Abstract
Helicobacter pylori are bacteria that have coevolved with humans to be transmitted from person to person and to persistently colonize the stomach. Their population structure is a model for the ecology of the indigenous microbiota. A well-choreographed equilibrium between bacterial effectors and host responses permits microbial persistence and health of the host but confers risk of serious diseases, including peptic ulceration and gastric neoplasia.
The twin hallmarks of the interaction between Helicobacter pylori and humans are its persistence during the life of the host, and the host’s responses to its continuing presence. This conflict appears paradoxical, but both the microbes and the host adapt to the other in the form of a long-standing dynamic equilibrium (1, S1 [http://www.jci.org/cgi/content/full/113/3/321/DC1]). Our understanding of the phenomena underlying these interactions is growing. The relationships are important, both because of the major role of H. pylori in promoting risk of peptic ulcer disease (2) and non-cardia adenocarcinoma of the stomach (3), and because of the emerging evidence that gastric H. pylori colonization has a protective role in relation to severe gastro-esophageal reflux disease and its sequelae, Barrett esophagus and adenocarcinoma of the esophagus (reviewed in ref. 4). New studies suggest other important impacts of H. pylori colonization on human physiology (5, 6).
We now present a general model for this host-microbial interaction and then turn to examples of specific operating mechanisms. Although H. pylori is unique in colonizing the human stomach, the principles governing the interaction are paradigms for understanding both commensalism and long-term parasitism. Such insights aid our understanding of disease processes as diverse as chronic inflammation, oncogenesis, and hormonal dysregulation and may be relevant to modern epidemic problems such as obesity and diabetes.
A general model of host-microbial persistence
Much evidence indicates that Helicobacter species are the indigenous biota of mammalian stomachs, and that H. pylori is the human-specific inhabitant (Figure 1a), having been present for at least tens of thousands of years, and probably for considerably longer (7–9). Therefore, coevolution of microbe and host might be expected, and for H. pylori, substantial evidence supports this notion (10), with important implications.
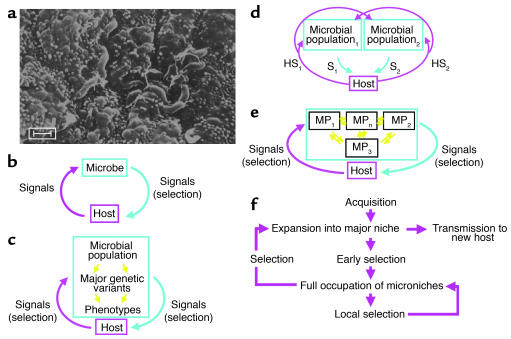
Figure 1.
Models of the cross-signaling between obligate parasites and their hosts. (a) H. pylori in the gastric (antral) niche, inhabiting the luminal mucus layer overlaying the epithelium. Scale bar: 1 μm. (b) Model 1: A coevolved microbe sends chemical and physical (contact) signals to its host. The host’s signals, including motion, temperature, and chemicals (including host-defense molecules), affect microbial growth. In a local niche, equilibrium can be reached if there is negative feedback in the cross-signaling 1, S1). The microbial signals may alter selection of growing host-cell populations (e.g., by antiapoptotic mechanisms). (c) Model 2: The microbial population includes genetic variants. Now, the host signals select for different bacterial phenotypes, and thus genotypes. (d) Model 3: Due to ongoing variation and selection, each microbe becomes a microbial population of related variants. The signals of each variant affect not only the local host signals, but also those affecting other microbial populations. This is represented by two distinct microbial populations, with each signaling the host (S1, S2) and inducing specific host signals (HS1, HS2). Increasing numbers of populations markedly and nonlinearly increase the complexity. (e) Model 4: The microbial populations are not clonal but can exchange genetic information; host selection, based on microbial genes or gene fragments rather than cells, determines the (dynamic) equilibria between the microbial populations (MPn). For the naturally competent, extensively recombining H. pylori cells, model 4 best reflects the fluidity of population structures during persistent colonization (11, S2, S3). (f) Schematic of adaptation to individual hosts. After H. pylori acquisition and expansion into a major niche, early selection allows occupation of microniches, where further local selection determines predominant populations. Both local selection and global selection determine overall population structure, and the probability of transfer of particular genotypes to new hosts. Local resource differences and barriers to diffusion of bacterial cells allow establishment of distinct subpopulations. External fluctuations, such as incomplete antibiotic treatment, can markedly change genotype distribution.
Microbial colonization of a host locale affects the surrounding tissue through the occupation of niches, utilization of resources, and excretion, all of which may be considered as signals to the host (Figure 1b). The host also signals the microbe in the form of pressure, temperature, and chemical milieu (including host-defense molecules). Although these signals could be uncoordinated, coevolution implies linkage, in which the signals of one party affect the signals of the other (Figure 1c). Microbial persistence requires equilibrium, which only can occur when negative feedback is present (1, S1). This simple model forms the basis for understanding H. pylori persistence, and microbial persistence in general. If the microbial population includes differing strains, as clearly occurs for H. pylori (11, S2, S3), then the host signals are selective forces (Figure 1c), as it is this selected microbial population rather than the individual cell that is the host-signaling entity (Figure 1d). Many bacterial populations are not entirely clonal, reflecting both point mutations and recombination; H. pylori is a particularly extreme example with both a high mutation rate and a very high recombinational frequency (12, 13). Thus, each host is colonized not by a single clone but rather by a cloud of usually closely related organisms (11), resembling the “quasispecies” observed with persistent RNA viruses, such as hepatitis C and HIV. This microbial variation affects the signals to the host; for example, within an H. pylori population, individual cells may or may not express specific host-interaction molecules (e.g., CagA) that affect host biology in a directed manner. Consequent host “signals,” ranging from increased nutrient supply through immune effectors to changes in the gastric microenvironment, are differentially selective for specific H. pylori genes. Thus, each host is colonized by a fluid bacterial gene pool, with genotype dominance determined by selection (Figure 1e). In sum, concepts of such highly plastic populations subject to host-specific selection provide models to explain the facility of H. pylori to persist, the presence of different strains as well as variants of these strains in individual hosts, and the ability of H. pylori to colonize essentially all humans (Figure 1f), despite our heterogeneity.
H. pylori adaptations that facilitate persistence
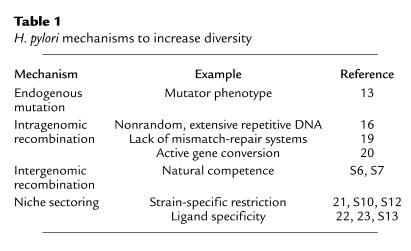
H. pylori mechanisms to increase diversity.
The remarkable diversity of H. pylori (12, S4) may be viewed as evidence of a versatile population, able to maximize resource utilization in a variety of niches and microniches and to avoid host constraints. Such constraints include not only host immunity but also developmental changes in the gastric epithelium, acidity, and nutrient availability. Generation of diversity typically involves endogenous (point) mutations, and recombination; H. pylori has mechanisms for each (Table 1). Mutation rates are not constant in bacterial populations but subject to environmental signals, and within large populations a small proportion of cells arise that have heightened mutation rates (S5). For H. pylori, most strains would be considered to have this hypermutator phenotype (13), which favors the emergence of variants after selective pressure. A good example of efficient adaptive point mutation by H. pylori is the rapid development in the bacterial population of high-level resistance to commonly used antibiotics, such as the macrolide clarithromycin (14).
