Abstract
Background
Up to 28% of patients undergoing carotid endarterectomy (CEA) are estimated to experience neurocognitive dysfunction following surgery. The complement cascade plays a central role in ischaemia-reperfusion injury. The authors investigated the effect of common polymorphisms in the complement component 3 (C3F) and complement factor H (CFH Y402H) genes on incidence of neurocognitive dysfunction post-CEA.
Methods
This study examined a nested cohort of prospectively recruited patients receiving elective CEA, who were genotyped for the C3F or Y402H polymorphisms. Each patient underwent a standard battery of eight neuropsychometric tests before, and 1 day and 30 days after, surgery.
Results
57 of 142 (40%) CEA patients had at least one copy of the C3F allele (C3F+), and 17 of 137 (12%) patients had two copies of the CFH Y402H allele (Y402H++). At postoperative day 1, patients were three times (OR 3.05, p=0.045) or six times (OR 6.41, p=0.006) more likely to experience moderate-to-severe neurocognitive dysfunction if they carried the C3F+ or Y402H++ genotype, respectively. Patients with both risk genotypes had an almost eightfold risk of dysfunction (OR 7.67, p=0.046). Right-hand-dominant C3F+ subjects undergoing right-side CEA performed significantly worse on tests of visuospatial function than C3F− subjects. At day 30, C3F+ and Y402H++ genotypes trended towards significance as predictors of dysfunction (p=0.07 and p=0.22, respectively).
Conclusion
The C3F and Y402H polymorphisms are strong independent predictors of moderate-to-severe neurocognitive dysfunction at 1 day following CEA. Furthermore, patients undergoing right-sided CEA are predisposed to deficits associated with cortex ipsilateral to the operative carotid artery.
INTRODUCTION
A growing body of evidence indicates that up to 28% of patients experience neuropsychological dysfunction 1 day following carotid endarterectomy (CEA).1–3 While the molecular bases for postoperative cognitive dysfunction following CEA remain unclear, they are proposed to stem from cerebral hypoperfusion due to cross-clamping of the carotid artery, postoperative hyperperfusion or microembolisation of atheroma, thrombi or gaseous bubbles in the course of plaque removal.2,4
Recent studies implicate a genetic basis for post-CEA cognitive dysfunction.5,6 The presence of at least one apolipoprotein E (APOE)-ε4 allele, which is associated with plasma lipoproteins and is involved in the metabolism and transport of CNS lipids, has been associated with increased risk of cognitive dysfunction 1 month after CEA.5 A more recent study demonstrated that an inducible nitric oxide synthase (iNOS) promoter polymorphism afforded protection against moderate/severe cognitive dysfunction 1 month after CEA.6 Based on our current understanding of these genes, the APOE-ε4 polymorphism may be related to neuronal membrane repair and remodelling after injury, while the iNOS promoter polymorphism may be related to global cerebroprotective ‘baseline preconditioning.’
Despite developing insights into a genetic basis for post-CEA cognitive dysfunction, no study has examined genes that are thought to be directly related to inflammatory and complement-mediated injury after CEA. Recent evidence from animal models of cerebral ischaemia has defined complement components C3a and C5a as critical mediators in ischaemic injury, demonstrating neuroprotective benefits in mice genetically deficient in these components and their receptors, as well as with administration of anticomplement drug.7,8 As data emerge to support a role for complement modulation in ischaemic neuroprotection, the identification of complement’s influence on human mechanisms of ischaemic injury becomes increasingly important, particularly with regards to outcome prediction and the development of therapeutic targets. We therefore sought to identify whether two known complement-related genes, Complement Component 3 (C3) and Complement Factor H (CFH), are associated with neurocognitive dysfunction following CEA. The substitution of a single DNA base pair in the C3 protein (rs2230199) defines two allelic variants—C3S (slow) and C3F (fast)—described according to the differential motility of the proteins in gel electrophoresis.9 In the CFH gene, which produces a protein responsible for inhibition of the common complement pathway, a single nucleotide polymorphism at position 1277 in exon 9 (rs1061170) results in a T-to-C nucleotide substitution that changes a tyrosine amino acid at position 402 to histidine (Y402H) in the CFH protein.10
METHODS
Subjects
A nested cohort of 142 CEA patients was prospectively recruited between 1998 and 2007. A control group of 71 similarly aged and educated patients undergoing lumbar laminectomy with a comparable anaesthetic regimen was contemporaneously recruited. These patients represent a subgroup of an ongoing study of neurocognitive dysfunction following CEA, all of whom were given informed consent for this institutional review board-approved investigation.1–3,5,6 While all CEA patients were genotyped for the C3F polymorphism, genetic material was available only for 137 patients during the later arm of the study that genotyped for the CFH Y402H polymorphism. All patients were native English speakers with no history of drug abuse, axis I psychological disorders or previous ipsilateral CEA. Demographic and intraoperative variables were recorded.
Surgery and anaesthesia
All subjects underwent general anaesthesia with routine monitoring.1,2 Fentanyl and midazolam were administered for preinduction sedation. All CEA subjects received an inhalational agent (isoflurane or sevoflurane with 70% nitrous oxide for approximately 45 min before carotid cross-clamping). Intra-operative electroencephalography was used to monitor for cerebral ischaemia during the endarterectomy, and members of the neurovascular and vascular service performed all CEAs. Out of the six patients who had symptomatic stenosis, all underwent CEA on the symptomatic side. All patients were extubated without complication in the operating room and recovered in a postoperative care or neurological intensive care unit.
