linear electron flow and fluorescence estimates of electron transport should be one-to-one under non-photorespiratory conditions. In practice, this relationship deviates from one-to-one due to interspecific variation in the values of standard constants such as leaf absorptance (Baker, 2008), and measurement of the relationship under non-saturating CO2 where significant alternative electron transport sinks may be present. However, for simplicity, it is often assumed that standard constants are accurate and do not vary during experiments.
An alternative approach is to conduct pre-experimental calibrations to provide estimates of electron transport from photosystem II (PSII) fluorescence (Lawlor and Tezara, 2009). While empirical calibration has the potential to improve estimates of electron transport, it can also introduce systematic errors (biases) in the calculation of the total electron transport rate, and thus gm. The impact of calibration issues, such as non-linearity, on the calculation of gm has not been thoroughly assessed.
The present study examined whether the differences between two common methods used for measuring gm is the result of biases in the calibration of the variable J method. However, the challenges inherent in the variable J method have long been recognized (Harley et al., 1992), with the Harley criterion providing an indication of how sensitive the gm values are to errors when using this method (Harley et al., 1992). The original sensitivity analyses of Harley et al. (1992) demonstrated that the relationship of gm to CO2 was sensitive to errors in the values of mitochondrial respiration, the photo-compensation point, and the fluorescence estimate of the total electron transport rate. However, this analysis was not extended to a broad range of Cis, as subsequent studies do, and the sensitivity of the gm response to CO2 to errors has not been compared with the size of biases present in the calibration procedure.
Therefore, the goal of this study was to understand the conditions under which gm can be accurately measured using the variable J technique. Fluorescence with gas exchange measurements is calibrated using classical methods for the widely used genome model plant poplar (Populus trichocarpa Torr. & Gray). Consistent with the original literature, significant variation in the calibration relationship was found, such that there is the potential for systematic error when calibrations are applied to a broad range of environmental conditions. Photosynthetic models are then used to assess the effects of biases on the response of gm to CO2. Finally, new calibration techniques by which these biases may be reduced when estimating a single value of gm for a leaf, or comparing species, are suggested. However, it is demonstrated that the variable J method should be used with caution when measuring the response of gm to CO2 and light, as any bias in the estimation of electron transport rates results in changes in the relationship of gm with CO2.
Materials and methods
Plant material and growing conditions
Poplar plants were propagated from cuttings and grown in environmentally controlled growth chambers. Metal–halide and high pressure sodium lighting (400 μmol m−2 s−1) was provided for 14 h per day. Temperatures in the chambers were maintained between 20 °C and 24 °C, and humidity was kept at 70%. The cuttings were placed in 3785 cm3 pots in Farfard 3B potting soil which included Osmocote Plus slow release fertilizer as per the manufacturer's instructions (15/9/12/1 N/P/K/Mg plus trace elements: S, B, Cu, Fe, Mn, Mo, and Zn; Scotts Company, OH, USA). The pots were watered daily and fertilized weekly with Peters Excel All Purpose soluble fertilizer (21/5/20 N/P/K plus trace elements: B, Cu, Fe, Mn, Mo, and Zn; Scotts Company). Plants were measured after 4–9 months of growth in March–May of 2010 (all experiments), and a second set of plants in November 2010 (extra CO2 response curves).
Gas exchange and fluorescence measurements
Gas exchange and fluorescence measurements were done on young fully expanded leaves using a 2 cm2 LI-COR LI-6400 fluorescence chamber and gas exchange system (LI-COR, Lincoln, NE, USA). Plants were allowed to acclimate to the gas exchange system in a laboratory growth chamber for >30 min, until stomatal conductance was stable. Unless otherwise noted, general measurement conditions were as follows: photosynthetic photon flux density (PPFD), 400 μmol m−2 s−1 with no blue light component (Loreto et al., 2009); reference CO2, 400 μmol mol−1; Tl, 24.9±0.8 °C; vapour pressure deficit (VPD), 1.47±0.49 kPa; flow, 150 μmol s−1. Single flash fluorescence measurement settings were used and adjusted according to the optimal values obtained from the flash and measuring intensity procedures in the LI-6400 manual (Anon, 2004). All measurements were corrected for leaks using empirically determined leak corrections for dry poplar leaves under measurement conditions (CO2Scorrected=CO2Suncorrected+9.868×10−7×CO2R2+5.291×10−4×CO2R–1.008).
The Laisk method was used to measure non-photorespiratory respiration in the light (Rd) and the apparent photo-compensation point (Ci*) (Warren, 2006). In this method, the y and x value of the average intersection of three CO2 responses are taken as Rd and Ci*. The CO2 responses were measured at reference CO2 concentrations of 150, 100, 75, and 50 μmol mol−1, at three light levels 400, 175, and 75 μmol m−2 s−1. Six replicate sets of measurements were made providing mean (±SD) values of 0.42±0.21 μmol m−2 s−1 for Rd, and 36.0±1.9 μmol mol−1 for Ci*. In theory, the Ci* values should be increased by Rd/gm to obtain an estimate of the photocompensation point Γ* (von Caemmerer, 2000), but as no independent value for gm was available, the transformation was not performed and Γ* was taken to be equal to Ci*. A sensitivity analysis, described below, confirmed that minor variation in the value for Γ* did not greatly affect the values of gm (Table 1).
Table 1.
Sensitivity analysis for the calibration and calculation of gm at ambient CO2 (400 μmol mol−1) and 400 μmol m−2 s−1 PPFD
| Calibration type | Parameter varied | Apparent gm (mol m−2 s−1) | % increase with calibration | |
| No calibration standard parameters | ||||
| 1a | α=0.85, β=0.5 | NA | 0.166±0.018 | 0 |
| 1b | α=0.85, β=0.5 | Rd+95% CIa | 0.173±0.020 | 4.1 |
| 1c | α=0.85, β=0.5 | Rd–95% CI | 0.160±0.017 | –3.7 |
| 1d | α=0.85, β=0.5 | Γ*+95% CI | 0.181±0.021 | 9.0 |
| 1e | α=0.85, β=0.5 | Γ*–95% CI | 0.154±0.016 | –7.5 |
| Measured leaf absorptance | ||||
| 2 | α=0.831 (measured), β=0.5 | NA | 0.182±0.022 | 9.4 |
| Light response at 1% O2 and ambient CO2 calibration | ||||
| 3a | Linear fitb | NA | 0.341±0.087 | 104.5 |
| 3b | Linear–sigmoidal | NA | 0.306±0.079 | 83.6 |
| CO2 response at 1% O2 and 400 μmol m−2 s−1 light calibration | ||||
| 4 | Linear–sigmoidal | NA | 0.199±0.017 | 19.8 |
All percentage increases were expressed relative to the mean for scenario 1a which used standard calibration parameters (α=0.85, β=0.5). Scenarios 1b–1e represent gm values calculated using the standard calibration parameters and measured variation in Rd and Γ*. Scenario 2 represents the shift in gm due to measuring, and not assuming α=0.85. Scenarios 3a and 3b represent gm values calculated using calibrations estimated from different plots and regression fits to light response curves. Scenario 4 represents gm values calculated using variation in CO2 to generate a linear–sigmoidal calibration curve. Values represent means and standard errors for six replicates.
Rd and Γ* were estimated from six replicate sets of Laisk curves: in 1b–1e the mean values were used plus or minus the 95% confidence interval of the mean for the replicates (Rd, 0.42±0.17 μmol m−2 s−1 and Γ*, 36.0±1.5 μmol mol−1);
Linear fit to high light data from the efficiency plot (Fig. 1C) for data where is <0.05.
