Abstract
Platelet activation occurs in response to vessel injury and is important for the arrest of bleeding. Platelet activation during disease states leads to vascular occlusion and ischemic damage. The P2Y12 receptor, activated by ADP, plays a central role in platelet activation and is the target of P2Y12 receptor antagonists that have proven therapeutic value.
The vessel wall contains a continuous lining of endothelium that serves as a barrier between the circulating platelets and the prothrombotic subendothelial matrix (1). Upon vessel injury, the endothelial layer is disrupted and the circulating platelets are exposed to subendothelial proteins such as vWF, collagen, and vitronectin, among others (1). The platelets initially interact with the subendothelium through adhesive receptors, such as GPIb-IX-V receptors, that mediate rolling and tethering of the platelets to vWF at the site of vascular injury.
Next, the platelet collagen receptors α2β1 and GPVI mediate a more firm adhesion and cause further platelet activation. These initial interactions with the subendothelium cause the release of contents from the platelet dense granules, which contain platelet agonists such as ADP, and the α-granules, which contain fibrinogen, factor V, and P-selectin (1). The release of the granule contents causes further platelet activation, but it also fuels the coagulation response as a result of the release of factor V and fuels the inflammatory response through the exposure of P-selectin on the platelet surface. The platelet also generates lipid mediators such as thromboxane A2. ADP elicits its effects on the platelet through the P2Y1 and P2Y12 receptors (2), whereas thromboxane A2 activates the thromboxane-prostanoid (TP) receptor on the platelet surface (1). The released dense granule contents cause further platelet activation and recruitment of circulating platelets to the site of injury. Platelets interacting with these mediators also undergo platelet shape change, a process of actin cytoskeletal reorganization that changes the platelets from a disc shape to a round shape with long, filopodial extensions that form a meshwork of platelets in the platelet plug (3). Also, tissue factor is exposed, which initiates the coagulation response that results in formation of thrombin. Thrombin activates platelets via interactions with the proteinase-activated receptor-1 (PAR1) and PAR4 receptors (4) and also cleaves fibrinogen to form fibrin. Fibrin further stabilizes the accumulating platelet plug at the site of injury, resulting in a stable hemostatic plug.
Interactions of the platelets with collagen, vWF, ADP, thromboxane A2, and thrombin cause intracellular platelet signaling that leads to the activation of the heterodimeric integrin αIIbβ3, also known as the fibrinogen receptor (5). The intracellular platelet signaling from these agonists causes the fibrinogen receptor to change from a low-affinity state to a high-affinity state that binds fibrinogen (6). Fibrinogen binds to the platelets via the activated fibrinogen receptor, and this cross-linking of platelets to fibrinogen results in platelet aggregates that accumulate and arrest bleeding at the site of injury (Figure 1). Thus, platelet activation is the product of many signals originating from many receptors, which each contribute to the formation of a platelet plug.
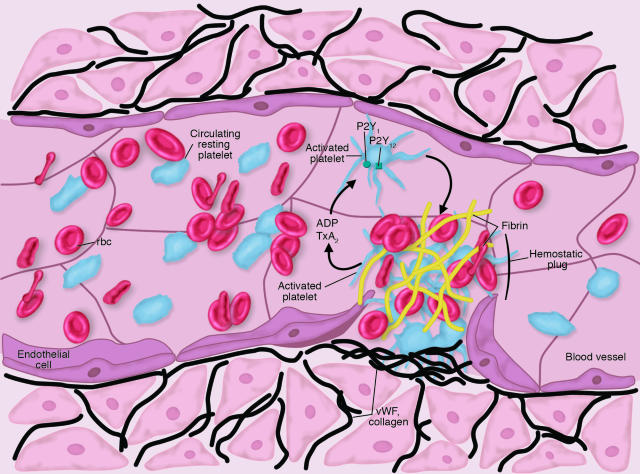
Figure 1.
The hemostatic process. Upon vessel injury, platelets roll and become tethered to the vessel wall by interactions with vWF and collagen (noted as black strands). These interactions cause platelet shape change, and release of ADP from dense granules. The activated platelet also generates thromboxane A2 (TxA2). Both ADP and TxA2 are agonists that cause further platelet activation and accumulation of platelets at the site of injury. Vessel injury also causes exposure of tissue factor, which catalyzes the coagulation response. This response results in the formation of thrombin, which further activates platelets and cleaves fibrinogen to form fibrin. The combination of activated platelets and fibrin at the site of injury forms a stable hemostatic plug that arrests bleeding.