C3 and CFH genotyping
DNA extracted from buffy coats of whole blood samples was amplified via PCR. For C3, 5′CGGAAGACCAAGAAT AATGGGCAGGC3′ was used as the sense primer and 5′TCTGTCTGGATGAAGAGGTACCCG3′ as the antisense primer. For CFH, the sense primer was 5′TGAGCAAATTTA TGTTTCTCATTTACT3′, while the antisense primer was 5′CATTCTCCATACATGTAACTGTGGT3′. PCR was performed with an Eppendorf Mastercycler Gradient thermal cycler (Hamburg, Germany). Cycling conditions were initial denaturation at 95°C for 5 min, then 35 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s and extension at 72°C for 45 s; this was followed by a final extension step at 72°C for 10 min. PCR products were treated with QIAquick PCR Purificantion kits (Qiagen, Alameda, California). Sequencing was performed with the aforementioned primers on 20 ng of PCR products using BigDye Termination v3.1 Cycle Sequencing kits (ABI, Foster City, California), and sequencing products were analysed on an ABI 3730xl capillary instrument. Sequence traces were aligned, and the single nucleotide polymorphism determined using Chromas 2.01 software (Digital River, Eden Prairie, Minnesota).
Neuropsychometric evaluation
All patients underwent a battery of eight neuropsychometric tests before surgery and at 1 day and 30 days postoperatively: (1) Controlled Oral Word Association, a test of verbal fluency; (2) Boston Naming, a test of verbal function and language skills; (3) Copy Portion of Rey Complex Figure, a test of visuospatial organisation and construction; (4) Immediate Recall Portion of Rey Complex Figure, a test of visuospatial memory; (5) Trails Making Part A, a test of attention, visual scanning and motor function; (6) Trails Making Part B, a test of complex conceptual switching; (7) Hopkins Total Recall or Buschke Selective Reminding Test Sum Total Recall, both tests of verbal learning and memory; (8) Grooved Pegboard, a test of manual dexterity administered twice to evaluate function of the dominant and non-dominant hand.11 Depending on their year of enrolment, subjects completed either Hopkins Total Recall or Buschke Selective Reminding Test Sum Total Recall, but not both. These two instruments are tests of verbal learning and memory, and 62% of subjects completed the Buschke Selective Reminding Test. This battery allows for a global assessment of higher neocortical functioning.
Statistical analysis
Each neuropsychometric test was scored individually for the CEA and lumbar laminectomy groups.1,2,5,6 A change score was calculated for each patient by computing the difference between preoperative and postoperative raw scores on each test. Subsequently, each change score was converted to a Z-score normalised against the scores of the lumbar laminectomy patient population in order to control for effects of anaesthesia, operative pain and performance changes accounted for by test readministration. The following formula was used: Z score=(change score–mean change score of lumbar laminectomy population)/SD of change score in the lumbar laminectomy cohort. Individual scores were averaged across all eight tests to compute a global change score accounting for general cognitive function. Global scores below 1.5SD of the mean performance of the control laminectomy patients were determined to represent moderate-to-severe neurocognitive dysfunction as reported in previous studies.6
JMP statistical software (SAS Institute) was used to perform univariate and multivariate analyses. The Fisher exact tests and Pearson χ2 tests were used for categorical variables, and t tests were used for continuous variables when evaluating univariate statistics. Nominal logistic regression was used with a forward stepwise model to adjust for factors previously reported to affect performance on neuropsychometric tests and cognitive outcome following CEA. These factors included age,3,5,6,12 years of education,12 diabetes mellitus,3,5 history of cerebrovascular accident12 and presence of the APOE-ε45 and iNOS promoter6 polymorphisms. Variables achieving univariate p<0.10 were included in the regression. A value of p<0.05 was used in both univariate and multivariate analysis to establish statistical significance.
RESULTS
C3F polymorphism
Forty-five patients had one copy of the C3F allele (heterozygous), 12 patients had two copies of C3F (homozygous), and 57 of the total 142 CEA patients (40%) had at least one copy of the C3F allele (denoted C3F+). The frequency of C3F (0.24) in the predominantly White population of this study (93%) did not differ significantly from the previously reported frequency in Caucasians (0.20; p=0.23 by χ2 test),9 and the C3 genotypes were in Hardy–Weinberg equilibrium (p=0.60 by χ2 test).