Table 1.
H. pylori mechanisms to increase diversity
H. pylori
cells are also highly competent for uptake of DNA from other H. pylori strains (S6, S7, S8). Analysis of H. pylori sequences shows strong evidence of recombination between strains, to the degree that clonal lineages are largely obscured (12, 15). Substantial intragenomic recombination occurs, based largely on the presence of repetitive DNA sequences (16, 17). Repetitive DNA allows for high-frequency deletion and duplication, including slipped-strand mispairing (18, S9). However, because H. pylori is naturally competent, any genetic element that is lost may be regained from either unaffected sectors of the population of that strain or from another strain (17, S10). A lack of mismatch-repair systems (19) may increase the frequency of random variation, but it also may facilitate gene conversion, which minimizes genomic diversity of those alleles present in multiple copies (20). Thus, H. pylori can maximize diversity of sequences under strong selective pressure, while maintaining alleles that are critical for its lifestyle.
Introduction of a new H. pylori strain into an already colonized host increases the total diversity in the population, since each resembles a quasispecies; however, transformation of one strain by the other reduces diversity. All H. pylori strains contain multiple restriction-modification systems, but rarely do any two have the same complement (21, S11). Thus, there are restriction barriers to transformation, a property that may maximize coexistence of parallel gene pools, by slowing genetic exchange (S12). Local selection also may add to genetic diversity within an individual stomach; separate gastric microniches are likely colonized by subpopulations that have particular attributes to maximize fitness, for example, ligand specificity for local receptors (22, 23, S13).
Human interaction domain 1: cag island.
In 1989, a strain-specific H. pylori gene, cagA, was identified (24), which now has been recognized as a marker for strains that confer increased risk for peptic ulcer disease (25, 26) and gastric cancer (27, S14). No homologs are known for cagA in other Helicobacter species or in other bacteria, suggesting that it reflects a human gastric-specific gene. cagA (S15, S16) is a marker for the 35- to 40-kb cag (pathogenicity) island that is flanked by 39-bp direct DNA repeats (S17, S18). Strains without the island possess a single copy of the 39-bp sequence, in a conserved gene (glutamate racemase [glr]), and through transformation the entire island may be restored or lost (28). H. pylori strains with partial cag islands also have been identified, and variation in island size and genotype within individual hosts is well described (S3, S19, S20).
The island contains genes encoding a type IV secretion system, which in other bacteria inject macromolecules (i.e., DNA and proteins, such as pertussis toxin) into host cells (29). One substrate for the type IV system in H. pylori is the cagA product (30, 31, S21–S23), which is injected into epithelial cells, both in vitro (30, 31, S22, S23) and in vivo (32) (Figure 2). In many strains, the CagA protein contains tyrosine-phosphorylation sites (30, 31, 33, S21–S23) that are recognized by the host cell Src kinase (34). Once phosphorylated, CagA interacts with SHP-2, a tyrosine phosphatase (35), which affects spreading, migration, and adhesion of epithelial cells (32). This phenomenon can be assessed in vitro by a change in epithelial cell morphology to the scattered, or “hummingbird,” phenotype (31).
Figure 2.
CagA interaction with epithelial cells. H. pylori cells with intact cag islands, including an active type IV secretion system, possess a pilus composed of CagY protein. The cagA product is injected into the cytoplasm of the host cell, where tyrosine (Y) residues near its COOH-terminus are phosphorylated. Phosphotyrosine-CagA interacts with several major signal-transduction pathways in the host cell (40, S113), affecting phenotypes including cell morphology, proliferation, and apoptosis (see text). ERK, extracellular signal–regulated kinase; PTPase, protein-tyrosine phosphatase; P, phosphate.
The injected CagA protein also interacts with Grb2 and activates the Ras/MEK/ERK pathway, leading to the phenotypes of cell scattering (in AGS cells) and proliferation (in MDCK cells) (36). Tyrosine-phosphorylated CagA binds and activates C-terminal Src kinase (Csk) via its SH2 domain, which in turn inactivates the Src family of protein-tyrosine kinases. Since this signaling may induce apoptosis, the Csk pathway may attenuate the other CagA interactions (37). By inactivating Src, tyrosine-phosphorylated CagA induces dephosphorylation of cortactin, which then colocalizes with filamentous actin (F-actin), in the tip and base of hummingbird protrusions (38). Thus, the H. pylori CagA protein interacts with several of the major signal-transduction pathways present in epithelial cells. H. pylori cells with the cag island deleted have remarkably little interaction with AGS cells in tissue culture (39); conversely, the cag apparatus promotes antiapoptotic pathways, which may aid persistence by slowing turnover of the epithelial cells to which they are attached.
There is extensive H. pylori variation in this major interactive modality; clonality and the lack thereof each imply important, albeit different, selective pressures. In individual strains, parts of the cag island, including cagA, may be deleted (S3, S19, S20); cagA itself shows phylogeographic variation with Eastern, Western, and hybrid genotypes (S24). The DNA sequences encoding the tyrosine-phosphorylation motifs are variable in number and flanked by repetitive elements, allowing their deletion or duplication, which affects the phenotype of the injected CagA protein (33). Thus, H. pylori populations possess extensive repertoires that permit variation of Cag phenotypes in response to particular hosts, microniches within these hosts, or changing environmental circumstances. Nevertheless, antibody responses to CagA remain relatively constant over at least 20 years (40) in an individual host, implying an overall stability in the interactive relationship, best represented in Figure 1e.
Human interaction domain 2: vacA.
Culture supernatants from some H. pylori strains release a high–molecular weight multimeric pore-forming protein, VacA, that causes massive vacuolation in epithelial cell lines (41, S25). As with cagA, no close homologs of vacA exist in other Helicobacter species or in other bacteria (42, S26–S28), which suggests its importance in the specific relationship of H. pylori with the human stomach. However, unlike cagA, vacA is conserved among all H. pylori strains, although significant polymorphism exists (43). vacA alleles possess one of two types of signal region, s1 or s2, and one of two mid-regions, m1 or m2, occurring in all possible combinations (Figure 3). Research has focused on the most interactive (vacuolating) type, s1/m1.
Figure 3.