Low oxygen, non-photorespiratory conditions, were obtained by mixing air with nitrogen gas using a Wösthoff gas mixer to achieve a 1% O2 content. This was tested using CO2 drawdown as an indicator of the 5% mixing ratio necessary to produce 1% O2 from 21% O2 air, and further verified using an Ocean Optics USB4000-FL-450 Fiber Optic Spectrophotometer oxygen probe. Initial experiments demonstrated that 1% O2 was the highest oxygen concentration at which the slope of the variable J calibration relationship did not change with successive dilution of O2 at ambient CO2, indicating that further reductions in O2 would not further inhibit photorespiration.
Calibration relationships and CO2 responses
Light or CO2 response curves were measured under non-photorespiratory conditions to calibrate the relationship between fluorescence-derived electron transport rates (Jraw) and photosynthesis (). Nine light response curves were measured at ambient CO2 (400 μmol mol−1) and 1% O2, starting at a PPFD of 2000 μmol m−2 s−1 and reducing PPFD to 1500, 1000, 800, 600, 500, 400, 350, 300, 250, 200, 150, and 100 μmol m−2 s−1 with 4–6 min intervals between measurements. Five CO2 response curves were measured at 400 μmol m−2 s−1 PPFD and three at 1000 μmol m−2 s−1 PPFD at 1% O2. CO2 was reduced from 400 μmol mol−1 to 100 μmol mol−1 in decrements of 75 μmol mol−1, and after an 8 min re-acclimation at 400 μmol mol−1 increasing CO2 to 600, 800, 1000, 1500, and 2050 μmol mol−1. Gas exchange was measured at each CO2 concentration after the cuvette CO2 concentration was stable for >120 s. A second similar series of CO2 responses was measured after the first under 21% O2. The leaf absorptance of 10 leaves was measured using a Taylor integrating sphere (LI-COR 1800-12).
Estimation of ‘variable J’ gm
Values for gm were estimated from the following standard formulae used in the ‘variable J’ technique (Harley et al., 1992; Valentini et al., 1995; von Caemmerer, 2000), and using variants of the calibrations detailed below. Mesophyll conductance to CO2 is estimated as the ratio of the net photosynthetic rate (A) and the difference in CO2 mole fraction from the intercellular airspaces (Ci) and the chloroplastic sites of photosynthesis (Cc):
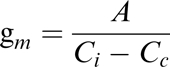 |
(1) |
As A and Ci are provided by standard gas exchange measurements, estimation of Cc remains as the difficult-to-measure unknown in this equation. Cc is estimated assuming that Rubisco specificity to O2 and CO2 (Sc/o) remains constant, and that the ratio of the carboxylation rate (Vc) to the oxygenation rate (Vo) varies in direct proportion to the concentration of CO2 at the site of carboxylation (Cc) in the chloroplast or the concentration of oxygen (O) which is assumed not to vary. Thus:
| (2) |
where Γ* is the photo-compensation point (=0.5×O/Sc/o), measured using the Laisk method. Vc can be estimated as the sum of the measured A, a value for Rd assumed to be constant and equal to that measured using the Laisk method, and half of Vo:
| (3) |
Vo is included in Vc, as for every two oxygenations one CO2 is released, leading to gross photosynthesis being underestimated by A. The total electron transport (Jtotal) is the sum of the reductant required for Vc, Vo, and any alternative electron transport sinks (Valt). Under many conditions four electrons are used per CO2 molecule fixed (Baker, 2008), and it is known that two photorespiratory cycles release one CO2, thus the rate of photorespiration can be estimated by rearranging this equation:
| (4) |
Here Jtotal includes Valt; by calibration of the total electron transport rate estimated from fluorescence (Jraw) with measurements of A+Rd under non-photorespiratory conditions—where Valt and Vo are assumed to be absent—a calibrated electron transport rate (Jcal) can be obtained. Under photorespiratory conditions Jcal then represents the sum of Vc and Vo, such that:
| (5) |
From a theoretical perspective Cc is relatively well defined, but see Parkhurst (1994) and Evans (2009) for issues with describing CO2 fluxes or fluorescence with an average number representing different depths in the leaf. However, it is the practical estimation of Jcal and Valt that remains controversial and which represents a potential source of error in the calculation of gm. To obtain an accurate value for Jcal, the raw measurements of chlorophyll fluorescence (Jraw) must be calibrated and in doing so account for Valt under the experimental conditions as follows. Fluorescence of PSII provides an initial estimate of total electron flux through the electron transport chain:
| (6) |
where 0.425 is the product of 0.85, the standard assumed value for leaf absorptance (α), and 0.5, the standard fraction of quanta absorbed by PSII relative to PSI (β), and ΦPSII the quantum efficiency of PSII measured from fluorescence [ΦPSII=(Fm′–Fs′)/Fm′]. If measured values for leaf absorptance are available, the assumed α, and the estimate for Jraw, can be improved. However the calibration procedures described below are often used to estimate a value for αβ and therefore α is not typically necessary. Jraw then can be related to under appropriate non-photorespiratory conditions—normally at 1% O2—where Vo is negligible. From this relationship, the empirical values for αβ can be found and thus provide a calibrated estimate of total electron flux (Jcal). Under non-photorespiratory conditions Equations 5 and 6 become:
| (7) |
assuming a linear relationship. Thus the corrected value for αβ is m×0.425. Alternatively, this equation is often converted from electron transport rates to quantum efficiencies by solving for ΦPSII, preferably when no intercept is present:
| (8) |
where is the quantum efficiency of photosynthesis ([A+Rd]/PPFD), m′ is the slope of the efficiency relationship, and the calibrated value for αβ is 4m′. In practice, either of these relationships (Equation 7 or 8) are used for the calibration of Jraw, with the fitted slopes providing an estimate of the value of αβ for the calibration conditions. The intercept is usually assumed to be zero. Alternatively, the presence of a non-zero y-intercept can be tested: if present, it represents alternative electron transport at the photo-compensation point.
This calibration procedure is based upon the assumptions that: (i) α and β are constant across the range of experimental variation; (ii) it is possible to estimate alternative electron transport as a constant proportion of total electron flux estimated as the intercept of the relationship; and (iii) the non-photorespiratory measurement conditions do not alter alternative electron transport relative to the experimental conditions. If either αβ or alternative electron transport vary with the environmental condition used to create the relationship (light or CO2), non-linearities should be present in the relationship. An alternative is then to fit a non-linear function to the calibration data, such as the following linear–sigmoidal function:
| (9) |
Analysis of sensitivity of ‘variable J’ gm magnitude to calibration scenarios
To test whether calibration variants have significant effects on the calculation of gm, a sensitivity analysis was performed. Apparent shifts in gm due to changing the calibrations were calculated as follows for gas exchange measurements made on six leaves under ambient conditions (400 μmol mol−1 CO2 and a PPFD of 400 μmol m−2 s−1). (1) Standard calibration using assumed α and β values (0.85 and 0.5) as is often used for the variable J method, with the following variants: (1a) the mean Rd and Γ* values measured using the Laisk method with six replicates; (1b) Rd plus and (1c) Rd minus the 95% CI of the mean; (1d) Γ* plus and (1e) Γ*minus the 95% confidence interval of the mean. (2) Standard calibration using a measured α (0.831) and assumed β value (0.5). (3) Calibrations fit to light response data measured under non-photorespiratory conditions at ambient CO2: (3a) using a linear fit, passing through the origin on the efficiency plot, but only using data points below a of 0.05 as suggested by Seaton and Walker (1990) and (3b) a linear–sigmoidal fit to the combined light response data on the rate plot. (4) Calibrations fit to the CO2 response data measured at 400 μmol m−2 s−1 PPFD and under non-photorespiratory conditions, using the linear–sigmoidal function. Fitted parameters for the calibration functions are provided in the Results. The non-linearity of the calibrations was assessed by comparing the Akaike Information Criterion (AIC) values between linear–sigmoidal fits and linear fits, where fits with the lowest AIC values have greatest support with model complexity taken into account (Burnham and Anderson, 2004). The R statistical program was used for these analyses (R_Development_Core_Team, 2010). An apparent value for gm was calculated for each of the six replicate leaves for all of the scenarios or parameter changes described above.