Pathophysiologic conditions, such as atherosclerotic plaque rupture, can lead to aberrant platelet activation resulting in arterial thrombosis, which can cause myocardial infarction and ischemic stroke (6). The importance of ADP in this process has been demonstrated both by antiplatelet drugs that target the P2Y12 receptor (2) and by patients with dysfunctional P2Y12 receptors (7). Antagonism of the P2Y12 receptor with either ticlopidine or clopidogrel is clinically effective in the prevention of myocardial infarction, ischemic stroke, and vascular death (8). Despite the established role of the P2Y12 receptor in the hemostatic response, the full implications of P2Y12 receptor antagonism in the prevention of thrombosis remain incompletely understood. It is hoped that more clinically effective P2Y12 antagonists will prevent the incidence of ischemic events that stem from aberrant platelet activation and therefore will be used as improved and suitable treatments for thrombosis.
The central role of the P2Y12 receptor: ex vivo effects
Prior to the cloning of the P2Y12 receptor, drugs that selectively target this receptor had been widely used as antiplatelet agents (2). Ex vivo studies used platelets treated with clopidogrel or reversible antagonists of the P2Y12 receptor and led to the conclusion that the P2Y12 receptor is crucial to several platelet functions. Thus far, studies have identified a potentiating role for the P2Y12 receptor in dense granule secretion (9), fibrinogen-receptor activation (10–14), and, as reported in a recent issue of the JCI, thrombus formation (15, 16), identifying it as a central mediator of the hemostatic response. This receptor is also important for the irreversible platelet aggregation induced not only by ADP, but also by thromboxane A2 and the PAR1-selective peptide agonist SFLLRN (17, 18). The P2Y12 receptor also causes inhibition of stimulated adenylyl cyclase (19, 20) but does not play any role in ADP-induced platelet shape change and intracellular calcium mobilization (17, 18). Furthermore, Gi signaling that is mediated by P2Y12 receptor activation can lead to platelet aggregation when either Gq or G12/13 pathways are simultaneously stimulated (10, 11, 14), or by itself when exposed to high concentrations of ADP (100 μM) (21). The P2Y12 receptor plays a crucial role in ADP-mediated generation of thromboxane A2, another important platelet activator (22). Signaling events downstream of the P2Y12 receptor also potentiate agonist-induced dense granule release and procoagulant activity (17). In addition, α-granule release and subsequent expression of P-selectin on activated platelets depend on P2Y12 activation (23, 24). Interestingly, all the functions of the P2Y12 receptor can be mimicked by epinephrine, which stimulates members of the Gi family of G proteins by binding to the platelet α2A receptor (17). Thus, the P2Y12 receptor plays a central role in platelet activation, in the recruitment of other platelets to the site of injury subsequent to the adhesion of platelets to vWF and collagen, and in the enhancement of the efficiency of platelet activation by other agonists such as thrombin and thromboxane A2, which are generated as secondary platelet agonists (Figure 2).
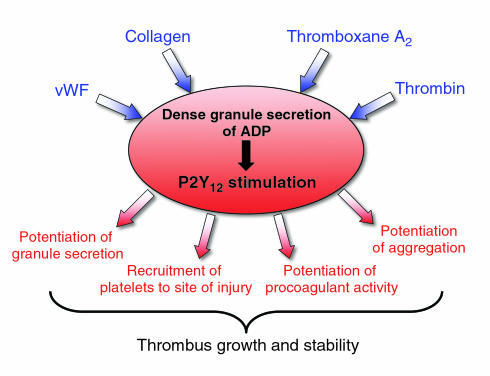
Figure 2.
The central role of the P2Y12 receptor in platelet activation. Exposure of platelets to vWF and collagen results in the adhesion of platelets and subsequent release of ADP from dense granules. Similarly, activation of platelets by thrombin or thromboxane A2 also results in release of ADP. Secreted ADP activates the P2Y12 receptor (as depicted) and the P2Y1 receptor (not shown). P2Y12 receptor activation likely affects thrombus growth and stability by recruiting platelets to the site of injury, and by potentiating dense granule release, procoagulant activity, and aggregation.