VacA polymorphism and function. (a) VacA polymorphism. The gene, vacA, is a polymorphic mosaic with two possible signal regions, s1 and s2, and two possible mid-regions, m1 and m2. The translated protein is an autotransporter with N- and C-terminal processing during bacterial secretion. The s1 signal region is fully active, but the s2 region encodes a protein with a different signal-peptide cleavage site, resulting in a short N-terminal extension to the mature toxin that blocks vacuolating activity and attenuates pore-forming activity. The mid-region encodes a cell-binding site, but the m2 type binds to and vacuolates fewer cell lines in vitro. (b) VacA biological activities. Secreted VacA forms monomers and oligomers; the monomeric form binds to epithelial cells both nonspecifically and through specific receptor binding, for example, to Ptprz, which may modulate cell signaling. Membrane-bound VacA forms pores. Following VacA endocytosis, large vacuoles form, but, although marked in vitro, these are rarely seen in vivo. VacA also induces apoptosis, in part by forming pores in mitochondrial membranes, allowing cytochrome c (Cyt c) egress. Although the presence of cytoplasmic VacA is implied rather than demonstrated, yeast two-hybrid experiments show potential for specific binding to cytosolic targets including cytoskeletal proteins, consistent with observed cytoskeletal effects. Finally, VacA has suppressive effects on immune cell function. The relative importance of these effects on H. pylori persistence and host interaction remains unclear.
VacA has several specific effects that may contribute to H. pylori persistence in the gastric niche. Firstly, it forms pores in epithelial cell membranes, allowing egress of anions and urea (44, 45, S29, S30). This is important since urea hydrolysis, catalyzed by H. pylori urease, protects against gastric acidity (S31). VacA also induces loosening of epithelial tight junctions, potentially allowing nutrients to cross the mucosal barrier to H. pylori’s gastric luminal niche (46, S32). Recent work in vitro suggests that VacA may help H. pylori persistence by specific immune suppression. VacA blocks phagosome maturation in macrophages (47), selectively inhibits antigen presentation in T cells (48, S33), blocks T cell proliferation, and downregulates Th1 effects by interacting with calcineurin to block signaling (49). Besides these actions that may benefit H. pylori persistence, VacA also has direct cell-damaging effects in vitro, inducing cytoskeletal changes, apoptosis, and suppression of epithelial cell proliferation and migration (50–52, S34, S35), as well as cell vacuolation. Whether these effects are germane in vivo is unknown, but cell damage could aid nutrient delivery from the gastric mucosa.
Which in vitro effects of VacA are most important for H. pylori persistence in vivo is unclear, and animal models have not clarified this. In piglets, gerbils, and mice, VacA-null strains persist without apparent disadvantage (S36–S38), although in competition experiments in mice, VacA-null mutants colonize less well than their VacA+ wild-type parents (S38). However, animal models have proved useful for characterizing the damaging effects of VacA. Although VacA is not necessary for gastric ulcer formation, in H. pylori–colonized Mongolian gerbils its presence increases the risk (53). Mice administered VacA orally develop gastric ulcers (54, S28), but mice deficient in the protein tyrosine phosphatase receptor type Z, polypeptide 1 (Ptprz–/– mice) do not (54). Ptprz is one of several putative VacA cellular receptors, and VacA-induced activation increases tyrosine phosphorylation of G protein–coupled receptor kinase-interactor 1 (Git1), leading ultimately to epithelial cell detachment (54). VacA also may have important effects on nonepithelial cells: in rats, only VacA+ strains induce macromolecular leakage from the gastric microcirculation (S39).
H. pylori strains with different forms of vacA exhibit varied phenotypes and have particular associations with gastro-duodenal diseases. The vacA signal region encodes the signal peptide and the N-terminus of the processed VacA toxin: type s1 VacA is fully active, but type s2 has a short N-terminus extension that blocks vacuole formation (55, 56) and attenuates pore formation in eukaryotic membranes (S40). vacA s2 strains are rarely isolated from patients with peptic ulcers or gastric adenocarcinoma (43, 57, S41, S42). The vacA mid-region encodes part of the toxin cell binding domain. s1/m2 forms of VacA bind to and vacuolate a narrower range of cells than s1/m1 forms and induce less damage, yet they also act as efficient membrane pores and increase paracellular permeability (56, S30, S32, S43). vacA s1/m1 strains are most closely associated with gastric carcinoma (58, 59, S44). Natural persistence of distinct polymorphic forms of vacA in diverse human populations implies that each offers a survival advantage. That particular forms of VacA potentially induce different levels of H. pylori–host interaction fits well with the general model in which less interactive strains cause diminished tissue injury and disease, and highly interactive strains, while benefiting from their interaction, are more likely to affect their niche and thus injure their host.
Interaction between the interacting domains.
The cag island and vacA are far apart on the H. pylori chromosome (19, 60), yet there is a strong statistical linkage between the s1 genotype of vacA and the presence of the cag island (43, S24); similarly, the s2 genotype is associated with lack of the cag island (43). Although these phenomena may reflect founder effects, the panmixis of H. pylori (12) suggests selection for the skewed relationships. This is not a simple interdependence of function: in a cag+/vacA s1/m1 strain, cag mutagenesis does not abolish vacuolating cytotoxin activity, nor does disruption of vacA abolish the cag+ phenotype (29, 31, 39, S45, S46). However, there are subtle quantitative effects: vacA disruption slightly reduces early tyrosine phosphorylation of CagA during epithelial cell interaction, whereas, in contrast, cag disruption slightly increases VacA-induced vacuolation (61). One contributor to these effects may be the colocalization on lipid rafts of tyrosine-phosphorylated Git1/Cat1 (substrate molecules of the VacA receptor phosphatase Ptprz) and tyrosine-phosphorylated CagA (61).
The minor interactions between CagA-induced and VacA-induced effects on epithelial cells are unlikely to explain their close linkage in H. pylori. An alternative hypothesis is that VacA selects for a functional cag island, since VacA-induced immune suppression might not permit adequate nutrition of the H. pylori population (Figure 1). The effect of cag+ strains in weakening epithelial tight junctions (62) may enhance nutrient flow to the bacteria and allow better VacA delivery to the mucosa. For the less potent s2 strains, this selection would be less substantial and might be counterbalanced by the phenotypic costs of maintaining the cag island. Whatever the true selective benefits VacA and cag offer each other, in conditions with multiple strains colonizing individual hosts, each major genotypic combination (cag+/vacA s1 or cag–/vacA s2) could occupy a relatively exclusive physiological niche. Recombination events mixing the loci would be selected against. With diminishing multiplicities of H. pylori colonization (7), the strain (and genotype) diversity within a host would be reduced, diminishing the resource base for the overall population. Thus, once H. pylori transmission within a human population declines, the decline may accelerate because of diminished vitality of the colonizing bacterial populations in individual hosts.
Immune evasion and manipulation by H. pylori.
If a microbe is to persist in a vertebrate host, its biggest challenge is to avoid clearance by the immune system. Transient H. pylori colonization has been documented in both primates and humans (63, 64, S47), implying that persistence does not inevitably follow acquisition. The race between H. pylori adaptation to a specific host (Figure 1) and the development of effective immunity also implies the feasibility of vaccine development. However, usually, following H. pylori acquisition, there is rapid host recognition in the form of both innate and acquired immune responses, including generation of specific local and systemic antibodies (65, S48–S51). Once chronicity is established, the immune stimulation appears remarkably constant; for example, antibody titers remain stable for over 20 years (40), consistent with a model of dynamic equilibrium (Figure 1). The ubiquity and duration of host recognition of H. pylori and yet the lifelong colonization by the bacterium demonstrate the effectiveness of H. pylori’s strategies to evade host immunity. The important first step is to survive without tissue invasion (Table 2), and the bulk of H. pylori cells, if not all of them (Figure 1a), reside in the gastric lumen (66, S52) beyond the reach of most host immune recognition and effector mechanisms (S48, S52, S53). However, even in this niche, some H. pylori cells establish intimate contact with the surface epithelium (S52, S54), some H. pylori proteins cross the epithelial barrier (67), and both innate and acquired immune systems are activated (65, S48–S50). Although it is not able to completely avoid immune activation, H. pylori has evolved mechanisms to reduce recognition by immune sensors, downregulate activation of immune cells, and escape immune effectors.