Cross-validation of ‘variable J’ gm with gm estimated from curve-fitting procedures
Values of mesophyll conductance to CO2 were measured for an additional 10 CO2 response curves using the same apparatus, corrections, and measurement conditions as detailed above. Added to the five initial CO2 response curves measured at 21% O2, these provided a total of 15 curves, with an average of 14 CO2 levels per curve. The measurements for the CO2 response curves were made simultaneously with the fluorescence measurements, by using the 2 cm2 LI-COR fluorescence chamber, a necessary compromise, as the goal of this experiment was cross-validation between the variable J and curve-fitting methods. Ideally, measurements for use in curve fitting should be made using larger leaf areas (Warren, 2006; Pons et al., 2009).
The Exhaustive Dual Optimization (EDO) curve-fitting technique of Gu et al. (2010), as implemented on the LeafWeb website, was employed for this analysis in cognizance of the curve-fitting parameterization issues raised in that paper. The technique is based upon the principle that the photosynthetic CO2 response curve can be represented by the minimum of a combination of three equations (Equation 10). These equations are non-rectangular hyperbolas that explicitly account for a non-infinite gm, and are based upon the original Farquhar–von Caemmerer–Berry-type photosynthetic functions:
| (10) |
The EDO approach uses functions for these three processes approximately similar to past curve-fitting approaches (Ethier et al., 2006; Warren, 2006), but applies these by assessing the possibility that any CO2 response curve point could be limited by any of the three processes, with some constraints. The technique then exhaustively searches for parameter estimates for all of these possible limitation states, and selects the optimal fit as the fit with a minimum of a cost function consistent with the form of Equation 10. For the EDO analysis, five parameters (gm, Vc,max, J, Rd, and the rate of triose phosphate utilization) were fit. To test for reliability of the parameter estimate for gm, the first and second derivatives of the cost function with respect to gm were tested to be zero and non-zero, respectively (Gu et al., 2010).
To enable comparison between the two methods, gm values for the curve-fitting procedure (representing the entire CO2 response curve) were compared with variable J gm values measured at ambient CO2 (ambient CO2 point on the CO2 response curve), or the interpolated gm value for a Ci of 600 μmol mol−1 (interpolated as the point at a Ci of 600 μmol mol−1 on the line connecting the measured gm and Ci value greater and less than 600 μmol mol−1).
Sensitivity analysis of ‘variable J’ gm to CO2 variation
The sensitivity of gm response to variation in CO2 to errors in the estimation of Rd or Jcal was assessed by introducing a constant offset into a Farquhar–von Caemmerer–Berry-type photosynthetic model that held gm constant. A region of the modelled gm to Ci response curve was defined from a Ci of 200–500 μmol mol−1 corresponding to values for which photorespiration should not be greatly inhibited, and thus measurements of Vo would be relatively accurate. A linear slope was fit to these data, and non-zero slopes were recorded in response to introducing positive or negative biases in Rd or Jcal.
The photosynthetic modelling was conducted using inputs of varying Cc and constant values of gm (0.3 mol m−2 s−1), Rd (0.42 μmol m−2 s−1), Γ* (36.0 μmol mol−1), Vcmax (70 μmol m−2 s−1), and J (108 μmol m−2 s−1). The values for these parameters were chosen to represent approximately a measured CO2 response curve for P. trichocarpa. The model calculations are provided online as a spreadsheet (Supplementary Spreadsheet S1 available at JXB online). Kc and Ko values and the standard Farquhar–von Caemmerer–Berry equations were taken from von Caemmerer (2000), and reference to them is provided in the spreadsheet. From these inputs, Vc,min was calculated from the limiting process, namely the minimum of Vc,c and Vc,j (the Rubisco and RuBP regeneration-limited carboxylation rates), and the photorespiration rate, Vo, calculated from Vc,min. The total RuBP regeneration rate, Jtotal/4, was calculated as the sum of Vc,min and Vo, assuming no alternative electron transport sinks and strict linear correspondence to fluorescence. Thus Jtotal/4 provides a value for Jcal/4, as per the variable J method. Ci was calculated using Fick's law and the calculated value for A. This model explicitly held gm constant; a bias was then introduced into the assumed value of Rd or modelled value for Jcal/4 (Jtotal/4), and from these new values a ‘biased’ estimate of gm was obtained using the formulae associated with the variable J method; the reverse of the initial calculations. Note that the formulae used in the variable J method given above (Equations 1–4) are algebraically the same as those used in the photosynthetic model just outlined. Thus the only difference between the biased estimate of gm and the value for gm when held constant is the introduction of a constant error in Rd or Jcal/4.
Results
Calibration of fluorescence estimates of electron transport rate with gas exchange
Data used to calibrate fluorescence with gas exchange can be expressed either as quantum efficiency plots or as rate plots using CO2 or electron equivalent units. As each has its advantages, the same light or CO2 response curve data were compared on both plots (Fig. 1). The calibration relationships relating photosynthetic rates in electron-equivalent units () to uncalibrated fluorescence estimates of electron transport (Jraw) under non-photorespiratory conditions were non-linear when measured across a broad range of light or CO2 conditions (Fig. 1). Light response curves demonstrated three phases of non-linearity: a subtle increase in Jraw relative to at low light, a large shift towards increased Jraw, but not , at intermediate light, and in some responses a return to the one-to-one line at the highest light levels (Fig. 1A). For the same data plotted as efficiency plots (note the reverse in direction representing increasing light), the same shifts resulted in curvature towards greater quantum efficiency of net photosynthesis () at low light (Fig. 1C). This method of plotting the same data emphasizes the second curvature towards greater PSII efficiency (ΦPSII) at very low light (∼100 μmol m−2 s−1).
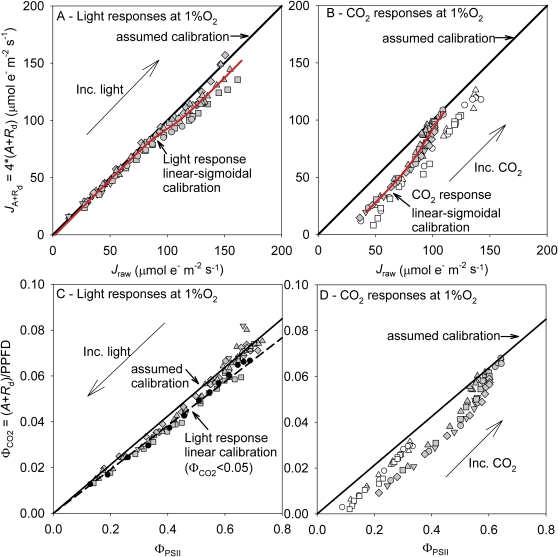
Fig. 1.