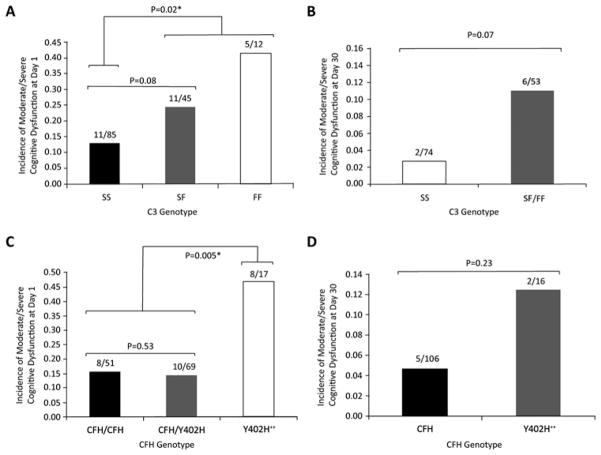
Patients with any C3F allele (C3F heterozygotes and homozygotes together, denoted C3F+) were more likely to have cognitive dysfunction at day 1 (28%) than those without any C3F allele (13%, p=0.02, figure 1A). The C3F+ grouping is maintained in all other data analyses because of this significant difference. There was no significant difference in dysfunction between those with no C3F allele and C3F heterozygotes; nor was there any difference between C3F heterozygotes and C3F homozygotes. At day 30, a higher percentage of C3F+ patients continued to exhibit neurocognitive dysfunction than non-carriers (11% and 3%, respectively), but this result only trended towards significance (p =0.07, figure 1B).
Figure 1.
C3F and CFH Y402H polymorphisms and incidence of postoperative neurocognitive dysfunction. C3F+ status is a significant predictor of moderate-to-severe neurocognitive dysfunction at 1 day postoperatively (A) and trends towards significance at 30 days (B). Y402H++ status is a significant predictor of moderate-to-severe neurocognitive dysfunction at 1 day postoperatively (C) and trends towards significance at 30 days (D). The bars denote incidence of dysfunction as a proportion of the respective patient cohort. All p values were obtained from the Fisher exact test.
The test-by-test z-score analysis of all right-hand-dominant CEA subjects at day 1 is displayed in table 1. At the first postoperative day, right-hand-dominant C3F+ patients undergoing right-side CEA performed significantly worse than C3F− patients on tasks of visuospatial attention (Trails Making Part A Test, p=0.03) and visuospatial memory (Rey Complex Figure Test Recall Portion, p=0.01). Test scores were not significantly different according to C3 genotype at day 30 (data not shown). By definition, the mean z-scores for the control group were zero for each test.
Table 1.
Mean (SE) 1 day z-scores for all right-hand-dominant carotid endarterectomy patients by operative side and C3 genotype
| 1 day test | Left-side carotid endarterectomy
|
Right-side carotid endarterectomy
|
||||
|---|---|---|---|---|---|---|
| C3S | C3F+ | p Value | C3S | C3F+ | p Value | |
| Controlled Oral Word Association Test | −0.06 (0.18) | 0.07 (0.20) | 0.31 | 0.16 (0.12) | 0.09 (0.22) | 0.39 |
| Boston naming | −0.21 (0.09) | −0.21 (0.16) | 0.50 | 0.04 (0.09) | −0.02 (0.20) | 0.38 |
| Rey, copy | −0.17 (0.17) | −0.56 (0.25) | 0.10 | −0.07 (0.21) | −0.59 (0.26) | 0.06 |
| Rey, recall | 0.06 (0.20) | −0.33 (0.23) | 0.10 | 0.39 (0.25) | −0.47 (0.29) | 0.01 |
| Trails A | −0.39 (0.35) | −0.02 (0.21) | 0.19 | 0.26 (0.22) | −0.89 (0.53) | 0.03 |
| Trails B | −0.26 (0.24) | −0.16 (0.23) | 0.39 | −0.14 (0.19) | −0.58 (0.27) | 0.09 |
| Buschke Consistent Long-Term Retrieval | −0.02 (0.21) | 0.13 (0.26) | 0.33 | 0.08 (0.11) | 0.44 (0.37) | 0.19 |
| Hopkins Total Recall | −0.58 (0.37) | −0.48 (0.47) | 0.43 | −0.56 (0.45) | −0.12 (0.33) | 0.22 |
| Grooved Pegboard tested on the dominant hand | −0.49 (0.34) | 0.11 (0.53) | 0.17 | −0.17 (0.18) | −0.66 (0.35) | 0.35 |
| Grooved Pegboard tested on the non-dominant hand | −0.29 (0.23) | −0.10 (0.32) | 0.31 | −0.16 (0.20) | −0.76 (0.44) | 0.11 |
Bold values indicate significance by t test.
C3F+ refers to subjects possessing at least 1 F allele.
Preoperative performance on each neuropsychometric test did not differ between the C3 genotypes (data not shown), but C3F+ patients were less likely to have previously undergone contralateral CEA (p=0.02; table 2). In total, 27 of 142 (19%) patients experienced moderate-to-severe neurocognitive dysfunction at day 1, and 8 of 127 (6%) at day 30.
Table 2.
