Abstract
Immunotherapy, particularly the adoptive cell transfer (ACT) of tumor infiltrating lymphocytes (TIL), is a very promising therapy for metastatic melanoma. Some patients unable to receive TIL have been successfully treated with autologous peripheral blood lymphocytes (PBL), genetically modified to express HLA class I antigen restricted, melanoma antigen-reactive T-cell receptors; however, substantial numbers of patients remain ineligible due to the lack of expression of the restricting HLA class I allele. We sought to overcome this limitation by designing a non-MHC-restricted, chimeric antigen receptor (CAR) targeting the high molecular weight-melanoma associated antigen (HMW-MAA), which is highly expressed on over 90% of human melanomas but has a restricted distribution in normal tissues. HMW-MAA-specific CARs containing an antigen recognition domain based on variations of the HMW-MAA-specific monoclonal antibody (mAb) 225.28S and a T-cell activation domain based on combinations of CD28, 4-1BB, and CD3ζ activation motifs were constructed within a retroviral vector to allow stable gene transfer into cells and their progeny. Following optimization of the HMW-MAA-specific CAR for expression and function in human PBL, these gene-modified T cells secreted cytokines, were cytolytic, and proliferated in response to HMW-MAA expressing cell lines. Furthermore, the receptor functioned in both CD4+ and CD8+ cells, was non-MHC-restricted, and reacted against explanted human melanomas. To evaluate this HMW-MAA-specific CAR in patients with metastatic melanoma, we developed a clinical-grade retroviral packaging line. This may represent a novel means to treat the majority of patients with advanced melanoma, most notably those unable to receive current ACT therapies.
Keywords: high molecular weight-melanoma associated antigen, chimeric antigen receptor, melanoma, adoptive cell transfer, immunotherapy
Introduction
Greater than half of patients treated in protocols combining lymphodepletion and adoptive cell transfer (ACT) of autologous tumor-infiltrating lymphocytes (TIL) experienced objective tumor regression.(1, 2) This potent melanoma therapy, however, has been limited by the requisite surgery to procure tumor-reactive TIL and by ex vivo identification and expansion of these cells. Therefore, an alternative strategy to insert tumor antigen-reactive T-cell receptor (TCR) genes into peripheral blood lymphocytes (PBL) was investigated.
TCR gene-modified T cells are capable of activation, cytokine secretion, and targeted lysis.(3, 4) We reported the first clinical trials using autologous PBL modified to express a tumor antigen-reactive TCR in the treatment of patients with metastatic cancer resulted in objective tumor regressions.(5, 6) These strategies, however, remain less effective than TIL, suggesting that further modifications are needed. Furthermore, only a minority of patients with advanced melanoma are eligible for current protocols, as they must express human leukocyte antigen (HLA)-A*0201 and have tumors that express a common melanoma-associated antigen.
Unlike a conventional TCR, a chimeric antigen receptor (CAR) is capable of relaying excitatory signals to T cells in a non-MHC-restricted manner. These hybrid proteins, composed of an extracellular antigen recognition domain fused to an intracellular T-cell activation domain,(7) may therefore be used in patients regardless of their HLA genotype. The non-HLA-restricted antigen recognition is achieved by harnessing the antigen-binding properties of monoclonal antibodies (mAb); this recognition is also independent of antigen processing, thus bypassing a potential mechanism by which tumor cells can evade the immune system in vivo.(8) Several clinical trials using CAR-transduced T cells have been reported.(9-12)
High molecular weight-melanoma associated antigen (HMW-MAA, gene name CSPG4) is a cell-surface proteoglycan expressed on greater than 90% of melanomas. It is postulated that HMW-MAA contributes to the malignant phenotype of melanoma cells via enhancement of their spreading, invasion, and migration.(13) The expression of HMW-MAA in adult tissues is restricted to hair follicles, basal cells of the epidermis, endothelial cells, and activated pericytes. Given its expression on the majority of melanoma lesions with limited inter- and intra- lesional heterogeneity and its restricted expression on normal tissues, HMW-MAA represents a clinically attractive target for CAR-based immunotherapy.