Calibration plots for the rate of photosynthesis versus electron transport estimated from fluorescence (A and B), or for the photosynthetic versus fluorescence quantum efficiencies (C and D), measured under non-photorespiratory conditions (1% O2) using light response curves (A and C) or CO2 response curves with 400 μmol m−2 s−1 or 1000 μmol m−2 s−1 PPFD (grey and white symbols) (B and D). Jraw was calculated using standard parameters (Jraw=αβ×PPFD×ΦPSII, α=0.85, β=0.5, thus αβ=0.425). Three lines are shown: the line where Jcal=Jraw (solid line; all panels), the average linear fit for nine light responses on the efficiency plot for points below a of 0.05 (dashed line; C) and the average fitted linear–sigmoidal curve fit to the data of the nine light or five CO2 responses (A and B). Different symbols represent measurements on different leaves. In C, one representative response curve is highlighted in black to illustrate regions of concave curvature at low s and a final increase in ΦPSII at high (low light). Arrows demonstrate the direction in which light or CO2 increases on the different calibration plots. (This figure is available in colour at JXB online.)
Due to the curvature of the light response data from low to high light, two calibration relationships were fit. In the first method, a linear calibration was fit to each replicate light response on the efficiency plot forcing each line to pass through the origin (intercepts were not significantly different from the origin over this range of PPFD) and using data below a of 0.05 (here an average PPFD of >500 μmol m−2 s−1), consistent with the suggestions of Seaton and Walker (1990) and resulting in αβ=0.383. If the calibration was done using an assumed value for α of 0.85, as is common, the calibrated β value would be 0.451 rather than 0.5. The measured value of α was 0.831, resulting in an estimate of 0.461 for the β value for higher light intensities. For comparative purposes, the one-to-one line was considered as the standard ‘calibration’ (αβ=0.425), as it is common to assume this value for αβ with no further calibration. In the second calibration, a linear–sigmoidal curve was fit to all of the light response replicates simultaneously for the rate plot (Equation 9, a linear–sigmoidal fit: a=11.1, b=99.9, c=1.87, d=8.31, adjusted R2=0.974, AIC value=597.47). A linear fit to the same data resulted in a marginally higher AIC value (598.81) and similar adjusted R2 (0.974).
It is common to use light response curves to calibrate the variable J method and then make use of them in studies using other experimental stimuli, for example variation in CO2. The carbon dioxide response curves measured at low O2 and 400 μmol m−2 s−1 PPFD did not resemble the light response curves at lower CO2 concentrations, or at higher light (Fig. 1B). A large shift was observed in the opposite direction to the light response curves, consistent with an increase in alternative electron sinks as may be expected by the lack of photosynthetic ability to use reductant under conditions of high light, low CO2, and low O2. As the high CO2 points were measured after the low CO2 points, and showed high efficiency nearing the one-to-one line, photoinhibition was not apparent (also Fv′/Fm′ returned to pre-low CO2 exposure levels). The relationship on the efficiency plot was not linear; therefore, the CO2 response curves were only calibrated using a linear–sigmoidal function on the rate plots (Equation 9, a linear–sigmoidal fit: a=22.8, b=95.3, c= –0.51, d= –8.6, adjusted R2=0.958, AIC value=357.7). A linear fit to the same data resulted in a considerably higher AIC value (367.6) and lower adjusted R2 (0.949), the difference between AIC values of ∼10 signifying that the linear–sigmoidal fit had more support than the linear fit, despite taking into account the extra parameters in the linear–sigmoidal model (Burnham and Anderson, 2004). At a higher PPFD of 1000 μmol m−2 s−1, CO2 responses showed greater deviation from the one-to-one line, with ∼40 μmol e– m−2 s−1 of apparent electron transport for little assimilation at the lowest CO2 levels. To the authors’ knowledge, robust data have not been presented to validate that the calibration relationship is the same between conditions of varying light and CO2, apart from Hassiotou et al. (2009) whose results largely confirm those in Fig. 1. Most studies use a single saturating flash to measure Fm', potentially introducing additional non-linear effects with changing light (Markgraf and Berry, 1990; Earl and Ennahli, 2004). However, measurements of varying light demonstrated that using multiple saturating flashes rather than a single flash did not linearize the calibration functions, and rather the size of the discrepancy between and Jraw was slightly enhanced at high PPFDs (Fm' increases while Fs remains constant). As the key CO2 calibrations were done at moderate PPFD (400 μmol m−2 s−1), these were not affected.
Size of calibration biases on Jcal and gm
From the data shown in Fig. 1, five types of calibrations were performed: the three linear or linear–sigmoidal functions, the standard assumed calibration parameters from the literature, and the measured leaf absorbtance and assumed β. The magnitude of the errors in Jcal on the rate plots, and particularly the efficiency plots, is both difficult to visualize and hard to relate to the magnitude of the measured quantities. Therefore, data were expressed as the residuals for the rate relationship, rescaled to units of CO2 uptake, and plotted against the PPFD or CO2 used to generate the points (Fig. 2A, B). The residuals were calculated as Jcal/4–A, where Jcal is the electron transport rate calibrated using one of the five types of calibration.
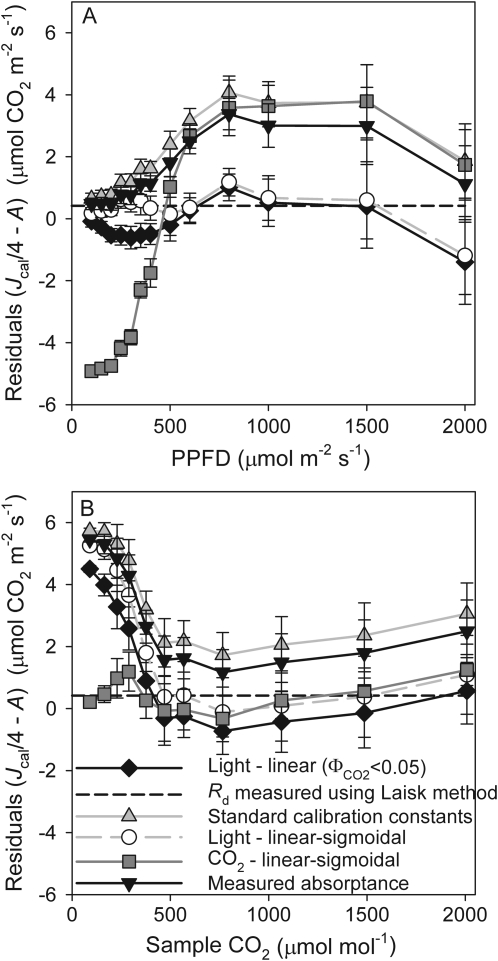
Fig. 2.
The average residuals of the calibrated rate of electron transport (Jcal), rescaled to units of CO2 uptake, relative to the observed photosynthetic rate for light (A) or CO2 response curves (B) measured under non-photorespiratory conditions. To allow for possible trends in Rd, residuals were calculated as Jcal/4–A. Points represent the mean and standard errors for five or more replicate light curves (the same data as in Fig. 1A and C) or five CO2 response curves (the same data as in Fig. 1B and D).
The linear–sigmoidal calibrations applied to the same environmental variation to which they were fit produced the smallest residuals, and did not have any systematic errors across a broad range of light or CO2, possibly apart from 2000 μmol m−2 s−1 PPFD (Fig. 2A). The linear higher light calibration produced few residuals at high light, but consistently underestimated A by ∼1 μmol CO2 m−2 s−1 at low light. The standard calibration, assuming αβ=0.425, performed poorly, with significant overestimates of A of up to 4 μmol CO2 m−2 s−1 under all but low light conditions. The standard calibration was even worse for low CO2 conditions (Fig. 2B), resulting in residuals as high as 6 μmol CO2 m−2 s−1. The calibration with assumed value for β and measured α had a similar pattern to the standard calibration although the residuals were improved.