Key demographic and perioperative variables
| C3S (N=85 at 1 day) | C3F+ (N=57 at 1 day) | p Value | CFH (N=120 at 1 day) | Y402H++ (N=17 at 1 day) | p Value | |
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Age | 70.3 (8.9) | 71.0 (9.8) | 0.34 | 70.1 (9.1) | 71.9 (10.6) | 0.25 |
| Male | 59 (69.4%) | 39 (68.4%) | 0.71 | 82 (68.3%) | 12 (70.6%) | 0.91 |
| White | 77 (90.6%) | 55 (96.5%) | 0.16 | 113 (94.2%) | 15 (88.2%) | 0.31 |
| Years of education | 14.7 (3.36) | 15.0 (2.68) | 0.27 | 15.0 (3.14) | 13.4 (2.74) | 0.02* |
| Right-hand-dominant | 81 (95.3%) | 53 (92.0%) | 0.41 | 113 (94.2%) | 16 (94.1%) | 0.66 |
| Medical history | ||||||
| Obesity | 24 (28.6%) | 11 (19.6%) | 0.16 | 31 (26.3%) | 3 (17.7%) | 0.33 |
| Diabetes mellitus | 16 (18.8%) | 8 (14.0%) | 0.31 | 20 (16.7%) | 4 (23.5%) | 0.34 |
| Hypertension | 57 (67.1%) | 40 (70.2%) | 0.42 | 80 (66.7%) | 13 (76.5%) | 0.30 |
| Hypercholesterolaemia | 56 (65.9%) | 41 (71.9%) | 0.28 | 81 (67.5%) | 13 (76.5%) | 0.33 |
| Previous cerebrovascular accident | 45 (52.9%) | 23 (40.4%) | 0.10 | 57 (47.5%) | 9 (52.9%) | 0.44 |
| Previous myocardial infarction | 17 (20.0%) | 10 (17.54%) | 0.45 | 24 (20.0%) | 3 (17.7%) | 0.56 |
| Previous CEA | 10 (11.8%) | 1 (1.75%) | 0.03* | 10 (8.3%) | 1 (5.9%) | 0.59 |
| Peripheral vascular disease | 9 (10.8%) | 4 (6.90%) | 0.56 | 11 (9.32%) | 1 (5.90%) | 1.00 |
| Symptomatic CEA | 2 (2.35%) | 4 (6.90%) | 0.22 | 4 (3.31%) | 2 (11.8%) | 0.16 |
| Ipsilateral stenosis (%) | 86.5 (10.0) | 88.4 (7.82) | 0.28 | 86.1 (9.47) | 90.9 (6.82) | 0.02* |
| Contralateral stenosis (%) | 32.7 (32.5) | 42.9 (30.8) | 0.13 | 36.2 (32.3) | 43.0 (32.8) | 0.47 |
| Genetic variables | ||||||
| Apolipoprotein E-ε4 | 19 (25.0%) | 11 (20.8%) | 0.37 | 28 (25.5%) | 2 (12.5%) | 0.21 |
| Inducible nitric oxide synthase promoter | 21 (26.6%) | 13 (24.5%) | 0.48 | 30 (26.6%) | 3 (18.8%) | 0.37 |
| Intraoperative variables | ||||||
| Right-side CEA | 45 (52.9%) | 30 (52.6%) | 0.55 | 62 (51.7%) | 10 (58.8%) | 0.39 |
| Surgery duration (min) | 160.9 (42.9) | 153.3 (32.3) | 0.11 | 160.0 (40.5) | 145.8 (29.1) | 0.04* |
| Clamp duration (min) | 44.1 (16.0) | 40.6 (15.6) | 0.10 | 43.6 (16.1) | 39.5 (15.4) | 0.16 |
| Fentanyl (μg/kg) | 2.17 (1.11) | 2.47 (1.32) | 0.08 | 2.32 (1.22) | 2.11 (1.19) | 0.26 |
| Midazolam (mg/kg) | 0.033 (0.014) | 0.036 (0.017) | 0.16 | 0.035 (0.015) | 0.029 (0.011) | 0.02* |
| EEG change | 4 (4.71%) | 1 (1.75%) | 0.33 | 3 (2.50%) | 2 (11.8%) | 0.12 |
| Shunt placed | 4 (4.71%) | 1 (1.75%) | 0.33 | 3 (2.50%) | 2 (11.8%) | 0.12 |
Values are expressed as number (percentage of cases or SD).
CEA, carotid endarterectomy; CFH, one or no Y402H alleles; C3F+, presence of any (one or two) C3F allele; C3S, no C3F allele; Y402H++, two Y402H alleles.
Statistically significant; p values are from the t test for continuous variables and Fisher exact test or Pearson χ2 test for categorical variables.
Y402H polymorphism
Sixty-nine of 137 (50%) CEA patients had one copy of the Y402H allele, and 17 of 137 (13%) had two copies (Y402H++). The frequency of the Y402H allele was 0.38, which was not significantly different from that reported for a large cohort of white patients in the Rotterdam study (0.36; p=0.72 by χ2 test),13 and the CFH genotypes were in Hardy–Weinberg equilibrium (p=0.83 by χ2 test).
Y402H homozygotes (denoted Y402H++) were more likely to have neurocognitive dysfunction at day 1 (47%) than Y402H/CFH heterozygotes and CFH homozygotes grouped together (denoted CFH) (15%, p<0.01; figure 1C). There was no significant difference in rates of neurocognitive dysfunction between CFH homozygotes and Y402H/CFH heterozygotes, but both of these groups differed from the Y402H++ cohort (p=0.01 in each case). We maintain the CFH versus Y402H++ grouping in all other analyses because of this significant difference. Differences in neurocognitive scores were not significant at day 30 (figure 1D), and the test-by-test z-score analysis did not reveal any association between operative side of CEA and lateralised cognitive dysfunction (data not shown).