In this study, therefore, we developed a non-MHC-restricted CAR to target HMW-MAA. We next optimized this HMW-MAA-specific CAR for robust expression and function in human PBL. Finally, we generated a clinical-grade retroviral product for use in new ACT protocols for patients with metastatic melanoma who express HMW-MAA in their lesions.
Materials and Methods
Cell lines, lymphocytes, and tumor digests
Human melanoma lines were generated at the Surgery Branch, NCI (Bethesda, MD) and other cell lines were obtained from ATCC (Rockville, MD). Non-transformed human cell cultures were purchased from Lonza (Rockland, ME) and PBL were collected via leukaphereses. Single-cell suspensions of explanted human melanomas were prepared via overnight digestion with collagenase, hyaluronidase, and DNase. Tumor cells were cultured in RPMI 1640 medium plus 10% FBS (Biofluids, Rockville, MD) and penicillin/streptomycin (Invitrogen, Carlsbad CA). Lymphocytes were maintained in AIM-V medium (Invitrogen) plus 5% human AB serum (Valley Biomedical, Winchester, VA), 55 mM 2-mercaptoethanol (Invitrogen), penicillin/streptomycin (Invitrogen) and 300 IU/mL IL-2 (Chiron Corporation, Emerville, CA).
Generation of retroviral constructs, vector production, and T cell transduction
The human HMW-MAA-specific scFv, derived from the murine mAb 225.28S(14), was synthesized (GeneArt, Toronto, Canada) and cloned into to an anti-ErbB2 CAR vector(15) containing either CD28 and CD3ζ or a CD8α-chain hinge region with CD28, 4-1BB and CD3ζ signaling domains. Retroviruses were produced and transduction performed as described.(15)
Flow cytometry and functional assays
Conjugated mAb, specific for human HMW-MAA (R&D Systems, Minneapolis, MN) and human CD3 and CD8 (BD Biosciences, San Jose, CA), were used according to the manufacturers’ recommendations. Cytokine secretion, cytolysis, and T cell proliferation were performed as described.(15)
Results and Discussion
HMW-MAA expression
HMW-MAA is expressed on the vast majority of human melanomas.(13) As expected, all seven established human melanoma lines tested in this study expressed HMW-MAA, as determined by FACS analysis following staining with HMW-MAA-specific mAb (Figure 1A). Among the non-melanoma (lung, breast, ovarian, pancreatic, renal, prostate, colorectal, and brain) tumor lines, HMW-MAA was only detected on the SNB-75 glioma line (Figure 1B). Ex vivo cultured PBL and four normal cell cultures were also evaluated, with dermal fibroblasts exhibiting low expression (Figure 1C). 11 explanted melanoma tumors displayed HMW-MAA expression (representative data from eight patients shown in Figure 1D), supporting the use of HMW-MAA as a target for immunotherapy of melanoma.
Figure 1. HMW-MAA was highly expressed on human melanoma lines and explanted melanoma tumors.
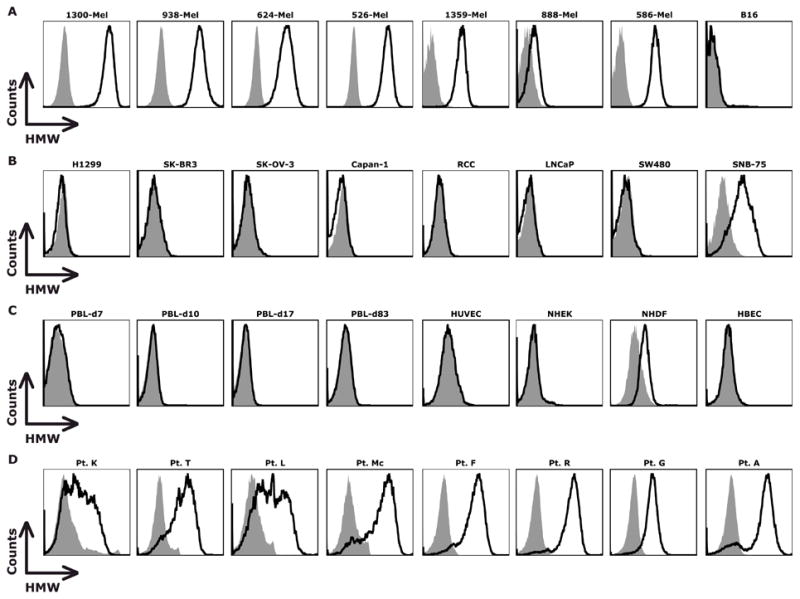
(A) HMW-MAA was detected on the human, but not murine (B16), melanoma lines by flow cytometry. (B) In non-melanoma lines, only a glioma line (SNB-75) expressed HMW-MAA. (C) Ex vivo activated PBL (7-83 days post-stimulation), endothelial cell (HUVEC), epidermal keratinocytes (NHEK), and bronchial epithelial cells (HBEC) did not express HMW-MAA; dermal fibroblasts (NHDF) displayed low HMW-MAA expression. (D) Eight explanted human melanomas expressed HMW-MAA.