The P2Y12 receptor couples primarily to Gαi2 and less prominently to other members of the Gi family, resulting in the inhibition of adenylyl cyclase (25). Epinephrine, through stimulation of the α2A receptor and resulting Gz signaling, also achieves the same effect (26). However, reduced levels of cAMP are not directly responsible for the downstream effects of P2Y12 receptor activation (25, 26). Gi signaling leads to activation of PI3K, Akt, Rap1b, and potassium channels (17). Mice lacking PI3K-γ show aberrations in platelet function only when low doses of ADP are used, but are provided protection from thromboembolism (27). Recent studies indicate that Rap1b, Akt, and potassium channels are important functional effectors downstream of P2Y12 receptor stimulation (28–31) (Figure 3).
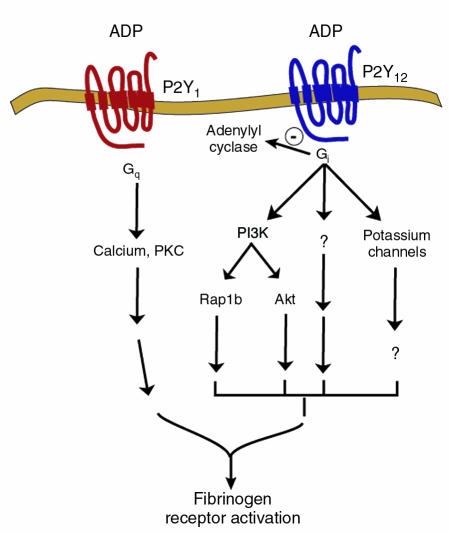
Figure 3.
Intracellular signaling events downstream of the P2Y1 and P2Y12 receptors. ADP binds to the P2Y12 receptor and causes a number of intracellular signaling events downstream of the Gi pathway that contribute to fibrinogen receptor activation and platelet aggregation. The P2Y12 receptor–mediated inhibition of adenylyl cyclase is not directly responsible for fibrinogen receptor activation. Potassium channels and PI3K are also activated by the P2Y12 receptor. Both Rap1b and Akt are signaling mediators that contribute to platelet aggregation and are activated in a PI3K-dependent manner. Other mediators of P2Y12 signaling remain to be elucidated.
Patients with defective P2Y12 receptor function
Patients with a defect in the gene encoding the P2Y12 receptor have a congenital bleeding disorder (7, 32–35). A number of patients have been identified that have decreased aggregation responses to ADP and to low doses of other agonists such as collagen and thrombin (32–35). These patients generally have normal platelet shape change responses to ADP but have impaired abilities to inhibit adenylyl cyclase activity (33). While patients with defective P2Y12 receptor function have dense granules that are normal in both numbers and content, platelet release of granules is generally decreased because of the potentiating effects of the P2Y12 receptor on granule secretion (34). Patients and mice lacking functional P2Y12 receptors have increased bleeding times (7, 32, 36). Patients who are heterozygous for the P2Y12 receptor bind intermediate amounts of 2-methylthio-ADP (2-MeSADP), an agonist of the P2Y1 and P2Y12 receptors, and also have extended bleeding times (7, 34, 37). Some patients with P2Y12 receptor deficiency have been shown to possess a truncated form of the receptor due to deletions in the gene, whereas other individuals have mutations that lead to impaired P2Y12 receptor function (38). Analysis of the P2Y12 receptor sequence from a patient with impaired ADP responses has identified amino acid residues important in P2Y12 receptor function (35). A G-to-A mutation in one allele changed Arg256 into Gln, while a C-to-T alteration resulted in Arg265 changing to Trp (35). Though receptor number and affinity for 2-MeSADP were unchanged, these mutations were demonstrated to lead to impaired Gi signaling in response to P2Y12 stimulation (35). Expressed P2Y12 receptors containing these mutations had a similar loss of function (35). Thus, these residues play an important role in the function of the P2Y12 receptor but have no effect on the binding of ADP.