Y402H++ patients were more likely to have a shorter surgery time (p=0.04), decreased dose of midazolam during surgery (p=0.02), slightly higher mean ipsilateral stenosis (p=0.02) and fewer years of education (p=0.02; table 2). Twenty-six of 137 (19%) patients experienced moderate/severe neurocognitive dysfunction at day 1, while seven of 122 (6%) did so at day 30.
Multivariate modelling
Presence of the C3F and Y402H++ polymorphisms was included in a multivariate logistic regression model adjusted for age, years of education, diabetes mellitus, history of cerebrovascular accident, previous contralateral CEA, degree of ipsilateral stenosis, duration of surgery, dose of midazolam, and presence of APOE-ε4 and iNOS promoter polymorphisms. Following stepwise forward elimination, C3F+ patients carried a threefold higher risk, and Y402H++ patients had a more than sixfold higher risk of neurocognitive dysfunction at day 1 (OR 3.01, p=0.045 and OR 6.41, p=0.006, respectively; table 3). Age was the only other factor that remained an independent predictor of dysfunction at the first postoperative day (OR 1.14, p=0.0003). The predictive validity of the whole final model reached p<0.0001. In a second multivariate model adjusting for the same factors above, patients who carried both C3F+ and Y402H++ genotypes (C3F+/Y402H++) had a more than sevenfold higher risk of experiencing neurocognitive dysfunction at day 1 (OR 7.67, p=0.046; table 3).
Table 3.
Multivariate logistic regression models of moderate/severe neurocognitive dysfunction after stepwise elimination for postoperative day 1
| OR (95% CI) | p Value | |
|---|---|---|
| Model 1 | ||
| Age | 1.14 (1.06 to 1.25) | 0.0003* |
| Years of education | *** | *** |
| Previous carotid endarterectomy | *** | *** |
| Previous cerebrovascular accident | *** | *** |
| Ipsilateral stenosis | *** | *** |
| Diabetes mellitus | *** | *** |
| Surgery duration | *** | *** |
| Midazolam dose | *** | 0.08 |
| Apolipoprotein E-ε4 | *** | *** |
| Inducible nitric oxide synthase | *** | *** |
| C3F+ | 3.05 (1.03 to 9.73) | 0.045* |
| Y402H++ | 6.41 (1.70 to 25.96) | 0.006* |
| Whole final model | *** | 0.0001* |
| Model 2† | ||
| Age | 1.12 (1.04 to 1.22) | 0.002* |
| Midazolam dose | *** | 0.12 |
| C3F+/Y402H++ | 7.82 (1.09 to 72.0) | 0.04* |
| Whole final model | *** | 0.0001* |
All independent variables listed were included in the model before stepwise elimination. All p values were determined from likelihood ratio tests; the OR for age is presented as per year. C3F+ refers to subjects with at least one F allele. Y402H++ refers to subjects with two copies of the Y402H allele. C3F+/Y402H++ refers to subjects with at least one F allele and two copies of the Y402H allele.
Statistically significant
Statistically non-significant independent variables are denoted ***.
For Model 2, only factors remaining after stepwise elimination are displayed.
Control group
There were no significant differences in key baseline and operative characteristics of the lumbar laminectomy group (mean age 68.1 years, 61.4% male, 98.6% white, 15.73 mean years of education, mean duration of surgery 150.04 min, mean fentanyl dose 2.43 μg/kg, mean midazolam dose 0.031 mg/kg) compared with the CEA cohort (table 2).
DISCUSSION
Postoperative neurocognitive dysfunction is a well-established phenomenon following cardiac bypass14 and CEA.1–3 Similar pathophysiological mechanisms of injury have been implicated in neurocognitive dysfunction and include ischaemia, reperfusion injury, inflammation and microembolic events. As in ischaemic stroke, acute and delayed neuronal response to ischaemia mediated by the inflammatory cascade, apoptosis and neuronal remodelling are critical processes, which may account for why patients respond differently to intraoperative ischaemia. The complement cascade is a process that has been defined in both humans and animals as a key mediator of inflammation, opsonisation and cytolysis following cerebral injury.15,16 In particular, C3a, a downstream cleavage product common to all three complement pathways,17 is elevated in human acute ischaemic stroke,18 and increases in systemic levels of C3a have been associated with worse clinical outcomes following subarachnoid haemorrhage.19 Furthermore, C3 genetic deletion in animal models of ischaemic stroke affords both a reduction in infarct volume and functional protection following ischaemic insult.15 Given the established role of C3, we hypothesised that genetic polymorphisms affecting complement levels and function would influence functional neurocognitive response to ischaemia in CEA.