Optimization of HMW-MAA-specific CAR
The HMW-MAA mouse mAb 225.28S is highly specific, displays a high association constant and efficiently detects human melanomas. This mAb detected HMW-MAA in 59 of 59 melanoma specimens,(14) and stained only the hair bulb and isolated basal cells in a panel of normal adult and fetal tissues.(16) Furthermore, radiolabelled forms of this mAb have safely imaged melanoma tumor deposits in more than 1000 patients.(13) Therefore, we constructed four 225.28S-based scFv cassettes, which differed by the order of the light chain variable region (VL) and heavy chain variable region (VH) and by one of two flexible linker peptides, GSTSGSGKPGSGEGS (linker 218) or GGGGSGGGGSGGGGS (linker G4S). These scFv cassettes were cloned into an MSGV1-based retroviral vector containing the CD28 and CD3ζ signaling domains (28z) to generate four HMW-MAA-specific CARs (Supplemental Figure S1). These four CARs were detected on the surface of transduced PBL and conferred reactivity against HMW-MAA-expressing tumors; the scFv with the VL-218-VH design (L2H), demonstrated both superior expression and function (Supplemental Table S2).
Next, we sought to optimize the intracellular, T-cell activation domain, while maintaining the L2H scFv cassette. Based on emerging evidence that 4-1BB (CD137) co-stimulation augments CAR function,(15) two modifications that included the 4-1BB signaling motif were evaluated (Supplemental Figure S3). As detailed in Supplemental Table S4, an initial screen of the three constructs identified superior transgene expression with L2H-28z and optimal function with the L2H-28z and L2H-CD8.28BBz constructs.
Function of HMW-MAA-specific CAR
A comprehensive comparison between the L2H-28z and L2H-CD8.28BBz HMW-MAA-specific CARs was performed. Again, L2H-28z provided superior gene transfer, in both percent and level of expression (Figure 2A). PBL transduced with either CAR secreted cytokines when cocultured with a panel of HMW-MAA-expressing melanoma lines but not those lacking HMW-MAA expression (Figure 2B). Although qualitatively similar, L2H-28z-transduced PBL displayed modest enhancement in IFN-γ and TNF-α secretion, compared to L2H-CD8.28BBz-transduced PBL. The L2H-28z construct demonstrated slightly greater cytolytic function (Figure 2C) and proliferation (Figure 2D) in response to melanoma targets than the L2H-CD8.28BBz construct. In contrast to our experience with an ErbB2-specific CAR(15), addition of the 4-1BB co-stimulatory motif did not improve any of the measured in vitro functions of this HMW-MAA-specific CAR. Inactivation of the CD28 internalization motif also failed to enhance expression or function (Supplemental Figure S5). Therefore, the L2H-28z format was selected as the optimal HMW-MAA-specific CAR. These data clearly demonstrate the ability of this HMW-MAA-specific CAR to redirect T cells to recognize melanoma lines and elicit effector functions, such as cytokine release, cytolysis, and proliferation.
Figure 2. PBL transduced to express an optimized HMW-MAA-specific CAR were redirected to target HMW-MAA-expressing melanoma lines.