Table 2.
Immune evasion by H. pylori
Innate immune system recognition of microorganisms involves Toll-like receptors (TLRs) that discriminate pathogen-associated molecular patterns (S55). TLR stimulation triggers proinflammatory signaling through NF-κB activation, and H. pylori has evolved to minimize such stimulation. TLR5 recognizes bacterial flagella such as those of Salmonella typhimurium but is not stimulated by H. pylori flagella (S56). TLR9 recognizes the largely unmethylated DNA of most bacteria (S57), but the highly methylated H. pylori DNA likely minimizes recognition (S11). H. pylori LPS is anergic compared with that of other enteric bacteria because of lipid A core modifications (S58–S61), and while it stimulates macrophage TLR4 (68, S61), it does not stimulate gastric epithelial TLR4 (69). Although H. pylori is relatively camouflaged from innate immune sensors on cell surfaces, cag+ strains do stimulate NF-κB activation in epithelial cells (70, S62), apparently through recognition by Nod1 (S63), an innate intracellular pathogen-recognition molecule that detects soluble components of bacterial peptidoglycan (71). How such components are delivered to the epithelial cytoplasm by the cag-encoded type IV secretion system remains unclear, but the resultant NF-κB–induced proinflammatory cytokine expression is an important and continuing stimulus to inflammatory cell infiltration and thus to pathogenesis (65, S49).
H. pylori
also activates the acquired immune system, as indicated by both humoral and cellular recognition of its antigens (72, S48, S50, S53), although it has evolved to substantially downregulate and avoid acquired immune effectors. Recognition by the acquired immune system requires antigen presentation, and H. pylori interferes with both uptake and processing of antigens, partially through a VacA effect (48). H. pylori also suppresses T cell proliferation and activation and induces selective T cell apoptosis, again partially through specific VacA effects on signaling (49, 73, S64–S66). It evades host adaptive responses by mimicry of the gastric epithelial fucosylated (Lewis) antigens (74, S67), and by antigenic variation of surface proteins including a critical pilus molecule, CagY (75). As this high-frequency antigenic variation occurs through mutation (usually slipped-strand mispairing) and intragenomic recombination between homologous sequences (19, 23, S9, S68), these genetic mechanisms are important contributors to immune evasion. Finally, H. pylori can also suppress less specific immune mechanisms such as phagocytosis (47, 76). The relative contributions of the different host manipulation and evasion strategies to H. pylori persistence are not established, possibly differing in individual hosts, but the existence of these varied mechanisms implies that immune surveillance of the gastric lumen is powerful, and that bacterial survival requires its subversion.
Host responses to H. pylori and their role in disease
The immune response to H. pylori and its importance in pathogenesis.
Despite the mechanisms H. pylori has evolved to avoid and downregulate host immune responses, substantial immune activation occurs following H. pylori infection. This is manifested by continuous epithelial cell cytokine signaling and gastric mucosal infiltration by neutrophils, macrophages, and lymphocytes, all of which are more pronounced in colonization with a cag+ strain (25, 65, 77, S69). There is a pronounced specific acquired immune response, including generation of antibodies and effector T cells, and although this includes both a Th1 and a Th2 component, mucosal cytokine profiles imply Th1 predominance (72, S50). This is unusual for extracellular, toxin-producing bacteria, which usually are met by B cell activation and high-level antibody production (Th2 responses). However, studies in mice suggest that the predominant Th1 response is appropriate to control H. pylori: Helicobacter colonization density is lower in mice with predominant Th1 responses, whether genetically programmed or manipulated by experimental helminth infection (78, 79, S70, S71).
Despite its apparent propriety, the immune response, and in particular its Th1 component, is a major factor in H. pylori–associated pathogenesis (78, 80, 81, S70). Mice with a predominant Th1 response develop more gastric inflammation during Helicobacter colonization than those with a Th2 response (79, 79, 81, S70, S71). Experiments that use T cell transfer between mice show that these effects are dependent on Th1 cells (78). Gastric inflammation and atrophic changes are abrogated in the absence of the key Th1 cytokine IFN-γ (81, S70) and are induced by IFN-γ infusion, even without Helicobacter (S72). In humans, peptic ulceration is rare during immune suppression with cyclosporin A (S73) and pregnancy (S74), a Th2-predominant state. One hypothesis is that the relative sparsity of H. pylori–associated disease in Africa despite high H. pylori prevalence (the “African enigma”) may be due to predominant Th2 responses to H. pylori among black Africans. These responses may be induced by endemic helminth infection (79) or may reflect a genetic predisposition selected by malaria (82).
The importance of heterogeneity in immune responses among human populations and individuals is further demonstrated by the contribution of cytokine polymorphisms to disease risk. Polymorphisms that increase the IL-1β response to H. pylori are associated with an increased risk of developing gastric atrophy, hypochlorhydria, and adenocarcinoma (83–85, S14, S75). Polymorphisms in TNF-α and IL-10 genes have a similar, but less pronounced, association (S14, S76). Thus the degree of activation of the immune response, which underlies H. pylori–associated pathology, is dependent on both H. pylori strain determinants and host genetic factors; the combined effect of these on disease outcome appears synergistic (S14), as predicted by the equilibrium model (Figure 1).
Effect of H. pylori–induced inflammation on acid homeostasis and its importance in upper-gastrointestinal diseases.
H. pylori–induced proinflammatory cytokine expression and inflammation affect various cell types in the stomach that are important in acid homeostasis, including somatostatin-producing D cells, gastrin-producing G cells, and acid-producing parietal cells (86, 87, S77–S79). H. pylori gastritis causes a reduction in somatostatin levels (87, S77, S80) and, since somatostatin negatively regulates gastrin, hypergastrinemia (88). Gastrin is a specific growth factor for H. pylori (89), so this potentially creates a positive-feedback loop. Gastrin expression may be enhanced by a direct stimulant effect of H. pylori–induced proinflammatory cytokines on G cells (S78). Removal of H. pylori reverses these effects (S81, S82) (Figure 4).