Bleeding times
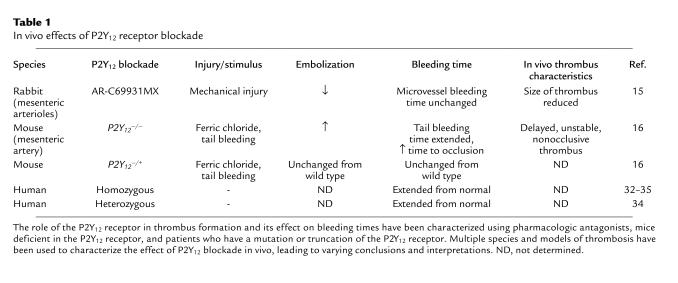
The P2Y12 receptor antagonist clopidogrel has been shown to be efficacious when occupying even 50% of the P2Y12 receptor population (39); thus an antagonist of the P2Y12 receptor could be an effective therapeutic even when 50% of the P2Y12 receptors are functioning. Patients deficient in the P2Y12 receptor, such that their platelets bind only intermediate levels of 2-MeSADP, have slightly prolonged bleeding times (7, 34, 37). The P2Y12 receptor has been cloned, and mice that are deficient in the P2Y12 receptor have been generated. Consistent with observations in patients deficient in the P2Y12 receptor, mice lacking the P2Y12 receptor have increased tail bleeding times (19, 36, 40, 41). However, heterozygous mice show little or no change in bleeding times (16). It is currently unclear why a 50% decrease in the population of functional P2Y12 receptors, either following clopidogrel treatment or in humans heterozygous for the P2Y12 receptor, prolongs bleeding time, while heterozygous mice show little change in bleeding time (Table 1).
Table 1.
In vivo effects of P2Y12 receptor blockade
Role of the P2Y12 receptor in shear-induced platelet activation
Pharmacologic blockade of the P2Y12 receptor in physiologic conditions of arterial flow revealed that this receptor is essential for platelet aggregation under shear conditions. Blood from a patient with defective P2Y12 receptors, or normal blood treated with the reversible P2Y12 receptor antagonist AR-C69931MX, exhibited small and loosely packed thrombi, whereas normal individuals formed large, densely packed thrombi in physiologic flow experiments (42). P2Y12 antagonism also decreased shear-induced platelet aggregation; however, greater inhibition was achieved by antagonism of both the P2Y12 and P2Y1 receptors (43). P2Y12 antagonism has also been shown to decrease P-selectin expression and microparticle formation that is initiated by platelet interactions with vWF (44). Thus, the P2Y12 receptor also plays a role in the formation of platelet aggregates under shear conditions and contributes to thrombus formation on surfaces coated with either collagen or vWF.
Clinical implications of P2Y12 blockade
Consistent with the central role of the P2Y12 receptor in thrombosis, P2Y12 receptor antagonists reduce occlusive thrombosis in animal models. Clinical studies using clopidogrel demonstrate a significantly reduced risk of peripheral artery disease, myocardial infarction, ischemic stroke, or vascular death, in comparison with aspirin therapy (8). Combination therapy with both clopidogrel and aspirin has been shown by the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events) study to result in enhanced beneficial effects, and this has led to FDA approval of clopidogrel for the treatment of some acute coronary syndromes (45). An ongoing trial for the Management of Atherothrombosis with Clopidogrel in High-Risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) is expected to test the effectiveness and safety of combined clopidogrel plus aspirin therapy versus clopidogrel only in patients who have experienced a transient ischemic attack or ischemic stroke (46). The results of this trial will further clarify the efficacy of combination therapy. Despite its beneficial effects, clopidogrel has been shown to cause the development of the immune-mediated syndrome thrombotic thrombocytopenic purpura (47).
The benefits of P2Y12 antagonism have been validated by multiple clinical trials, but some of the characteristics of the present P2Y12 antagonists could be improved. The metabolism of clopidogrel to its active form is required before the onset of drug action is achieved, and an interaction with the cholesterol-lowering drug atorvastatin, which also requires hepatic metabolism by cytochrome P450 3A4, has been identified (48, 49). Post hoc analysis of clinical studies where patients received both clopidogrel and atorvastatin found beneficial effects of clopidogrel in both the presence and the absence of atorvastatin, suggesting that the interaction between the two pharmacologic agents does not alter the clinical effect of clopidogrel (50). Another study also found no difference in the clinical outcome of patients taking clopidogrel with atorvastatin or with other statins (51). The clopidogrel metabolite irreversibly blocks P2Y12 receptor function for the lifespan of the platelet. Hence, prior to surgery, clearance of clopidogrel-treated platelets is necessary to prevent bleeding complications. Thus, new research efforts are aimed at discovering faster-acting, reversible P2Y12 receptor antagonists that would allow more control over antiplatelet treatments.