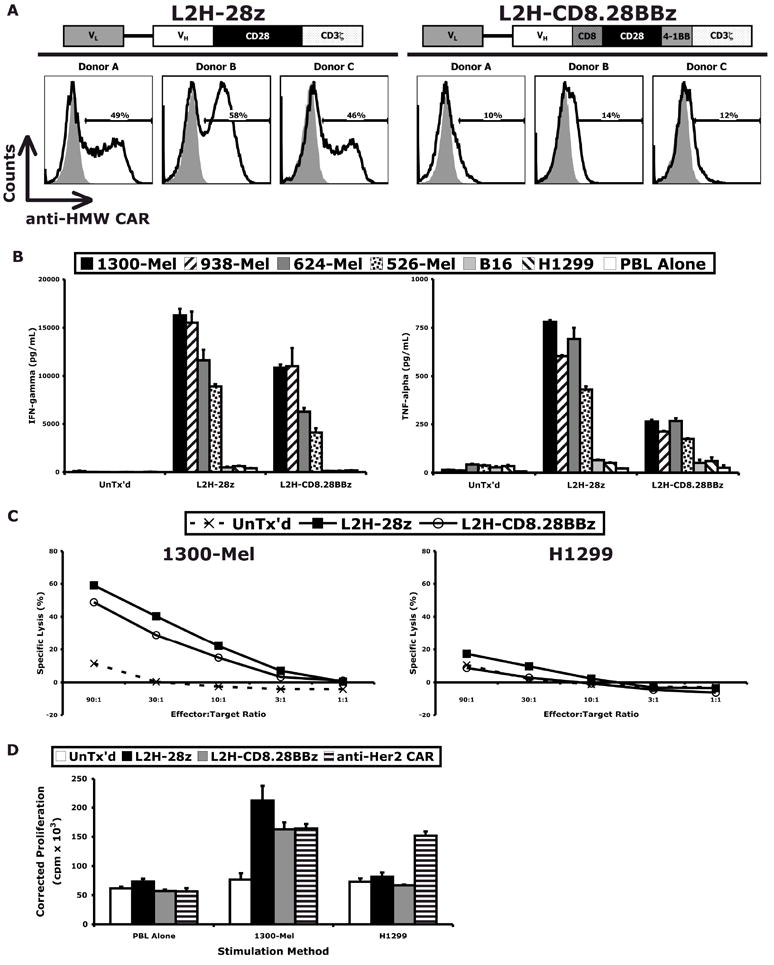
(A) Diagrams of optimized HMW-MAA-specific CARs and cell-surface expression of each construct in PBL transduced with the corresponding retroviral vector. HMW-MAA-specific CAR-transduced, but not untransduced, T cells were redirected to target HMW-MAA-expressing melanoma lines but not non-HMW-MAA-expressing cells, as demonstrated by (B) cytokine release, (C) lysis, and (D) proliferation assays.
HMW-MAA-specific CAR functions in CD4+ and CD8+ T lymphocytes and confers non-MHC-restricted recognition of resected human melanomas
The function of the HMW-MAA-specific CAR was evaluated in enriched populations of CD4+ and CD8+ T cells. Cytokine release was similar in the CD4+ and CD8+ cells (Figure 3A) demonstrating the efficacy of this receptor in both T cell subsets. We also tested its efficacy against a panel of resected human melanomas with mixed HLA-A haplotypes. In generating single-cell tumor suspensions, cell-surface HMW-MAA expression was transiently lost but reemerged after washing and overnight culture. Samples with viable tumor were assayed for HMW-MAA expression (Figure 3B) and co-cultured with HMW-MAA-specific CAR-transduced T cells in cytokine release assays (Figure 3C). Of the four tumors tested, two had high expression (Pt. T and Pt. C) and two had moderate expression (Pt. M and Pt. K). HMW-MAA-specific CAR-transduced T cells recognized all four, and the IFN-γ secretion correlated with HMW-MAA expression (P < 0.001), as displayed in Figure 3D. Furthermore, tumors from patients with disparate HLA-A haplotypes were recognized, supporting the non-MHC-restricted activation of the HMW-MAA-specific CAR.