Though in vitro evidence regarding functional differences between the C3F/S allotypes remains inconclusive, a number of genetic association studies provide compelling evidence of important differences in immunological function. In vitro, C3F appeared to have a lower activity than C3S in a sheep erythrocyte haemolytic assay but did not demonstrate a difference in binding properties to major complement receptors.20–22 Clinically, the presence of the C3F allele has been reported to correlate with the development of IgA nephropathy,23 age-related macular degeneration,24 systemic vasculitis25 and Crohn’s disease.26 C3F also appears to correlate with early rupture of intracranial aneurysms27 and the incidence of myocardial infarction.28,29 Studies examining the role of C3F in development of myocardial infarction have hypothesised that the polymorphism increases the susceptibility of atherosclerotic plaques to rupture.28,29
Complement Factor H is responsible for inhibition of the common complement pathway by regulating cleavage of complement component 3 (C3) and the resulting products. CFH binds directly to the pro-inflammatory C-reactive protein (CRP), inactivates the opsonin C3b on self-cells and also inhibits the assembly of an active C3 convertase. The Y402H polymorphism decreases the binding affinity of CFH for CRP and other substrates, leading to excessive activation of the complement cascade.10
Y402H has been most thoroughly implicated in susceptibility to age-related macular degeneration (AMD), contributing to an RR that ranges from 2.45 in heterozygotes to 7.4 in homozygote Y402H carriers.30 AMD shares pathological and epidemiological similarities with atherosclerosis,31 as inflammation plays an important role in plaque formation and destabilisation.32 Furthermore, the presence of Y402H is associated with an increased incidence of ischaemic stroke and coronary heart disease in Caucasians31 as well as increased cardiovascular mortality.33
Our results demonstrate that both the C3F+ and Y402H++ genotypes are predictive of moderate-to-severe neurocognitive dysfunction at 1 day following CEA. While significantly associated with dysfunction at 1 day following CEA, the presence of these polymorphisms only trended towards worse neurocognitive function at 30 days. The identification of a significant difference at 30 days may have been limited by the loss of 10% of the original cohort to follow-up and by the markedly lower incidence of neurocognitive dysfunction at 30 days (6%) compared with 1 day (19%) post-CEA. An analysis of studies on postoperative cognitive dysfunction concluded that in the majority of cases, cognitive deficits resolved by 6 months after surgery.34 Despite delayed resolution of acquired cognitive deficits, there still exists clear evidence that the incidence of neurocognitive injury, even at early time points, correlates with important life changes for patients after surgery. In a recent study of 701 patients followed for a median of 8.5 years, cognitive impairment at 1 week after non-cardiac surgery was significantly associated with premature employment termination due to disability as well as voluntary early retirement.35 Additionally, a larger study of 1064 patients demonstrated that cognitive dysfunction at hospital discharge was predictive of mortality at 3 months after non-cardiac surgery.12
We chose to assess for neurocognitive dysfunction at postoperative day 1 because the general recovery time for patients undergoing CEA is relatively rapid, as most patients are discharged home within 1–2 days after surgery unless preoperative stroke deficits result in placement issues or the need for lengthy inpatient rehabilitation. We suggest that day 1 is perhaps the most relevant time point to evaluate for cognitive dysfunction because it may influence the length of patient stay in a more heavily monitored setting, length of in-hospital stay, patient-reported in-hospital experience or longer-term social outcomes. For our CEA cohort, the use of metrics that assess instrumental activities of daily living may prove to be a sensitive approach for detecting significant lifestyle changes. Future studies must evaluate these in-patient and longer-term outcomes in relationship to postoperative day 1 dysfunction in order to determine the predictive value of assessing cognitive function at this time point.
As shown in table 1, the test-by-test analysis in C3F carriers demonstrates that right-side CEA preferentially damages visuospatial functions (Rey Complex Figure Recall Portion and Trails Making Part A Tests) which normally localise to the right hemisphere in right-hand-dominant subjects.36,37 Lateralisation of cognitive dysfunction supports our hypothesised mechanisms of neuronal injury, which relate directly to transient ischaemia and subsequent reperfusion resulting from carotid cross-clamping, and the potential for side-specific microembolic events related to carotid plaque removal.
An inherent limitation of our study can be attributed to differences in baseline characteristics among the patients analysed. When stratified by CFH genotype, the Y402H++ group had a slightly higher mean ipsilateral stenosis (90.9% vs 86.1%). This difference is both minor and clinically insignificant because there is no difference in indication for surgery or outcomes for patients within this range of stenosis. Furthermore, it does not seem to point towards a more severe generalised atherosclerotic burden in the Y402H++ group because they did not have a higher degree of contralateral stenosis, nor did they have more peripheral vascular disease. Y402H++ patients also had reduced surgery times and doses of midazolam. Both of these intraoperative variables would be expected to decrease the incidence of neurocognitive dysfunction and therefore are unlikely to have contributed to the higher rate of dysfunction observed in patients with two Y402H alleles. The Y402H++ group was also characterised by a significantly lower average number of years of education, which may predispose to worse performance on neuropsychometric testing in general. These factors were included in the multivariate model to control for its effects on the primary outcome. Since C3F+ patients had a higher likelihood of having undergone a contralateral CEA before the study procedure, the multivariate model was also used to adjust for this covariate. Ultimately, nominal logistic regression demonstrated that the predictive value of the C3F+ or the Y402H++ genotype was independent of the aforementioned factors, and C3F+ and Y402H++ remained robust predictors of neurocognitive dysfunction independent even of each other at 1 day postoperatively. Moreover, the likelihood of injury was even greater in patients who had both a C3F+ and Y402H++.