The variation between these calibration curves resulted in large differences in apparent gm values when applied to measured photosynthetic data for ambient CO2 and moderate light (Table 1). Values ranged by 104% from a minimum of the standard assumed calibration to that of the linear calibration. Linear–sigmoidal fits to light or CO2 response curves were intermediate. This variation in apparent gm due to the underlying calibration was larger than variation in gm caused by changes in Rd or Γ* when adjusted by the 95% CIs of the mean values (Table 1).
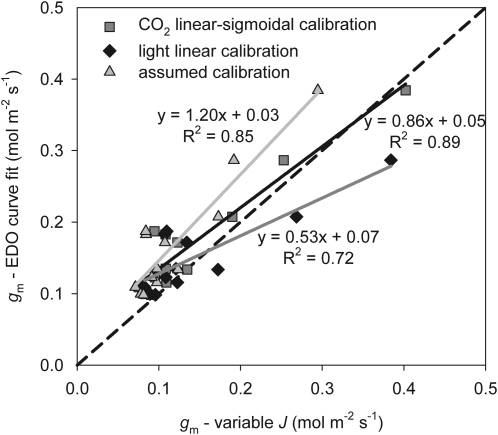
Correspondence between ‘variable J’ gm and curve-fitting gm
The gm values calculated by the variable J and EDO approach curve-fitting method were most highly correlated, and the points nearest the one-to-one line for the CO2 linear–sigmoidal calibration (Fig. 3). The assumed and linear-light calibrations resulted in correlations between the variable J gm and the curve-fitting gm values, but resulted in greater deviation from the one-to-one relationship. In addition, the linear-light calibration resulted in a negative value of gm, which was removed from the analysis. Two gm values were removed from all analyses due to the EDO curve-fitting analysis providing high gm values (>0.5 mol m−2 s−1), and this was consistent with a zero second derivative of the EDO cost function (the condition under which the parameter estimate is not reliable). For this analysis the variable J gm estimate was limited to measurements made at ambient CO2, while the curve-fitting estimate used the entire CO2 response curve data. When the variable J gm value representing a Ci of 600 μmol mol−1 was plotted against the curve-fit gm value, R2s were reduced and the variable J gm value was an underestimate for all calibrations. For the linear-light calibration a number of variable J gm estimates at high Ci were negative.
Fig. 3.
Cross-validation of gm values calculated from 15 CO2 response curves using three alternative calibrations for the variable J method and applied to the ambient CO2 measurement on the curve, and gm calculated from the Exhaustive Dual Optimization (EDO) approach for fitting Farquhar–von Caemmerer–Berry models of Gu et al. (2010). Experimental conditions were: PPFD, 400 μmol m−2 s−1; Tl, 24.9±0.8 °C; VPD, 1.47±0.49 kPa.
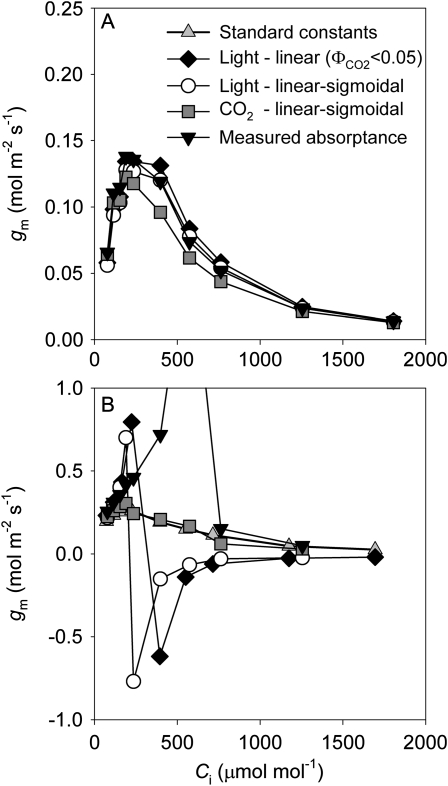
Response of gm to CO2
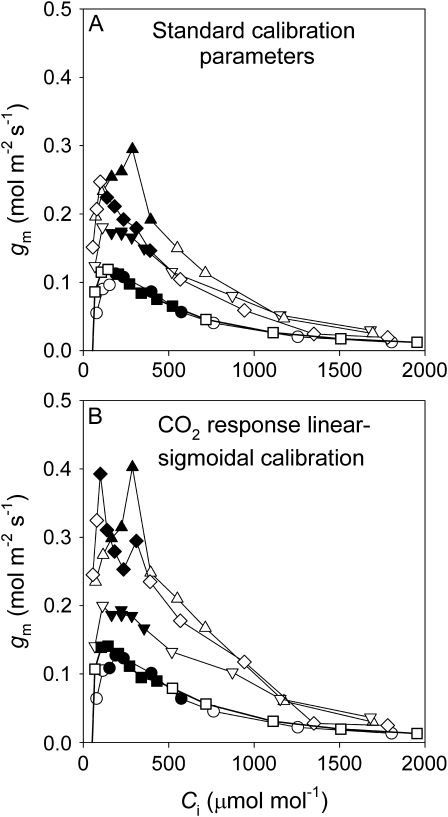
The response of gm to CO2 (detrended for stomatal conductance changes by using Ci) was highly variable when the five calibration protocols were compared (Fig. 4). In one of the five replicates (Fig. 4A), all five calibrations produced values for gm in the range of past reports (Niinemets et al., 2009); in the other four replicates the linear and linear–sigmoidal fit to the light response calibration resulted in negative or large (>1) values for gm at CO2 levels higher than ambient (one representative replicate is shown in Fig. 4B).
Fig. 4.
The observed response of gm to Ci illustrating either qualitative agreement amongst the four calibration options (representative of only one of five replicate CO2 response curves; A) or marked disagreement between calibration methods (a representative CO2 response curve for four of five replicates; B). Note that in B two of the calibration options result in negative values of gm due to overestimates of Cc (apparent Cc >Ci). These occur as gm=A/(Ci–Cc), thus underestimates of Jcal at high Ci due to the different calibrations lead to Cc approaching Ci, the denominator of the equation is small leading to gm approaching infinity, and when Cc becomes higher than Ci, gm instantly becomes negative. Experimental conditions were the same as in Fig. 3.
As only the standard calibration constants and the linear–sigmoidal fit to the CO2 response calibration gave reasonable values for gm for all replicates, these two calibration protocols were investigated in greater detail. Using either calibration, gm showed strong shifts, increasing from the lowest Ci values, remaining stable or slowly decreasing at ambient CO2 values, and decreasing strongly at high Cis (Fig. 5A, B). However, the Harley et al. (1992) criterion was violated for almost all points at high Ci. Nevertheless, the points that satisfy the Harley criterion (Harley et al., 1992) demonstrate a consistent negative response of gm to Ci.
Fig. 5.
Observed gm response to Ci for five replicate CO2 response curve using the standard calibration constants (A) and the linear–sigmoidal fit to the CO2 response calibration (B). Points that satisfy the Harley criterion (filled symbols) and points that had a Harley criterion of <10 or >50 (open symbols) are distinguished. Replicate curves are shown with the same symbols. Experimental conditions were the same as in Fig. 3.