Figure 3. HMW-MAA-specific CAR functions in CD4+ and CD8+ T cells and is non-MHC-restricted.
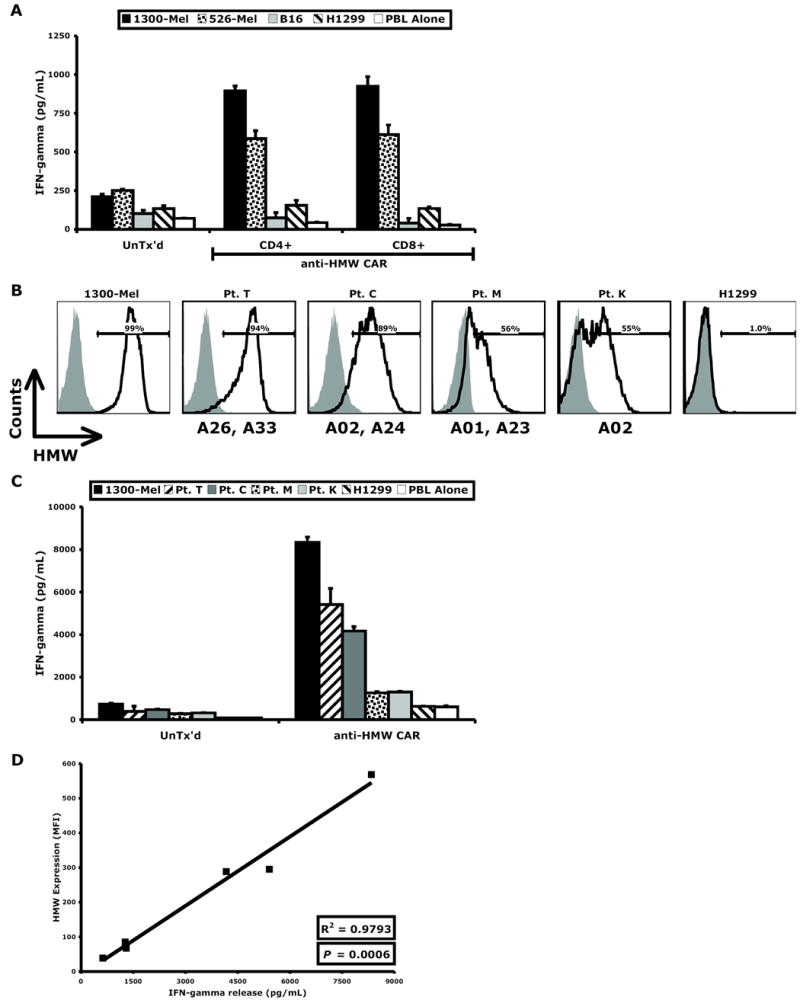
(A) HMW-MAA-specific CAR-transduced T cells were enriched for CD4+ and CD8+ populations, both of which displayed similar function. (B) Explanted melanoma tumors from four patients (Pt. T, Pt. C, Pt. M, and Pt. K) with disparate HLA-A haplotypes (indicated below histograms) expressed HMW-MAA by flow cytometry and (C) were recognized by HMW-MAA-specific CAR-transduced T cells. (D) IFN-γ released by HMW-MAA-specific CAR-transduced T cells correlated with the level of HMW-MAA expression of the target cells.
Clinical-grade HMW-MAA-specific CAR retrovirus redirects lymphocytes to target autologous melanoma
A HMW-MAA-specific CAR retroviral packaging line was developed for clinical-grade retrovirus production and screened to identify the highest titer clone. Based on transduction efficiency (Figure 4A) and cytokine release (Figure 4B), clone D8 was selected. To test the ability of clone D8 transduced T cells to lyse tumor targets, PBL from two patients were transduced and subject to cell lysis assay (Supplemental Figure S6). HMW-MAA CAR transduced cells specifically lysed melanoma line 1300 but not HMW-MAA negative line H1299 or two normal human diploid fibroblast cultures. Clinical-grade supernatant harvested from clone D8 was then used to transduce PBL from a non-HLA-A*02 donor (Pt. B), and demonstrated efficient gene transfer (75% CAR +, Figure 4C). As displayed in Figure 4D, transduction with the HMW-MAA-specific CAR redirected this patient’s lymphocytes to effectively target his autologous tumor.