In vivo analysis of thrombus growth and stability
With the established role of the P2Y12 receptor as the central point of thrombus formation, in vivo analysis will clarify the mechanism by which the P2Y12 receptor contributes to thrombus growth and stability. Multiple studies have begun to characterize the effects of either P2Y12 antagonism or P2Y12 knockout on the formation of a thrombus using various models of vessel injury. A recent report by van Gestel et al. (15) investigated the effect of the P2Y12 receptor antagonists AR-C69931MX and clopidogrel on in vivo thrombus growth and stability (Table 1). Mechanical injury of mesenteric arterioles in rabbits treated with AR-C69931MX or clopidogrel resulted in decreased thrombus height (20% reduction) within the vessel but no change in the bleeding time of the vessel (15). P2Y12 receptor blockade significantly reduced the total duration of embolization with fewer and smaller emboli being produced, and it also reduced the size of the initial thrombus without affecting its stability (15). Thrombin generation was decreased in the AR-C69931MX–treated mice, suggesting that the P2Y12 receptor contributes to the procoagulant response (15). In clopidogrel-treated mice, thrombosis scores were significantly reduced compared with those in controls (52). In addition, there was a delay in the time of initial thrombus formation in the clopidogrel-treated mice (52). Treatment with AR-C69931MX also decreased the reocclusion rate and improved myocardial tissue perfusion in a canine model of coronary electrolytic injury (53). Andre et al. (16) used several approaches to explore the effects of P2Y12 receptor deficiency on thrombus formation. FeCl3-induced vessel wall injury of the mouse mesenteric artery resulted in occlusion of the wild-type mouse vessel; however, the P2Y12-deficient mouse vessel remained unoccluded in eight out of nine mice (16). The appearance of the first thrombus was delayed and only small “unstable” thrombi formed in P2Y12–/– mice (16). There was increased embolization from the thrombus of P2Y12-deficient mice (16). Contrary to the observations of van Gestel et al. (15), more embolization occurred in the P2Y12–/– mice compared with wild-type or heterozygous mice. The differences between these studies are likely due to differences in both species (rabbit versus mouse) and experimental parameters (mechanical injury versus FeCl3 injury) and represent the beginning of our understanding of the effects of P2Y12 antagonism on thrombus formation in vivo.
Data on the role of the P2Y12 receptor in platelet activation and thrombus formation suggest that this receptor is important for the potentiation of many platelet responses and for the formation of a stable hemostatic plug. The tools available for the analysis of P2Y12 receptor function have facilitated characterization of the implications of P2Y12 antagonism, though the studies performed thus far also raise more questions. The differences in embolization between homozygous and heterozygous mice observed by Andre et al. (16) raise several important questions. Would complete blockade of the P2Y12 receptor be a better therapeutic goal, and would it be more beneficial than clopidogrel because of abolished receptor function? Why do results differ depending on the use of a P2Y12 receptor antagonist as opposed to the use of P2Y12 receptor–knockout mice? Is the reason for the increased bleeding times observed in patients and mice lacking the P2Y12 receptor due to unstable thrombus formation or the small size of the thrombus? What are the reasons for differences in embolization in the studies thus far? Answers to these questions are important because, ultimately, definition of the central role of the P2Y12 receptor would translate into the development of a more effective antithrombotic agent, and the answers would affect treatment modalities. The models for thrombus formation have provided varying results regarding the effect of P2Y12 antagonism and/or knockout on thrombus size and embolization. The method of vessel injury, vessel size, and species differences must be considered in the interpretation of the effects of P2Y12 antagonism on thrombus formation. Of course, it is the clinical data that will determine the effectiveness of such therapies, but it is the models of thrombosis that will guide the efforts toward new and improved P2Y12 antagonists. Thus, the large amount of data obtained using clopidogrel treatment, and the studies of van Gestel et al. (15) and Andre et al. (16), may herald the beginning of a new era in antithrombotics.
Acknowledgments
We wish to thank Todd M. Quinton for critically reviewing this manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: proteinase-activated receptor (PAR); 2-methylthio-ADP (2-MeSADP).