The effect of these genotypic variables on outcome is similar to that found in a previous study by Seitsonen et al, which examined the presence of the C3F and Y402H polymorphisms in a cohort of patients with age-related macular degeneration (AMD).38 In their analysis, C3F+ patients had an OR of 2.12, those with Y402H an OR of 5.45, and those with both C3F and Y402H an OR of 5.57 for the development of AMD. The results of our study mirrored this risk distribution, with the C3F+/Y402H++ combination representing the greatest risk factor for postoperative neurocognitive dysfunction (OR 7.67), followed by Y402H++ alone (OR 6.41) and C3F+ alone (3.04). The correlation of neurocognitive dysfunction with these risk alleles strongly implicates complement as a mediator of damage post-CEA, and a clinical trial of complement inhibitor, which is ongoing in the AMD setting,39 may be applicable in the CEA population.
The present study provides a clinically relevant risk correlation based on genetic variability, but further work must be done to understand the mechanisms of post-CEA cognitive decline. Though previous work from our group has shown no correlation between postoperative DWI restriction and neurocognitive dysfunction,40 we have demonstrated a robust association between MR perfusion asymmetry and neurocognitive dysfunction.41 In order to establish a causal relationship, future studies should explore detailed associations between localised perfusion deficits referable to cognitive deficits. Furthermore, localised and systemic complement activity, as well as downstream inflammatory effects, must be assessed to provide an understanding of why patients with the C3F and Y402H respond differently to ischaemic insults.
This study underscores the importance of complement disturbances in the development of neurological injury, and further exploration of the precise effect of the C3F and Y402H polymorphisms on C3 protein function is required to begin development of therapeutic targets. Notably, a large number of patients receiving CEA may derive only limited benefit from the surgery on account of old age, borderline stenosis or a lack of cerebrovascular autoregulatory dysfunction.42–44 Therefore, risk factors that predispose to cognitive deterioration may be significant in determining the proper mode of clinical management in this group. Our study indicates that genotyping for the C3F polymorphism in such patients can help with appropriate selection of surgical candidates.
Acknowledgments
The authors thank D O Quest, P C McCormick, J F McKinsey, N J Morrissey, R A Solomon for their support and operative treatment of the patient cohort. The authors also thank the Columbia Irving Institute Clinical and Translational Science Award (CTSA) Program for biostatistics consultation.
Funding ESC was supported in part by Grant Number R01-NS-040409-08 from the National Institutes of Health (NIH) and the Department of Neurological Surgery, Columbia University; New York, NY. EJH was supported in part by Grant Number R01-AG-017604-06 from the NIH and the Department of Anesthesiology, Columbia University; New York, NY. The Columbia Irving CTSA Program was supported by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. The contents of this project are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Competing interests None.
Ethics approval Ethics approval was provided by the Columbia University Medical Center Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Heyer E, Adams D, Solomon R, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–15. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyer E, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–22. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocco J, Wilson D, Komotar R, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–50. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirooka R, Ogasawara K, Sasaki M, et al. Magnetic resonance imaging in patients with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy. J Neurosurg. 2008;108:1178–83. doi: 10.3171/JNS/2008/108/6/1178. [DOI] [PubMed] [Google Scholar]
- 5.Heyer E, Wilson D, Sahlein D, et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology. 2005;65:1759–63. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yocum G, Gaudet J, Lee S, et al. Inducible nitric oxide synthase promoter polymorphism affords protection against cognitive dysfunction after carotid endarterectomy. Stroke. 2009;40:1597–603. doi: 10.1161/STROKEAHA.108.541177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducruet AF, Hassid BG, Mack WJ, et al. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28:1048–58. doi: 10.1038/sj.jcbfm.9600608. [DOI] [PubMed] [Google Scholar]
- 8.Kim GH, Mocco J, Hahn DK, et al. Protective effect of c5a receptor inhibition after murine reperfused stroke. Neurosurgery. 2008;63:125–6. doi: 10.1227/01.NEU.0000335079.70222.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poznansky M, Clissold P, Lachmann P. The difference between human C3F and C3S results from a single amino acid change from an asparagine to an aspartate residue at position 1216 on the alpha-chain of the complement component, C3. J Immunol. 1989;143:1254–8. [PubMed] [Google Scholar]
- 10.Boon C, van de Kar N, Klevering B, et al. The spectrum of phenotypes caused by variants in the CFH gene. Mol Immunol. 2009;46:1573–94. doi: 10.1016/j.molimm.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Lezak MD. Neuropsychological Assessment. 4. Oxford: Oxford University Press; 2004. [Google Scholar]
- 12.Monk T, Weldon B, Garvan C, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 13.de Jong F, Ikram M, Despriet D, et al. Complement factor H polymorphism, inflammatory mediators, and retinal vessel diameters: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2007;48:3014–18. doi: 10.1167/iovs.06-1460. [DOI] [PubMed] [Google Scholar]