Figure 4. A high-titer HMW-MAA-specific CAR retrovirus packaging clone enabled efficient gene transfer and redirected PBL to target a non-HLA-A*02 patient’s autologous tumor.
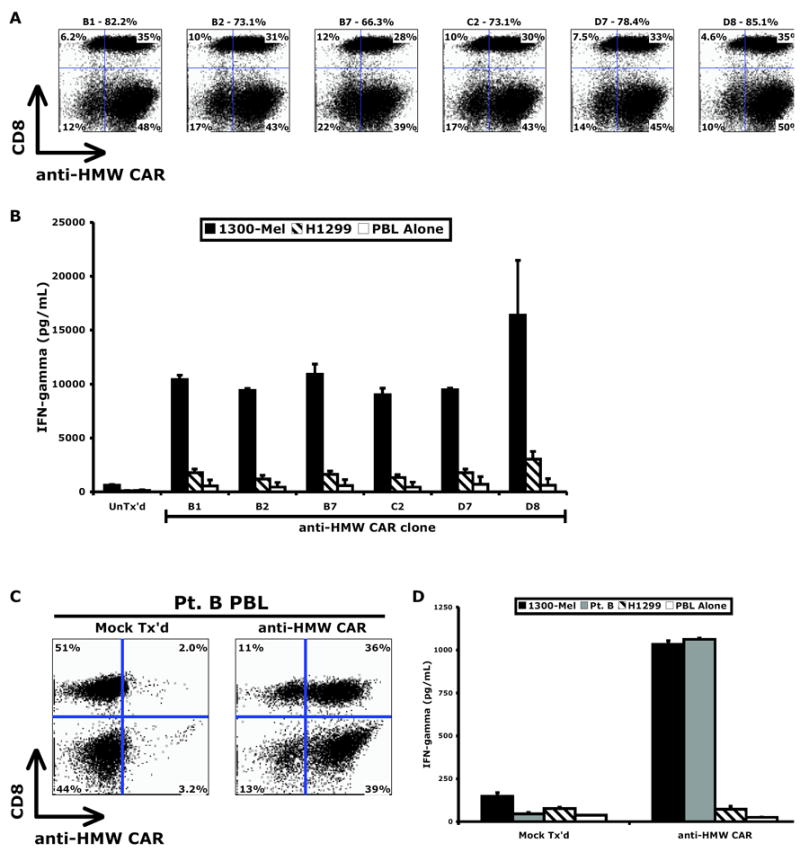
Of the six high-titer HMW-MAA-specific CAR retrovirus packaging clones, clone D8 conferred the greatest (A) transduction efficiency and (B) cytokine release against melanoma 1300-Mel. PBL of a non-HLA-A*02 patient (Pt. B) demonstrated robust (C) expression of the HMW-MAA-specific CAR and (D) recognition of the autologous tumor.
Concluding remarks
In this study, we developed a novel HMW-MAA-specific CAR that redirects lymphocytes to target human melanomas. While two groups have reported early-generation CARs targeting HMW-MAA, they were not suitable for clinical use. The first utilized an scFv derived from an antibody (clone 763.74) of lower affinity and failed to demonstrate T-cell activation by HMW-MAA-expressing tumors in the absence of anti-idiotype antibody prestimulation.(17) The second CAR (based on clone 225.28S) was limited by low expression and only shown to drive an EGFP reporter gene in a murine T cell line.(18) Following optimization for expression and function in human PBL, T cells expressing our HMW-MAA-specific CAR secreted cytokines, were cytolytic, and proliferated in response to HMW-MAA-expressing cell lines. Furthermore, the receptor functioned in both CD4+ and CD8+ cells, was non-MHC-restricted, and reacted against explanted human melanomas.