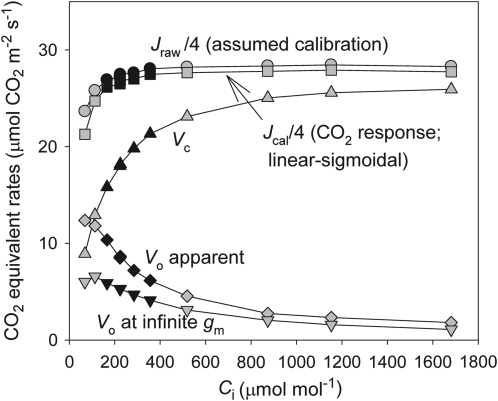
The potential for calibration biases to affect the calculation of gm can be illustrated by plotting Ci versus the parameters used to estimate Cc and gm (Fig. 6). As Ci increases, Vo tends towards zero due to competitive inhibition of photorespiration by CO2. As a result, Cc —proportional to the Vc/Vo ratio—is increasingly vulnerable to biases at high Ci. Specifically, as Vo decreases at higher CO2s, any errors in its estimation lead to an inflated Cc (calculated from the Vc/Vo ratio), as Cc tends towards Ci, gm values [calculated from A/(Ci–Cc)] rapidly become large. Once Cc, estimated with slight errors, is the same or larger than Ci, gm becomes infinite or negative. This explains the variability, high and negative values of gm in Fig. 4. Furthermore, as Vo tends towards zero, biases in Jcal/4 due to alternative electron transport sinks (or changes in Rd, α, or β) become increasingly important. In other words, small errors in the estimation of Jcal have increasing impact on the estimation of gm at high Ci, as Vo becomes small and the error to the Vo ratio increases.
Fig. 6.
Rates of the standard fluorescence estimate of electron transport (Jraw), the calibrated rate of electron transport expressed in CO2 equivalents, carboxylation (Vc), apparent photorespiration rate (Vo apparent=Jcal/4–Vc), and photorespiration calculated assuming Cc=Ci (Vo at infinite gm), for a measured response to CO2. Note the small shift in Vo necessary to result in an infinite gm at high CO2. Points that satisfy the Harley criterion (filled symbols) and those that did not (open symbols) are distinguished. Experimental conditions were the same as in Fig. 3.
Sensitivity of response of gm to CO2
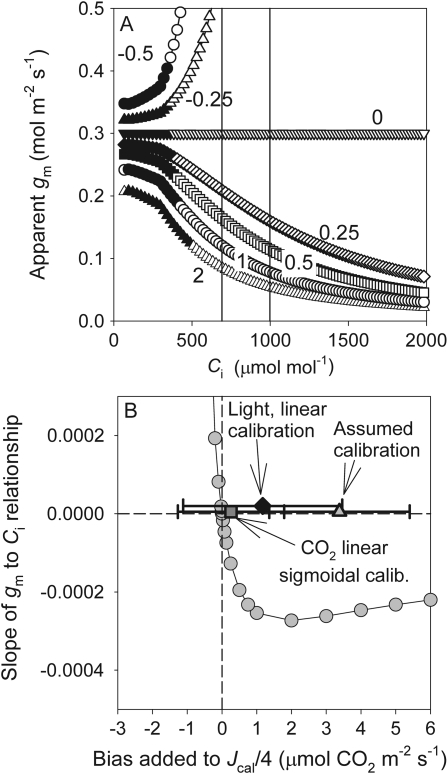
The sensitivity of gm to calibration biases was further investigated by introducing small biases in Jcal/4 into a photosynthetic model. The lines in Fig. 7A represent the apparent gm response for simulated data for which gm was held constant, but for which small systematic errors were introduced into the value for Jcal/4 and gm then calculated from the biased data. Any overestimation of Jcal/4 results in a lower gm and an apparent negative relationship with increasing Ci (Fig. 7A). In contrast, underestimating Jcal/4 results in a larger apparent gm. The presence of a positive or negative relationship between gm and Ci was a function of the small constant biases added to Jcal/4 (Fig. 7B). If, in the photosynthetic model, gm is assumed to be constant with CO2, then the residuals in Fig. 7B demonstrate that previously used calibration relationships would consistently result in apparent negative gm responses to CO2, while the linear–sigmoidal CO2 response calibration would result in both negative and positive relationships. If this assumption is true, the sensitivity analysis demonstrates that the bias in Jcal/4 necessary to result in an artefactual effect of Ci on gm is small (<0.5 μmol CO2 m−2 s−1) relative to the residuals typically observed in the calibration relationship (∼0.5–6 μmol CO2 m−2 s−1; Fig. 2A or B and Fig. 7B). If, however, gm is truly not constant, the observed slope of the response of gm to CO2 would still be sensitive to errors, and if errors were large enough would result in transitions from negative to positive or vice versa. The model is included as a spreadsheet in the Supplementary data at JXB online.
Fig. 7.
The sensitivity of the gm to Ci response to constant errors added to Jcal/4 for a photosynthesis model that had a constant gm (A) and the sensitivity of the slope of the gm to Ci response for the same errors in the photosynthesis model (B). Average and standard deviations of the residuals [Jcal/4–(A+Rd)] of three calibration options are shown in B. The slope of the gm to Ci response was defined as the linear fit to the modelled data within a range of Cis that would satisfy the criterion of Harley et al. (1992) (200–500 μmol mol−1). The residuals were calculated across the same range of Ci. Numbers in A represent the overestimate (error) added to Jcal/4 where zero error (and thus the true modelled relationship) had no relationship between gm and Ci. Vertical lines in A represent the Ci at which gm estimates shifted from high to negative values (see text for explanation).
Discussion
Can the variable J method measure the response of gm to CO2?
The nature of the observed response of gm to CO2 is highly sensitive to biases in the estimation of the calibrated total photosynthetic electron transport rate (Jcal). This was demonstrated using a sensitivity analysis of standard photosynthetic equations, to which a systematic bias was added. For example, the sensitivity is such that there is an apparent 23% decrease in gm over a 300 μmol mol−1 range of Ci when an ∼2% (∼0.5 μmol CO2 m−2 s−1) overestimate of Jcal/4 is included in the photosynthetic model, despite the modelled gm remaining constant (Fig. 7A). As the true modelled relationship was on the steepest portion of the sensitivity analysis (Fig. 7B), this demonstrates that if gm is indeed constant, then any bias in Jcal/4 will lead to artefactual positive or negative relationships of gm to Ci. If gm is dynamic, varying with CO2, the point of greatest sensitivity will drift, but the overall pattern of sensitivity demonstrated will remain. In this case, an observed relationship may represent a true response, but the slope will be sensitive to measurement errors and calibration biases. This sensitivity analysis provides similar results to those which Harley et al. (1992) presented in their fig. 6, and those which Hassiotou et al. (2009) presented in their supplementary material. Indeed, Harley et al. (1992) note that: ‘In all cases, the sensitivity to errors was relatively low between 100 and 300 μbar Ci, but outside this range the sensitivity was so great that the results could become unreliable.’ Despite these earlier cautions, subsequent researchers have continued to use this approach over a broad range of conditions. It is important to note that these considerations are applicable to any environmental variation that may affect photorespiration: CO2, temperature, light, stomatal closure, etc. For instance, a similar analysis could be done for the relationship of gm to PPFD, in which case the relationship would be sensitive to errors at PPFDs below light saturation where the errors become significant relative to photorespiration. Thus it is also the relationship of gm to light that is sensitive to errors when using the variable J method, although at saturating light intensities the presence of high rates of photorespiration leads to less sensitive estimates of the relationship of gm to light.