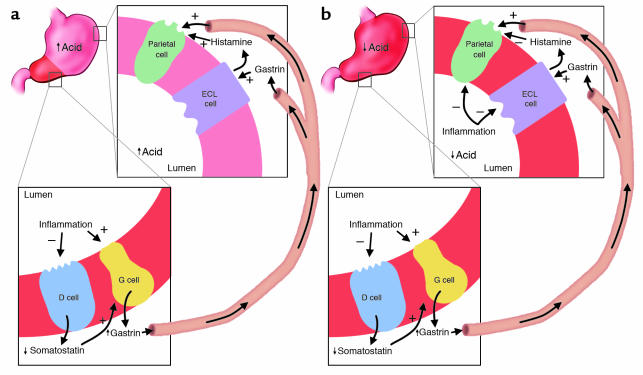
Figure 4.
Relation of topography of inflammation to gastric physiology and clinical outcome. (a) H. pylori–induced antral-predominant inflammation. Antral inflammation results in hypergastrinemia, which stimulates a physiologically intact corpus to secrete acid, increasing risk for duodenal ulceration. (b) H. pylori–induced pan-gastritis. The inflammatory process suppresses corpus acid production, despite the gastrin stimulus from the antrum. Hypochlorhydria enhances risk of gastric ulcer and adenocarcinoma but conversely decreases risk for severe gastro-esophageal reflux disease and its sequelae. ECL, enterochromaffin-like.
The effects of gastrin levels on acid homeostasis and disease depend crucially on the topographic distribution in the stomach of the H. pylori–induced inflammation (Figure 4). In antral-predominant gastritis, the enterochromaffin-like and parietal cells in the gastric corpus are relatively uninvolved; thus, high gastrin levels lead to greater acid secretion (90, S83, S84). Persistently increased gastrin levels also increase parietal cell mass, enhancing these effects (90, 91). The increased acid load delivered to the duodenum induces gastric metaplasia, a protective phenotypic change. H. pylori cannot colonize the normal duodenum but colonizes gastric metaplasia, with resultant inflammation and ulceration (92–94, S85). When the inflammation involves the corpus (pan-gastritis or corpus-predominant gastritis), H. pylori–induced inflammatory mediators suppress acid production both indirectly, by inhibiting enterochromaffin-like cell histamine production, and directly by inhibiting parietal cell function (86, S79). Reduced acid secretion further augments gastrin levels, which, while ineffective in raising acid production from the inflamed gastric corpus, provide an ongoing proliferative stimulus to gastric epithelial cells. Continuing proliferation and inflammation affect epithelial cell cycle characteristics (95, 96, S86–S88) and lead to progressive loss of gastric glands. Such atrophic changes markedly increase risk of gastric ulceration and non-cardia gastric adenocarcinoma (4, 97, S89) but, because acid production is lowered, are protective against duodenal ulceration, and probably against acid-induced complications of gastroesophageal reflux (98, 99).
The topographic distribution of gastritis during chronic H. pylori colonization is at least partly host specific; for example, polymorphisms leading to high IL-1β production are associated with pan-gastritis with its accompanying reduced acid production and gastric atrophy (84). However, environmental factors likely also play a crucial role; duodenal ulceration (and so presumably antral-predominant gastritis) is largely a 20th-century disease (100, S90) associated with socioeconomic development. Prior to 20th-century increases in duodenal ulcer incidence, the predominant gastritis pattern was probably that found commonly today in developing countries: pan-gastritis and progressive atrophy. As humans have coevolved with H. pylori over at least thousands of years (8, 9) and our genes cannot have evolved appreciably over the last century, unknown environmental influences such as older age at acquisition, reduced number of colonizing strains, changed proportion of strains preadapted by passage through family members, reduction in other microorganisms colonizing the stomach, and improved nutritional status must be responsible for this change. In even more recent times, absence of H. pylori from late 20th- and 21st-century stomachs in developed countries, perhaps for the first time in our evolutionary history, may have had further effects on human acid homeostasis and health. As the predominant historical result of colonization was pan-gastritis and reduced acid production, absence of H. pylori would be expected to increase mean acid production in the general population, and we speculate that this has contributed to the observed rise in acid-associated complications of gastro-esophageal reflux (severe reflux esophagitis, Barrett esophagus, and esophageal adenocarcinoma) in the late 20th century (101). In support of this, patients with severe or complicated acid-esophageal diseases have a reduced H. pylori prevalence, particularly of cag+ strains (101–103, S91), and a low prevalence of gastric atrophy (98, 99). Consequently, the current iatrogenic H. pylori removal may have important costs as well as benefits.
The ubiquity of H. pylori in unacculturated human populations has led to speculation that colonization also may be beneficial to the pre-reproductive host (S92, S93). We speculate that over the long course of human evolution, adult stomachs were mainly atrophic, and antral-predominant gastritis and hyperchlorhydria were largely conditions of childhood; children would be most likely to benefit from H. pylori colonization, by an enhanced acid barrier protecting against diarrheal pathogens (104). If so, there would be strong selection for maintenance of H. pylori in populations with poor sanitation; with improvement, such selection would be progressively lost.
Effects of H. pylori on leptin and ghrelin, hormones involved in appetite and satiety.
Recently, gastric H. pylori colonization has been shown to affect expression of leptin and ghrelin, hormones that control appetite and satiety (5, 6, S94) (Figure 5). Leptin is secreted from adipose tissue and from the stomach (S95, S96); gastric leptin is produced by chief and parietal cells, and released in response to meals and associated hormonal stimuli (105, S96, S97). Leptin signals satiety to the hypothalamus, causing reduced food intake, increased energy expenditure, reduced gastrin and acid secretion, and increased gastric mucosal cell proliferation (106, S98, S99). Ghrelin, produced in oxyntic glands, is released during fasting, and suppressed by feeding and leptin (107, 108). In rats, ghrelin stimulates food intake, reduces energy expenditure, and increases acid secretion (107, S100).
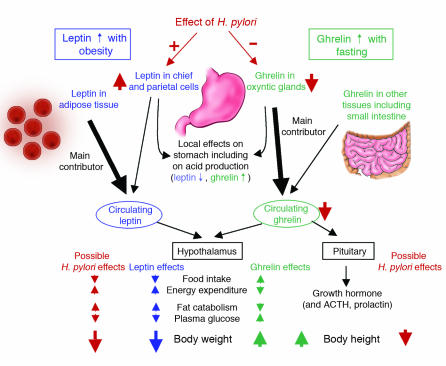
Figure 5.
The described effects of H. pylori on leptin and ghrelin, and postulated subsequent effects on satiety, energy expenditure, weight, and height. Although leptin and ghrelin have other important paracrine, autocrine, and endocrine effects, here we concentrate on actions that affect body habitus. The observed effects of H. pylori on leptin and ghrelin are based on observational and interventional (H. pylori eradication) human studies. Other observational human studies support the portrayed effects of H. pylori on weight and height.