References
- 1.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 2.Kunapuli SP, et al. ADP receptors: targets for developing antithrombotic agents. Curr. Pharm. Des. 2003;9:2303–2316. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- 3.Paul BZ, et al. Dynamic regulation of microtubule coils in ADP-induced platelet shape change by p160ROCK (Rho-kinase) Platelets. 2003;14:159–169. doi: 10.1080/0953710031000092794. [DOI] [PubMed] [Google Scholar]
- 4.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brass LF, Manning DR, Cichowski K, Abrams CS. Signaling through G proteins in platelets: to the integrins and beyond. Thromb. Haemost. 1997;78:581–589. [PubMed] [Google Scholar]
- 6.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 7.Cattaneo M, Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler. Thromb. Vasc. Biol. 1999;19:2281–2285. doi: 10.1161/01.atv.19.10.2281. [DOI] [PubMed] [Google Scholar]
- 8.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 9.Dangelmaier C, Jin J, Smith JB, Kunapuli SP. Potentiation of thromboxane A2-induced platelet secretion by Gi signaling through the phosphoinositide-3 kinase pathway. Thromb. Haemost. 2001;85:341–348. [PubMed] [Google Scholar]
- 10.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J. Biol. Chem. 2002;277:47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 12.Quinton TM, et al. Protein kinase C- and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. Biochem. J. 2002;368:535–543. doi: 10.1042/BJ20020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauffenstein G, et al. The P2Y(12) receptor induces platelet aggregation through weak activation of the alpha(IIb)beta(3) integrin: a phosphoinositide 3-kinase-dependent mechanism. FEBS Lett. 2001;505:281–290. doi: 10.1016/s0014-5793(01)02824-1. [DOI] [PubMed] [Google Scholar]
- 14.Nieswandt B, Schulte V, Zywietz A, Gratacap MP, Offermanns S. Costimulation of Gi- and G12/G13-mediated signaling pathways induces integrin alpha IIbbeta 3 activation in platelets. J. Biol. Chem. 2002;277:39493–39498. doi: 10.1074/jbc.M207256200. [DOI] [PubMed] [Google Scholar]
- 15.van Gestel MA, et al. In vivo blockade of platelet ADP receptor P2Y12 reduces embolus and thrombus formation but not thrombus stability. Arterioscler. Thromb. Vasc. Biol. 2003;23:518–523. doi: 10.1161/01.ATV.0000057809.32354.22. [DOI] [PubMed] [Google Scholar]
- 16.Andre P, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J. Clin. Invest. 2003;112:398–406. doi:10.1172/JCI200317864. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunapuli SP, Dorsam RT, Kim S, Quinton TM. Platelet purinergic receptors. Curr. Opin. Pharmacol. 2003;3:175–180. doi: 10.1016/s1471-4892(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 18.Gachet C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 2001;86:222–232. [PubMed] [Google Scholar]
- 19.Hollopeter J, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 20.Zhang FL, et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J. Biol. Chem. 2001;276:8608–8615. doi: 10.1074/jbc.M009718200. [DOI] [PubMed] [Google Scholar]
- 21.Ohlmann P, et al. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Galphaq. Blood. 2000;96:2134–2139. [PubMed] [Google Scholar]
- 22.Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood. 2002;99:193–198. doi: 10.1182/blood.v99.1.193. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Bath P, Heptinstall S. Effects of combining three different antiplatelet agents on platelets and leukocytes in whole blood in vitro. Br. J. Pharmacol. 2001;134:353–358. doi: 10.1038/sj.bjp.0704248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey RF, Judge HM, Wilcox RG, Heptinstall S. Inhibition of ADP-induced P-selectin expression and platelet-leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb. Haemost. 2002;88:488–494. [PubMed] [Google Scholar]
- 25.Daniel JL, Dangelmaier C, Jin J, Kim YB, Kunapuli SP. Role of intracellular signaling events in ADP-induced platelet aggregation. Thromb. Haemost. 1999;82:1322–1326. [PubMed] [Google Scholar]
- 26.Yang J, et al. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9984–9989. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch E, et al. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 28.Chrzanowska-Wodnicka M, Schoenwaelder S, White GC., II Rap1b regulates integrin IIb3 activity and platelet function: lessons from a knockout. Blood. 2003;102:773a. (Abstr.) [Google Scholar]