The residual variation in the different calibration relationships—which is a determinant of the error in Jcal/4—was up to 5 μmol CO2 m−2 s−1 at the extreme of using standard calibration constants, and about ±1 μmol CO2 m−2 s−1 when calibrated using a linear–sigmoidal function on CO2 response data (Fig. 2A, B). Thus, the magnitude of the errors in the calibrations was similar to, or considerably larger than, the error necessary to affect whether there is an apparent response of gm to Ci (Fig. 7B). It is broadly true then, given the large errors in our best estimates of Jcal/4, and the sensitivity of the gm to Ci relationship to any error, that it is difficult to measure the response of gm to Ci using the variable J method. That is, with the high overestimates of Jcal demonstrated for standard calibration methods over a moderate CO2 range (Fig. 7B), the variable J method is likely to produce steeper relationships between gm and CO2 than actually exist.
Variable J gm and partially independent gm values from the EDO curve-fitting approach corresponded well when the variable J technique was limited to use under ambient CO2 and with the non-linear calibrations reported here (Fig. 3). These results are consistent with the sensitivity analysis performed earlier (Table 1). That is, relative to the EDO curve-fitting gm values the linear-light calibration causes overestimates in variable J gm, the CO2 linear–sigmoidal calibration results in approximate correspondence, and the assumed calibration results in underestimates. This suggests that when appropriately calibrated the variable J method has value for studies comparing species, using unstressed plants at moderate light and ambient CO2, but should not be applied across a range of environmental conditions under which photorespiration is likely to vary. However, these non-linear calibrations remain empirical and do not address the implication that a non-linear response represents an unaccounted for fundamental change in photosynthetic functioning.
Why are the calibrations non-linear?
It is difficult to provide a retrospective review of whether the non-linearities in the calibration relationships observed here are present in the gm literature. For example, a literature review of 56 experimental studies of gm, published since 1992, found that 66% of these use the variable J method, and 44% use it as a sole technique. Of these, few studies provided calibration data, and if this was done even fewer calibrated the variable J method using environmental variation appropriate for the experiment at hand. Fewer performed brief sensitivity analyses, and finally no study attempted to calibrate the technique using non-linear functions. However, many of the non-linear effects described here have been previously described by Seaton and Walker (1990) and Oquist and Chow (1992). There are also indications of non-linearities in the calibration relationships used to calculate gm or Cc (Warren, 2006; Galle et al., 2009; Hassiotou et al., 2009; Loreto et al., 2009).
Seaton and Walker (1990) and Oquist and Chow (1992) demonstrated large non-linearities in light response curves, plotted as efficiency plots, measured under non-photorespiratory, saturating CO2 conditions and with oxygen electrodes. These curved relationships on efficiency plots are consistent with the sigmoidal patterns found on the rate plots, but there are clear differences in weighting of points between the plots. The reasons for the non-linearities are discussed by Oquist and Chow (1992) and include: (i) changing connectivity of PSII units, leading to more cycling of electrons between chlorophylls; (ii) at low light, mitochondrial respiration (Rd) may increase, but in the variable J calculations Rd is assumed to be constant and a single value usually estimated for all conditions from Laisk response curves; (iii) fluorescence parameters may be estimated from slightly shallower populations of chloroplasts than those that fix CO2, and the contributions of these populations of chloroplasts would change with light intensity (Warren, 2006; Evans, 2009). On the rate plots, possible alternative electron sinks are highlighted, resulting in non-linear shifts in the calibration relationship, and may represent little (Ruuska et al., 2000), or up to 24% of the total electron flux (Haupt-Herting and Fock, 2002). Two main processes are thought to account for alternative electron sinks (von Caemmerer, 2000), each accounting for up to 10% of total electron flux: reductant provided to nitrate assimilation (Rachmilevitch et al., 2004) and the Mehler reaction (Haupt-Herting and Fock, 2002).
These effects are highlighted when comparing light and CO2 responses measured under non-photorespiratory conditions (Fig. 1A, B). A priori this must be expected, as at low CO2, and particularly at high light, there is a limitation on reductant use, but high reductant supply that will result in large alterations of PSII heat dissipation and may result in up-regulation of alternative dissipative energy sinks, such as the Mehler reaction (Neubauer and Yamamoto, 1992). The quantitative effects of alternative energy sinks remain unclear (Ruuska et al., 2000); however, it is noted that relative to the errors (∼2% of Jtotal/4) necessary to cause apparent changes in gm, estimates of alternative electron sinks are large and therefore vital to account for.
Finally, it is not clear whether alternative electron sinks are changed when shifting from ambient to low O2 as required for the calibration curves (Pons et al., 2009). For instance, at high CO2 the calibration curve was closer to the one-to-one line than for high light points (Fig. 1). This may imply that alternative electron transport sinks are affected by the capacity of photosynthesis to dissipate absorbed light energy, or are directly affected by CO2. Considerable shifts in nitrate assimilation with age, CO2, and oxygen concentration occur in Arabidopsis, using an equivalent electron flux up to 10% of the photosynthetic rate (Rachmilevitch et al., 2004), and thus represent evidence of alternative electron transport shifts that could occur during the calibration procedure. If this is generally the case, it would be challenging to find conditions under which the variable J method can be calibrated. Indeed, the fitted α or β parameters for a non-linear calibration function cannot then be interpreted as physical constants as the non-linearity implies that they change with environmental conditions, or that they include alternative electron transport sinks. It appears that much work remains to be done, using independent methods, to understand the implications of the photosynthetic changes that occur when producing calibration relationships for the estimation of gm and Cc using the variable J method.
How should the variable J method be used?
The variable J method appears difficult to validate under circumstances of varying photorespiration due to the extreme sensitivity of gm under conditions of low photorespiration. However, the method when calibrated taking non-linearities into account did improve the estimates of gm under ambient CO2 relative to the EDO curve-fitting approach. Thus if the variable J method is to be used for comparing species (and not environmental variation) the following are imperative: (i) a calibration is done with conditions that match the experimental conditions (not a light calibration versus CO2 experiment); (ii) the calibration (and experiment) is limited to the linear region, for example <0.05; Seaton and Walker (1990), or non-linear functions are used, and if not linearity should be explicitly tested; (iii) the calibration is fit using rate plots, not the efficiency plots that emphasize low photosynthetic rate points disproportionately; and (iv) a sensitivity analysis is done that asks what size biases in the estimation of Jcal, or variation in the values for Rd and Γ*, are necessary to remove the observed effect or relationship, and are such errors plausible for the calibrations. Regardless of these improvements, the lack of knowledge of why the calibration response is curved, and whether alternative electron sinks are affected by changing O2 may preclude the use of the variable J method in most experiments.
Conclusion
The variable J method is sensitive to errors and must be used with caution in experiments where photorespiration varies. Nevertheless, none of the calibration or sensitivity scenarios tested here precludes an effect of any variable on gm; thus gm may be dynamic rather than constitutive, but these results suggest that we cannot know the magnitude or nature of changes with certainty using this technique. It is suggested to limit use of the variable J method to comparing species under conditions of moderate light and ambient CO2 with appropriate calibration, and not in experiments measuring responses to environmental factors that affect photorespiration. There is much research needed using independent methods to provide information on whether and how gm and alternative electron sinks respond to CO2, light, or O2. The region at which gm measured using the variable J method starts declining with CO2 (Flexas et al., 2007) or reduced light (personal observation) corresponds to the point where RuBP regeneration becomes limiting to photosynthesis. Although this may occur through a common mechanism related to RuBP regeneration, this effect is less apparent in gm measurements using carbon isotope discrimination (Flexas et al., 2007; Tazoe et al., 2009, 2011; Vrabl et al., 2009). The point where RuBP regeneration becomes limiting for both the light and CO2 response curves also corresponds to a decrease in photorespiration. Thus at this point the ratio of biases to photorespiration dramatically increases, causing artefacts to be introduced into the response of variable J gm to CO2 or light, if subtle biases are present in the calibration or measurements. Thus, it is suggested that positive biases in the calibration procedure result in the variable J method overestimating the slope of the relationship between gm and Ci—an explanation for the differences between studies using the variable J method and those using carbon dioxide discrimination.