Gastric leptin levels are higher in H. pylori–colonized than in noncolonized adults, and eradication leads to their reduction, although serum levels may not be affected (5, S94). Evidence conflicts as to whether serum ghrelin levels are higher in H. pylori–negative persons (109, S101), but they increase after H. pylori eradication (6). In an animal model, immunity to Helicobacter is associated with upregulation of adipocyte genes, including adipsin, resistin, and adiponectin (110). Inquiry in this field is at an early stage, but if early findings are confirmed, the implications may be important. Weight gain after H. pylori eradication is common (5), and these changes in hormonal levels may contribute (Figure 5). Similarly, obesity is increasing in developed countries, as H. pylori prevalence is falling. In developing countries, most children acquire H. pylori by age 5, and nearly all by age 10, whereas progressively fewer children in developed countries are becoming colonized (111, S102). Whether H. pylori genes represent microbial contributions to the complement of “thrifty” (calorie-conserving) genes of humans, and whether H. pylori disappearance plays a role in childhood (and adult) adiposity, remain to be determined.
Chronic effects of H. pylori on the gastric epithelium and carcinogenesis.
Persistent microbial colonization resulting in inflammation and cell damage is an important cause of carcinogenesis. Examples include H. pylori and distal gastric adenocarcinoma, Schistosoma haematobium and bladder carcinoma, and hepatitis B virus and hepatoma (112, S103–S105). In evolutionary terms, such cancers are probably neutral; their expression is mostly modern, possibly because of increased human lifespan, and they may be regarded as a cost of chronic colonization, which for gastric cancer occurs in about 1–3% of H. pylori–colonized persons.
H. pylori
–induced gastric carcinogenesis is more likely when the interaction between H. pylori and the host is more interactive; inflammation is more intense and the effects on epithelial cells are more damaging (S106). This may reflect colonization by more interactive H. pylori strains: cag+ strains induce more inflammation and cell cycle effects (4), and vacA s1/m1 strains cause more direct epithelial damage (27, 58, 59, S105). Host cytokine polymorphisms enhance the inflammatory response (83, 84, S14, S75). Damaging environmental factors, such as smoking and high-salt diets, further increase risk, whereas diets high in antioxidants are protective (113, S89, S107).
Although risk factors for gastric cancer now are well established, the mechanism of carcinogenesis is less clear. Carcinomas arise in stomachs with pan-gastritis; the more common intestinal type occurs following progressive atrophy (with loss of glands and hypochlorhydria), intestinal metaplasia, and dysplasia (S89, S108), whereas the diffuse type (S109) may arise de novo from H. pylori–colonized mucosa (112). H. pylori colonizes the atrophic stomach poorly, and intestinal metaplasia hardly at all, suggesting that the bacteria may create the environment for intestinal-type gastric carcinogenesis (atrophy and hypochlorhydria) rather than causing the cancer directly. In support of this concept, mutations in gastric carcinoma appear random, as expected from nonspecific DNA damage from environmental carcinogens (114, S110).
Disturbance of the epithelial cell proliferation/apoptosis balance is considered a risk factor for gastric atrophy and for neoplastic transformation. When cocultured with epithelial cell lines, H. pylori are antiproliferative and proapoptotic (115, S111), although cag signaling is essentially pro-proliferative (through MAPK signaling and expression of the transcription factor AP-1) (116, 117) and pro- and antiapoptotic (through NF-κB signaling) (70, 118). Animal models and human studies suggest that the net effect of H. pylori colonization is pro-proliferative and proapoptotic (95, 96, 119, 120, S87, S88). Pro-proliferative signaling increases cell replication and the chance of mutation, whereas apoptosis may be protective by inducing death of DNA-damaged cells. However, the consequences of both effects on the epithelial stem cell compartment is likely to be pro-proliferative (to replace apoptotic cells), potentially predisposing to senescence and atrophy or increased mutation and diffuse-type malignant transformation. Stem cell proliferation also may potentially arise more directly from H. pylori–induced hypergastrinemia, since gastrin is pro-proliferative for gastrointestinal epithelia; in H. pylori–infected gerbils, epithelial proliferation correlates well with serum gastrin levels (96).
Ultimately, carcinogenesis requires DNA damage, which can be induced directly by H. pylori products or indirectly through oxygen free radicals released by neutrophils (118, 121, 122). Gastric ascorbic acid, which neutralizes free radicals, is reduced in H. pylori–positive stomachs (S112), and H. pylori can also directly interfere with the epithelial mismatch-repair system (122). In the atrophic stomach, H. pylori colonization is sparse, but atrophy is associated with continuing epithelial proliferation and an inflammatory cell infiltrate. Reactive oxygen species survive longer in the low-acid environment of the atrophic stomach, and ascorbic acid concentrations remain low (123); colonization by oral and intestinal bacteria, which themselves can release reactive oxygen and nitrogen species, may occur. By leading to gastric atrophy, H. pylori may be permitting its replacement by more genotoxic bacteria in the postreproductive-age gastric niche.
While carcinogenesis may be merely an evolutionarily irrelevant consequence of H. pylori colonization, affecting individuals largely in their postreproductive years, we advance an alternative theory (124). Carcinogenesis may be one mechanism by which H. pylori and other commensal bacteria contributed to the fitness of premodern human populations, by the removal of senescent (postreproductive) individuals from the population in a programmed (“safe”) manner (124). This would lead to a selective advantage for colonized populations, as groups dominated by senescent individuals likely have reduced survival during times of scarcity, or epidemic disease.
Conclusions
The human body is teeming with microbes, especially bacteria, but their role in human physiology has been little explored (124, 125). H. pylori is unique in that it is both the major inhabitant of an ecological niche and is disappearing from human populations as a consequence of modernization. As such, the effects of H. pylori on physiology and pathophysiology can be measured and are a paradigm for our persistent indigenous biota, with both local and distant physiological effects.
Other microbes also may be disappearing from the human “microbiome” (125). We cannot yet ascertain this phenomenon because of the complexity of the indigenous flora, but parallels likely are present. Could extinction of H. pylori and other coevolved microbes have affected our physiological signaling? If so, could this in part be responsible for diseases that have increased in modern times, such as gastro-esophageal reflux, obesity, diabetes, asthma, and several malignancies?
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (R01GM63270, R01DK53707, R01CA97946, and R21DK063603), the Medical Research Service of the Department of Veterans Affairs, a Cancer Research United Kingdom grant, and the award of a Medical Research Council (United Kingdom) Senior Clinical Fellowship to John C. Atherton.
Note: Due to space constraints, a number of important references could not be included in this reference list. Interested readers can find a supplementary reference list at http://www.jci.org/cgi/content/full/113/3/321/DC1.
Footnotes
The Science in Medicine series is supported by a generous grant from the Doris Duke Charitable Foundation.
Conflict of interest: As co-discoverer of cagA and vacA, Martin Blaser may benefit from commercial exploitation of the intellectual property held by Vanderbilt University.
Nonstandard abbreviations used: C-terminal Src kinase (Csk); G protein–coupled receptor kinase-interactor 1 (Git1); Toll-like receptor (TLR).