- 29.Shankar H, et al. Role of G-protein coupled inwardly rectifying potassium channels (GIRKs) in P2Y12 receptor-mediated platelet functional responses. Blood. 2003;102:774a. doi: 10.1182/blood-2004-01-0069. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 30.Woulfe DS, et al. Mice lacking Akt2 have defects in platelet granule secretion and fibrinogen binding and reduced thrombus formation in vivo. Blood. 2003;102:207a. (Abstr.) [Google Scholar]
- 31.Kim, S., Jin, J., and Kunapuli, S.P. 2004. Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. In press. [DOI] [PubMed]
- 32.Nurden P, et al. An inherited bleeding disorder linked to a defective interaction between ADP and its receptor on platelets. Its influence on glycoprotein IIb-IIIa complex function. J. Clin. Invest. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992;80:2787–2796. [PubMed] [Google Scholar]
- 34.Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler. Thromb. Vasc. Biol. 2000;20:E101–E106. doi: 10.1161/01.atv.20.11.e101. [DOI] [PubMed] [Google Scholar]
- 35.Cattaneo M, et al. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster CJ, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cattaneo M, et al. Deficiency of (P-33)2MeS-ADP binding sites on platelets with secretion defect, normal granule stores and normal thromboxane A2 production. Evidence that Adp potentiates platelet secretion independently of the formation of large platelet aggregates and thromboxane A2 production. Thromb. Haemost. 1997;77:986–990. [PubMed] [Google Scholar]
- 38.Conley PB, Delaney SM. Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis. Curr. Opin. Hematol. 2003;10:333–338. doi: 10.1097/00062752-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Bennett JS. Novel platelet inhibitors. Annu. Rev. Med. 2001;52:161–184. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- 40.Savi P, et al. P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem. Biophys. Res. Commun. 2001;283:379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- 41.Takasaki J, et al. Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol. Pharmacol. 2001;60:432–439. [PubMed] [Google Scholar]
- 42.Remijn JA, et al. Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Arterioscler. Thromb. Vasc. Biol. 2002;22:686–691. doi: 10.1161/01.atv.0000012805.49079.23. [DOI] [PubMed] [Google Scholar]
- 43.Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- 44.Goto S, Tamura N, Eto K, Ikeda Y, Handa S. Functional significance of adenosine 5′-diphosphate receptor (P2Y(12)) in platelet activation initiated by binding of von Willebrand factor to platelet GP Ibalpha induced by conditions of high shear rate. Circulation. 2002;105:2531–2536. doi: 10.1161/01.cir.0000016703.93845.af. [DOI] [PubMed] [Google Scholar]
- 45.Mitka M. Results of CURE trial for acute coronary syndrome. JAMA. 2001;285:1828–1829. [PubMed] [Google Scholar]
- 46.Hacke W. From CURE to MATCH: ADP receptor antagonists as the treatment of choice for high-risk atherothrombotic patients. Cerebrovasc. Dis. 2002;13:22–26. doi: 10.1159/000047786. [DOI] [PubMed] [Google Scholar]
- 47.Bennett CL, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N. Engl. J. Med. 2000;342:1773–1777. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 48.Clarke TA, Waskell LA. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab. Dispos. 2003;31:53–59. doi: 10.1124/dmd.31.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Lau WC, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003;107:32–37. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 50.Saw J, et al. Lack of adverse clopidogrel-atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trial. Circulation. 2003;108:921–924. doi: 10.1161/01.CIR.0000088780.57432.43. [DOI] [PubMed] [Google Scholar]
- 51.Wienbergen H, et al. Comparison of clinical benefits of clopidogrel therapy in patients with acute coronary syndromes taking atorvastatin versus other statin therapies. Am. J. Cardiol. 2003;92:285–288. doi: 10.1016/s0002-9149(03)00626-x. [DOI] [PubMed] [Google Scholar]
- 52.Lenain N, Freund M, Leon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J. Thromb. Haemost. 2003;1:1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang K, et al. Blockade of the platelet P2Y12 receptor by AR-C69931MX sustains coronary artery recanalization and improves the myocardial tissue perfusion in a canine thrombosis model. Arterioscler. Thromb. Vasc. Biol. 2003;23:357–362. doi: 10.1161/01.atv.0000052669.50791.0b. [DOI] [PubMed] [Google Scholar]