Compared to intracellular melanocyte differentiation antigens such as gp100 and MART-1, HMW-MAA is expressed in a higher percentage of human melanomas and has a more restricted pattern of normal tissue expression(14, 19). Other cell-surface melanoma-associated antigens are less frequently expressed on human melanomas (such as GD2 or GD3 ganglioside) or are more frequently expressed on normal tissues (such as GM3 or GM2 ganglioside)(20). Despite these potential advantages for use in immunotherapy, and because on-target toxicity to normal tissues was observed using high-avidity TCRs against gp100 and MART-1 (6), care should be taken in the clinical application of any cell-based immunotherapy targeted to normal cell antigens and only patients with documented over expression of HMW-MAA should be considered as candidates for adoptive cell transfer trials. Toxicology studies in small animals would not be useful as the HMW-MAA antibodies used in this and other studies do not cross react with the murine HMW-MAA ortholog, AN2. While we did not observe lysis of normal diploid fibroblasts using HMW-MAA CAR transduced cells (Supplemental Figure S6), we have on occasion observed lysis of melanoma cell line 888 that expressed a similar low level of HMW-MAA (data not shown), suggesting that different threshold levels of HMW-MAA recognition may be observed with specific patients’ T cells.
The optimized HMW-MAA-specific CAR described in this study represents a novel means to treat the large number of melanoma patients unable to receive current ACT therapies. HMW-MAA is also expressed on other tumors of neural crest origin (e.g., astrocytomas, gliomas, neuroblastomas, and sarcomas), as well as, some forms of childhood leukemias and lobular breast carcinoma lesions. Furthermore, recent reports suggest that HMW-MAA may be up-regulated in pericytes associated with tumor neovasculature(21) making the potential application of HMW-MAA CAR-based cell therapies useful in a variety of malignancies.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Rosenberg SA, R N, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2(7):512–9. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 4.Stauss HJ, Cesco-Gaspere M, Thomas S, et al. Monoclonal T-cell receptors: new reagents for cancer therapy. Mol Ther. 2007;15(10):1744–50. doi: 10.1038/sj.mt.6300216. [DOI] [PubMed] [Google Scholar]
- 5.Morgan RA, D M, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 9.Pule MA, S B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 12.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor redirected cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–33. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 13.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24(4):267–96. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 14.Wilson BS, Imai K, Natali PG, Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. International journal of cancer. 1981;28(3):293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, W Q, Yang S, Kochenderfer J, Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA, Morgan RA. A Herceptin-based CAR with modified signaling domains leads enhanced survival of transduced T lymphocytes and anti-tumor activity. 2009 doi: 10.4049/jimmunol.0900447. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natali P, Bigotti A, Cavalieri R, Wakabayaski S, Taniguchi M, Ferrone S. Distribution of a cross-species melanoma-associated antigen in normal and neoplastic human tissues. J Invest Dermatol. 1985;85(4):340–6. doi: 10.1111/1523-1747.ep12276944. [DOI] [PubMed] [Google Scholar]
- 17.Reinhold U, Liu L, Ludtke-Handjery HC, et al. Specific lysis of melanoma cells by receptor grafted T cells is enhanced by anti-idiotypic monoclonal antibodies directed to the scFv domain of the receptor. J Invest Dermatol. 1999;112(5):744–50. doi: 10.1046/j.1523-1747.1999.00586.x. [DOI] [PubMed] [Google Scholar]
- 18.Losch FO, Muller R, Mutschler B, et al. Activation of T cells via tumor antigen specific chimeric receptors: the role of the intracellular signaling domain. Int J Cancer. 2003;103(3):399–407. doi: 10.1002/ijc.10826. [DOI] [PubMed] [Google Scholar]
- 19.Natali P, B A, Cavalieri R, Wakabayaski S, Taniguchi M, Ferrone S. Distribution of a cross-species melanoma-associated antigen in normal and neoplastic human tissues. J Invest Dermatol. 1985;85(4):340–6. doi: 10.1111/1523-1747.ep12276944. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Cordon-Cardo C, Zhang HS, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73(1):42–9. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer research. 2008;68(19):8066–75. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