Supplementary data
Supplementary data are available at JXB online.
Spreadsheet S1. Sensitivity analysis of modelled photosynthetic response to CO2, with gm held constant.
Acknowledgments
This research was funded in part by USDA-CREES grant number 2006-35100-7263, a Spanish Ministry of Education and Research project AGL2005-06927-CO2-01/AGR (to AP), and a Giorgio Ruffolo Fellowship in the Sustainability Science Program at Harvard University (to MG) for which the Italian Ministry for Land, Environment and Sea is gratefully acknowledged. We thank Jessica Savage for comments on the manuscript, and Lianhong Gu and the creators of the EDO curve-fitting website at http://www.leafweb.ornl.gov.
Glossary
Abbreviations
- α
leaf absorptance
- β
fraction of quanta absorbed by PSII
- ΦPSII
quantum efficiency of PSII
- Φco2
quantum efficiency of gas exchange
- Γ*
photo-compensation point
- A
net photosynthetic rate
- Ac
Rubisco-limited photosynthetic rate
- Aj
RuBP regeneration-limited photosynthetic rate
- ATPU
triose phosphate utilization-limited photosynthetic rate
- Cc
chloroplastic CO2 concentration
- Ci
intercellular CO2 concentration
- Ci*
apparent photo-compensation point
- EDO
exhaustive dual optimization procedure
- gm
mesophyll conductance to CO2
- Kc
Rubisco Michaelis–Menten constant for carboxylation
- Ko
Rubisco Michaelis–Menten constant for photorespiration
- J
electroxn transport rate
- JA+Rd
rate of J needed to account for measured A+Rd
- Jcal,
calibrated fluorescence-derived J
- Jraw
uncalibrated fluorescence-derived J
- Jtotal
modelled or measured total J to carboxylation and photorespiration
- O
oxygen mole fraction
- PPFD
photosynthetic photon flux density
- Rd
mitochondrial respiration in the light
- Sc/o
relative specificity of Rubisco
- Tl
leaf temperature
- Vc
rate of carboxylation
- Vc,c
rate of carboxylation limited by Rubisco
- Vc,j
rate of carboxylation limited by RuBP regeneration
- Vc,min
minimum of Vc,c and Vc,j
- Vcmax
maximum rate of Rubisco carboxylation
- Vo
rate of photorespiration
- Valt
alternative J in CO2 equivalents
- VPD
vapour pressure deficit
References
- Anon . Using the LI-6400 portable photosynthesis system. Lincoln, NE: LI-COR Biosciences; 2004. [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference—understanding AIC and BIC in model selection. Sociological Methods and Research. 2004;33:261–304. [Google Scholar]
- Earl HJ, Ennahli S. Estimating photosynthetic electron transport via chlorophyll fluorometry without Photosystem II light saturation. Photosynthesis Research. 2004;82:177–186. doi: 10.1007/s11120-004-1454-3. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA. Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant, Cell and Environment. 2006;29:2168–2184. doi: 10.1111/j.1365-3040.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- Evans JR. Potential errors in electron transport rates calculated from chlorophyll fluorescence as revealed by a multilayer leaf model. Plant and Cell Physiology. 2009;50:698–706. doi: 10.1093/pcp/pcp041. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmes J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment. 2007;30:1284–1298. doi: 10.1111/j.1365-3040.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Galle A, Florez-Sarasa I, Tomas M, Pou A, Medrano H, Ribas-Carbo M, Flexas J. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? Journal of Experimental Botany. 2009;60:2379–2390. doi: 10.1093/jxb/erp071. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gu LH, Pallardy SG, Tu K, Law BE, Wullschleger SD. Reliable estimation of biochemical parameters from C3 leaf photosynthesis–intercellular carbon dioxide response curves. Plant, Cell and Environment. 2010;33:1852–1874. doi: 10.1111/j.1365-3040.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany. 2009;60:2303–2314. doi: 10.1093/jxb/erp021. [DOI] [PubMed] [Google Scholar]
- Haupt-Herting S, Fock HP. Oxygen exchange in relation to carbon assimilation in water-stressed leaves during photosynthesis. Annals of Botany. 2002;89:851–859. doi: 10.1093/aob/mcf023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Tsonev T, Centritto M. The impact of blue light on leaf mesophyll conductance. Journal of Experimental Botany. 2009;60:2283–2290. doi: 10.1093/jxb/erp112. [DOI] [PubMed] [Google Scholar]
- Markgraf T, Berry J. Measurement of photochemical and non-photochemical quenching: correction for turnover of PS2 during steady-state photosynthesis. In: Baltscheffsky M, editor. Current research in photosynthesis. Vol. IV. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 279–282. [Google Scholar]
- Neubauer C, Yamamoto HY. Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiology. 1992;99:1354–1361. doi: 10.1104/pp.99.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U, Diaz-Espejo A, Flexas J, Galmes J, Warren CR. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany. 2009;60:2249–2270. doi: 10.1093/jxb/erp036. [DOI] [PubMed] [Google Scholar]
- Oquist G, Chow WS. On the relationship between the quantum yield of photosystem-II electron-transport, as determined by chlorophyll fluorescence and the quantum yield of CO2-dependent O2 evolution. Photosynthesis Research. 1992;33:51–62. doi: 10.1007/BF00032982. [DOI] [PubMed] [Google Scholar]
- Parkhurst DF. Diffusion of CO2 and other gases inside leaves. New Phytologist. 1994;126:449–479. doi: 10.1111/j.1469-8137.1994.tb04244.x. [DOI] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany. 2009;60:2217–2234. doi: 10.1093/jxb/erp081. [DOI] [PubMed] [Google Scholar]
- R_Development_Core_Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Rachmilevitch S, Cousins AB, Bloom AJ. Nitrate assimilation in plant shoots depends on photorespiration. Proceedings of the National Academy of Sciences, USA. 2004;101:11506–11510. doi: 10.1073/pnas.0404388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. Journal of Experimental Botany. 2000;51:357–368. doi: 10.1093/jexbot/51.suppl_1.357. [DOI] [PubMed] [Google Scholar]
- Seaton GGR, Walker DA. Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proceedings of the Royal Society B: Biological Sciences. 1990;242:29–35. [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell and Environment. 2007;30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Badger MR, Evans JR. Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. Journal of Experimental Botany. 2009;60:2291–2301. doi: 10.1093/jxb/erp035. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Von Caemmerer S, Estavillo GM, Evans JR. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell and Environment. 2011;34:580–591. doi: 10.1111/j.1365-3040.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- Valentini R, Epron D, De Angelis P, Matteucci G, Dreyer E. In-situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey Oak (Quercus cerris L) leaves—diurnal cycles under different levels of water-supply. Plant, Cell and Environment. 1995;18:631–640. [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Collingwood, Australia: CSIRO Publishing. 2000 [Google Scholar]
- Vrabl D, Vaskova M, Hronkova M, Flexas J, Santrucek J. Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. Journal of Experimental Botany. 2009;60:2315–2323. doi: 10.1093/jxb/erp115. [DOI] [PubMed] [Google Scholar]
- Warren C. Estimating the internal conductance to CO2 movement. Functional Plant Biology. 2006;33:431–442. doi: 10.1071/FP05298. [DOI] [PubMed] [Google Scholar]
- Warren CR. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany. 2008;59:1475–1487. doi: 10.1093/jxb/erm245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.