- 14.Newman MF, Mathew JP, Grocott HP, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. doi: 10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- 15.Mocco J, Mack W, Ducruet A, et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–17. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 16.Komotar RJ, Starke RM, Arias EJ, et al. The complement cascade: new avenues in stroke therapy. Curr Vasc Pharmacol. 2009;7:287–92. doi: 10.2174/157016109788340677. [DOI] [PubMed] [Google Scholar]
- 17.van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: Roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 18.Mocco J, Wilson DA, Komotar RJ, et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 19.Mack WJ, Ducruet AF, Hickman ZL, et al. Early plasma complement C3a levels correlate with functional outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;61:260–1. doi: 10.1227/01.NEU.0000255518.96837.8E. [DOI] [PubMed] [Google Scholar]
- 20.Arvilommi H. Capacity of complement c3 phenotypes to bind on to mononuclear cells in man. Nature. 1974;251:740–1. doi: 10.1038/251740a0. [DOI] [PubMed] [Google Scholar]
- 21.Bartok I, Walport MJ. Comparison of the binding of C3S and C3F to complement receptors types 1, 2, and 3. J Immunol. 1995;154:5367–75. [PubMed] [Google Scholar]
- 22.Welch TR, Beischel L, Kleesattel A. Functional consequences of the genetic polymorphism of the third component of complement. J Pediatr. 1990;116:S92–7. doi: 10.1016/s0022-3476(05)82709-x. [DOI] [PubMed] [Google Scholar]
- 23.Rambausek M, van den Wall Bake A, Schumacher-Ach R, et al. Genetic polymorphism of C3 and BF in IGA nephropathy. Nephrol Dial Transplant. 1987;2:208–11. [PubMed] [Google Scholar]
- 24.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 25.Finn JE, Zhang L, Agrawal S, et al. Molecular analysis of C3 allotypes in patients with systemic vasculitis. Nephrol Dial Transplant. 1994;9:1564–7. [PubMed] [Google Scholar]
- 26.Elmgreen J, Sørensen H, Berkowicz A. Polymorphism of complement c3 in chronic inflammatory bowel disease. Predominance of the c3f gene in Crohn’s disease. Acta Med Scand. 1984;215:375–8. doi: 10.1111/j.0954-6820.1984.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 27.Ostergaard J, Bruun-Petersen G, Kristensen B. The C3-F gene in patients with intracranial saccular aneurysms. Acta Neurol Scand. 1986;74:356–9. doi: 10.1111/j.1600-0404.1986.tb03525.x. [DOI] [PubMed] [Google Scholar]
- 28.Csaszar A, Duba J, Melegh B, et al. Increased frequency of the C3*F allele and the Leiden mutation of coagulation factor V in patients with severe coronary heart disease who survived mycardial infarction. Exp Clin Immunogenet. 2001;18:206–12. doi: 10.1159/000049199. [DOI] [PubMed] [Google Scholar]
- 29.Szamosi T, Mihai K, Peto J, et al. Potential markers of the atherosclerotic process in high-risk childern. Clin Biochem. 1991;24:185–7. doi: 10.1016/0009-9120(91)90541-l. [DOI] [PubMed] [Google Scholar]
- 30.Moshfeghi DM, Blumenkranz MS. Role of genetic factors and inflammation in age-related macular degeneration. Retina. 2007;27:269–75. doi: 10.1097/IAE.0b013e31802e3e9b. [DOI] [PubMed] [Google Scholar]
- 31.Volcik K, Ballantyne C, Braun M, et al. Association of the complement factor H Y402H polymorphism with cardiovascular disease is dependent upon hypertension status: The ARIC study. Am J Hypertens. 2008;21:533–8. doi: 10.1038/ajh.2007.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 33.Mooijaart S, Koeijvoets K, Sijbrands E, et al. Complement Factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Exp Gerontol. 2007;42:1116–22. doi: 10.1016/j.exger.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Newman S, Stygall J, Hirani S, et al. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–90. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz J, Christensen K, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 36.Ferro J. Hyperacute cognitive stroke syndromes. J Neurol. 2001;248:841–9. doi: 10.1007/s004150170067. [DOI] [PubMed] [Google Scholar]
- 37.Mesulam M. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seitsonen SP, Onkamo P, Peng G, et al. Multifactor effects and evidence of potential interaction between complement factor H Y402H and LOC387715 A69S in age-related macular degeneration. PLoS One. 2008;3:e3833. doi: 10.1371/journal.pone.0003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anonymous. Potentia Pharmaceuticals I: Safety of Intravitreal POT-4 Therapy for Patients with Neovascular Age-Related Macular Degeneration. Bethesda, MD: National Library of Medicine (US); 2008. p. NCT00473928. [Google Scholar]
- 40.Heyer EJ, DeLaPaz R, Halazun HJ, et al. Neuropsychological dysfunction in the absence of structural evidence for cerebral ischemia after uncomplicated carotid endarterectomy. Neurosurgery. 2006;58:474–80. doi: 10.1227/01.NEU.0000197123.09972.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson DA, Mocco J, D’Ambrosio AL, et al. Post-carotid endarterectomy neurocognitive decline is associated with cerebral blood flow asymmetry on post-operative magnetic resonance perfusion brain scans. Neurol Res. 2008;30:302–6. doi: 10.1179/016164107X230540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson G, Eliasziw M, Barr H, et al. The North American symptomatic carotid endarterectomy trial: Surgical results in 1415 patients. Stroke. 1999;30:1751–8. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 43.Halm E, Chassin M, Tuhrim S, et al. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464–71. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 44.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and tia risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–67. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]