References
- 1.Blaser MJ, Kirschner D. Dynamics of Helicobacter pylori colonization in relation to the host response. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8359–8364. doi: 10.1073/pnas.96.15.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura AMY, et al. Helicobacter pylori cagA seropositivity and gastric carcinoma risk in a Japanese American population. J. Infect. Dis. 2002;186:1138–1144. doi: 10.1086/343808. [DOI] [PubMed] [Google Scholar]
- 3.HelicobacterCancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of twelve case-control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Azuma T, et al. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–640. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser MJ. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology in the modern era. Gut. 1998;43:721–727. doi: 10.1136/gut.43.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghose C, et al. East Asian genotypes of Helicobacter pylori: strains in Amerindians provide evidence for its ancient human carriage. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15107–15111. doi: 10.1073/pnas.242574599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falush D, et al. Traces of human migration in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 10.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Invest. 2001;107:767–773. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuipers EJ, et al. Quasispecies development of Helicobacter pylori observed in paired isolates obtained years apart in the same host. J. Infect. Dis. 2000;181:273–282. doi: 10.1086/315173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suerbaum S, et al. Free recombination with Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkholm B, et al. Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14607–14612. doi: 10.1073/pnas.241517298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjolund M, Wreiber K, Andersson DI, Blaser MJ, Engstrand L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann. Intern. Med. 2003;139:483–487. doi: 10.7326/0003-4819-139-6-200309160-00011. [DOI] [PubMed] [Google Scholar]
- 15.Falush D, et al. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15056–15061. doi: 10.1073/pnas.251396098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aras RA, Kang J, Tschumi A, Harasaki Y, Blaser MJ. Extensive repetitive DNA facilitates prokaryotic genome plasticity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13579–13584. doi: 10.1073/pnas.1735481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aras RA, Takata T, Ando T, Van der Ende A, Blaser MJ. Regulation of the HpyII restriction-modification system of Helicobacter pylori by gene deletion and horizontal reconstitution. Mol. Microbiol. 2001;42:369–382. doi: 10.1046/j.1365-2958.2001.02637.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Ge Z, Rasko DA, Taylor DE. Lewis antigens in Helicobacter pylori: biosynthesis and phase variation. Mol. Microbiol. 2000;36:1187–1196. doi: 10.1046/j.1365-2958.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomb JF, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 20.Pride DT, Blaser MJ. Concerted evolution between duplicated genetic elements in Helicobacter pylori. J. Mol. Biol. 2002;316:629–642. doi: 10.1006/jmbi.2001.5311. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Morgan RD, Roberts RJ, Blaser MJ. Identification of type II restriction and modification systems in Helicobacter pylori reveals their substantial diversity among strains. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9671–9676. doi: 10.1073/pnas.97.17.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilver D, et al. Helicobacter pylori adhesion binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 23.Pride DT, Meinersmann RJ, Blaser MJ. Allelic variation within Helicobacter pylori babA and babB. Infect. Immun. 2001;69:1160–1171. doi: 10.1128/IAI.69.2.1160-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cover TL, Dooley CP, Blaser MJ. Characterization and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crabtree JE, et al. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 26.Nomura AMY, Perez-Perez GI, Lee J, Stemmerman G, Blaser MJ. Relationship between H. pylori cagA status and risk of peptic ulcer disease. Am. J. Epidemiol. 2002;155:1054–1059. doi: 10.1093/aje/155.11.1054. [DOI] [PubMed] [Google Scholar]
- 27.Blaser MJ, et al. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 28.Kersulyte D, Chalkauskas H, Berg DE. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 1999;31:31–43. doi: 10.1046/j.1365-2958.1999.01140.x. [DOI] [PubMed] [Google Scholar]
- 29.Tummuru MKR, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 30.Odenbreit S, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 31.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazaki S, et al. The CagA protein of Helicobacter pylori is translocated into epithelial cells and binds to SHP-2 in human gastric mucosa. J. Infect. Dis. 2003;187:334–337. doi: 10.1086/367807. [DOI] [PubMed] [Google Scholar]
- 33.Aras RA, et al. Natural variation in populations of persistently colonizing bacteria affect human host cell phenotype. J. Infect. Dis. 2003;188:486–496. doi: 10.1086/377098. [DOI] [PubMed] [Google Scholar]
- 34.Selbach M, Moese S, Hauck CR, Meyer TF, Backert S. Src is the kinase of Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- 35.Higashi H, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 36.Mimuro H, et al. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 37.Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 2003;278:3664–3670. doi: 10.1074/jbc.M208155200. [DOI] [PubMed] [Google Scholar]
- 38.Selbach M, et al. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515–528. doi: 10.1093/emboj/cdg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillemin K, Salama N, Tompkins L, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15136–15141. doi: 10.1073/pnas.182558799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Perez GI, et al. Evidence that cagA+ Helicobacter pylori strains are disappearing more rapidly than cagA- strains. Gut. 2002;50:295–298. doi: 10.1136/gut.50.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 42.Cover TL, Tummuru MKR, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 43.Atherton J, et al. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto H, Czajkowsky DM, Cover TL, Szabo G, Shao Z. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 45.Tombola F, et al. The Helicobacter pylori VacA toxin is a urea permease that promotes urea diffusion across epithelia. J. Clin. Invest. 2001;108:929–937. doi:10.1172/JCI200113045. doi: 10.1172/JCI13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papini E, et al. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Invest. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 2003;5:25–40. doi: 10.1046/j.1462-5822.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 48.Molinari M, et al. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 50.Pai R, Cover TL, Tarnawski AS. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem. Biophys. Res. Commun. 1999;262:245–250. doi: 10.1006/bbrc.1999.1194. [DOI] [PubMed] [Google Scholar]
- 51.Kuck D, et al. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cover TL, Krishna US, Israel DA, Peek RM., Jr Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 53.Ogura K, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujikawa A, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 55.Letley DP, Atherton JC. Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J. Bacteriol. 2000;182:3278–3280. doi: 10.1128/jb.182.11.3278-3280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letley DP, Rhead JL, Twells RJ, Dove B, Atherton JC. Determinants of non-toxicity in the gastric pathogen Helicobacter pylori. J. Biol. Chem. 2003;278:26734–26741. doi: 10.1074/jbc.M304071200. [DOI] [PubMed] [Google Scholar]
- 57.Atherton JC, Peek RM, Tham KT, Cover TL, Blaser MJ. The clinical and pathological importance of heterogeneity in vacA, encoding the vacuolating cytotoxin of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 58.Kidd M, Lastovica AJ, Atherton JC, Louw JA. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999;45:499–502. doi: 10.1136/gut.45.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miehlke S, et al. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int. J. Cancer. 2000;87:322–327. [PubMed] [Google Scholar]
- 60.Alm RA, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 61.Asahi M. Helicobacter pylori CagA containing ITAM-like sequences localized to lipid rafts negatively regulates VacA-induced signaling in vivo. Helicobacter. 2003;8:1–14. doi: 10.1046/j.1523-5378.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 62.Amieva MR, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dubois A, et al. Transient and persistent experimental infection of non-human primates with Helicobacter pylori: implications for human disease. Infect. Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Perez GI, et al. Transient and persistent Helicobacter pylori colonization in Native American children. J. Clin. Microbiol. 2003;41:2401–2407. doi: 10.1128/JCM.41.6.2401-2407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peek RM, et al. Heightened inflammatory response and cytokine expression to cagA+Helicobacter pylori strains. Lab. Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- 66.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 67.Mai UEH, et al. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leucocytes and are present in gastric mucosa. J. Exp. Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maeda S, et al. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 2001;276:44856–44864. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- 69.Backhed F, et al. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J. Infect. Dis. 2003;187:829–836. doi: 10.1086/367896. [DOI] [PubMed] [Google Scholar]
- 70.Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J. Biol. Chem. 2000;275:39779–39785. doi: 10.1074/jbc.M007617200. [DOI] [PubMed] [Google Scholar]
- 71.Giardin SE, et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 72.Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, et al. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 2001;167:926–934. doi: 10.4049/jimmunol.167.2.926. [DOI] [PubMed] [Google Scholar]
- 74.Wirth HP, Yang M, Peek RM, Tham KT, Blaser MJ. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology. 1997;113:1091–1098. doi: 10.1053/gast.1997.v113.pm9322503. [DOI] [PubMed] [Google Scholar]
- 75.Aras RA, et al. Plasticity of repetitive DNA sequences within a bacterial (type IV) secretion system component. J. Exp. Med. 2003;198:1349–1360. doi: 10.1084/jem.20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen LA, Schlesinger LS, Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 2000;191:115–128. doi: 10.1084/jem.191.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atherton JC, Tham KT, Peek RM, Cover TL, Blaser MJ. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J. Infect. Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 78.Mohammadi M, et al. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 79.Fox JG, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 80.Guiney DG, Hasegawa P, Cole SP. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 2003;71:4163–4166. doi: 10.1128/IAI.71.7.4163-4166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smythies LE, et al. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 82.Blaser MJ. Malaria and the natural history of Helicobacter pylori infection. Lancet. 1993;342:551. (Letter). doi: 10.1016/0140-6736(93)91674-b. [DOI] [PubMed] [Google Scholar]
- 83.Machado JC, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 84.El-Omar E, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 85.El-Omar EM, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 86.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. [Google Scholar]
- 87.Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- 88.Levi S, et al. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989;1:1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- 89.Chowers MY, et al. A defined human gastrin sequence stimulates the growth of Helicobacter pylori. FEMS Microbiol. Lett. 2002;217:231–236. doi: 10.1111/j.1574-6968.2002.tb11480.x. [DOI] [PubMed] [Google Scholar]
- 90.El-Omar EM, et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 91.Gillen D, et al. The acid response to gastrin distinguishes duodenal ulcer patients from Helicobacter pylori-infected healthy subjects. Gastroenterology. 1998;114:50–57. doi: 10.1016/s0016-5085(98)70632-8. [DOI] [PubMed] [Google Scholar]
- 92.Hamlet A, Olbe L. The influence of Helicobacter pylori infection on postprandial duodenal acid load and duodenal bulb pH in humans. Gastroenterology. 1996;111:391–400. doi: 10.1053/gast.1996.v111.pm8690204. [DOI] [PubMed] [Google Scholar]
- 93.Khulusi S, et al. Pathogenesis of gastric metaplasia of the human duodenum: role of Helicobacter pylori, gastric acid, and ulceration. Gastroenterology. 1996;110:452–458. doi: 10.1053/gast.1996.v110.pm8566592. [DOI] [PubMed] [Google Scholar]
- 94.Ohkusa T, et al. Helicobacter pylori infection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. Gut. 2003;52:797–803. doi: 10.1136/gut.52.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peek RM, Jr, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 96.Peek RM, Jr, et al. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48–59. doi: 10.1016/s0016-5085(00)70413-6. [DOI] [PubMed] [Google Scholar]
- 97.Kuipers EJ, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 98.Yamaji Y, et al. Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastric cancer: analysis of 5732 Japanese subjects. Gut. 2001;49:335–340. doi: 10.1136/gut.49.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koike T, et al. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut. 2001;49:330–334. doi: 10.1136/gut.49.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baron JH, Sonnenberg A. Period- and cohort-age contours of deaths from gastric and duodenal ulcer in New York 1804-1998. Am. J. Gastroenterol. 2001;96:2887–2891. doi: 10.1111/j.1572-0241.2001.04243.x. [DOI] [PubMed] [Google Scholar]
- 101.El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–333. doi: 10.1136/gut.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chow WH, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 103.Vaezi MF, et al. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am. J. Gastroenterol. 2000;95:2206–2211. doi: 10.1111/j.1572-0241.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 104.Rothenbacher D, Blaser MJ, Bode G, Brenner H. An inverse relationship between gastric colonization by Helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectional study. J. Infect. Dis. 2000;182:1446–1449. doi: 10.1086/315887. [DOI] [PubMed] [Google Scholar]
- 105.Sobhani I, et al. Vagal stimulation rapidly increases leptin secretion in human stomach. Gastroenterology. 2002;122:259–263. doi: 10.1053/gast.2002.31385. [DOI] [PubMed] [Google Scholar]
- 106.Goiot H, et al. Antral mucosa expresses functional leptin receptors coupled to STAT-3 signaling, which is involved in the control of gastric secretions in the rat. Gastroenterology. 2001;121:1417–1427. doi: 10.1053/gast.2001.29581. [DOI] [PubMed] [Google Scholar]
- 107.Asakawa A, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 108.Lee HM, et al. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 109.Nishi Y, et al. The relationship between ghrelin and Helicobacter pylori infection. Helicobacter. 2003;8:402. (Abstr.) [Google Scholar]
- 110.Mueller A, et al. Protective immunity against Helicobacter is characterized by a unique transcriptional signature. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12289–12294. doi: 10.1073/pnas.1635231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parsonnet J. The incidence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1995;9:45–51. [PubMed] [Google Scholar]
- 112.Nomura A, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 113.Hansson LE, et al. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology. 1993;105:1098–1103. doi: 10.1016/0016-5085(93)90954-b. [DOI] [PubMed] [Google Scholar]
- 114.Simpson AJ, Caballero OL, Pena SD. Microsatellite instability as a tool for the classification of gastric cancer. Trends Mol. Med. 2001;7:76–80. doi: 10.1016/s1471-4914(01)01916-5. [DOI] [PubMed] [Google Scholar]
- 115.Wagner S, et al. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 116.Meyer-ter-Vehn T, et al. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- 117.Naumann M, et al. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 1999;274:31655–31662. doi: 10.1074/jbc.274.44.31655. [DOI] [PubMed] [Google Scholar]
- 118.Maeda S, et al. Analysis of apoptotic and antiapoptotic signaling pathways induced by Helicobacter pylori. Mol. Pathol. 2002;55:286–293. doi: 10.1136/mp.55.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fan XJ, et al. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J. Exp. Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rudi J, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Invest. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smoot DT, et al. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis. 2000;21:2091–2095. doi: 10.1093/carcin/21.11.2091. [DOI] [PubMed] [Google Scholar]
- 122.Kim JJ, et al. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;23:542–553. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- 123.Ruiz B, et al. Vitamin C concentration in gastric juice before and after anti-Helicobacter pylori treatment. Am. J. Gastroenterol. 1994;89:533–539. [PubMed] [Google Scholar]
- 124.Blaser MJ. The ecology of Helicobacter pylori in the human stomach. J. Clin. Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.