Abstract
Several genetic, neurodevelopmental, and pharmacological animal models of schizophrenia have been established. This short review examines the validity of one of the most used pharmacological model of the illness, ie, the acute administration of N-methyl-D-aspartate (NMDA) receptor antagonists in rodents. In some cases, data on chronic or prenatal NMDA receptor antagonist exposure have been introduced for comparison. The face validity of acute NMDA receptor blockade is granted inasmuch as hyperlocomotion and stereotypies induced by phencyclidine, ketamine, and MK-801 are regarded as a surrogate for the positive symptoms of schizophrenia. In addition, the loss of parvalbumin-containing cells (which is one of the most compelling finding in postmortem schizophrenia brain) following NMDA receptor blockade adds construct validity to this model. However, the lack of changes in glutamic acid decarboxylase (GAD67) is at variance with human studies. It is possible that changes in GAD67 are more reflective of the neurodevelopmental condition of schizophrenia. Finally, the model also has predictive validity, in that its behavioral and transmitter activation in rodents are responsive to antipsychotic treatment. Overall, although not devoid of drawbacks, the acute administration of NMDA receptor antagonists can be considered as a good model of schizophrenia bearing a satisfactory degree of validity.
Keywords: phencyclidine, ketamine, MK-801, antipsychotics
Clinical Evidence of NMDA Hypofunction in Schizophrenia
Psychiatric illnesses can be considered as uniquely human in that (1) they affect complex higher brain functions and (2) their diagnostic is usually carried out by structured interviews following standardized procedures, which obviously cannot be reproduced in animals. Therefore, animal models of psychiatric disorders face the main difficulty of replicating in the animal brain, and the alterations that are presumed to occur in the human brain. However, animal models are a crucial tool to study these changes inasmuch as the animal brain can be accessed directly.
The hypothesis of N-methyl-D-aspartate (NMDA) receptor hypofunction in schizophrenia stemmed from the clinical observation that a single subanesthetic dose of noncompetitive NMDA antagonists, such as phencyclidine (PCP) and ketamine, induced in healthy individuals psychotic and negative symptoms, as well as cognitive impairment, that resemble those present in schizophrenia.1,2 The present review will address the advantages and limitations of using NMDA receptor antagonists to model schizophrenia by comparing their actions in the clinical and preclinical settings.
Postmortem studies have shown alterations of subunit expression as well as trafficking complex and intracellular signaling proteins of NMDA receptors in prefrontal cortex of subjects with schizophrenia.3 Furthermore, recent imaging studies using a novel SPECT tracer for the NMDA receptor have reported the first in vivo evidence of an NMDA receptor reduction in medication-free schizophrenic patients.4 Besides, ketamine also induced a reduction of NMDA receptors in the human brain, which strongly correlated with the scores of negative symptoms.5
An important issue to be considered herein is the difference between acute and chronic dosage of NMDA antagonists. As reviewed by Jentsch and Roth,6 repeated use of PCP leads to a more persistent schizophrenic symptomatology. As a result, these authors propose that sustained exposure to PCP may better simulate the chronic symptoms of schizophrenia, such as the negative symptoms and cognitive deficits. In contrast, an acute regimen would rather resemble first-episode schizophrenia. Altogether, from a clinical viewpoint, the NMDA hypofunction model of schizophrenia possesses strong similarities in several major symptoms with schizophrenia. Indeed, PCP and ketamine recreate the symptoms of schizophrenia more accurately than do other psychotomimetic drugs, such as amphetamine or d-lysergic acid diethylamide (LSD).
NMDA Antagonism-Induced Sensory Gating Changes in Humans
Deficits in information processing have become the focus of interest because of their relevance in the functional impairment experienced by schizophrenic patients. Abnormalities in the corticostriato-thalamocortical circuitry may account for such deficits. Thus, thalamic gating deficits would result in a sensory overload of cortical structures and the subsequent cognitive deficiency. Schizophrenic patients exhibit robust deficits in sensory gating processes that can be measured in different paradigms, such as reductions of the prepulse inhibition (PPI) of the acoustic startle reflex7 and deficits in mismatch negativity.8 Reductions of mismatch negativity were noticed also following acute ketamine administration to healthy subjects.9 However, the administration of ketamine to healthy volunteers showed no change or an increase in PPI,10 which is in contrast to the effects consistently observed in rodents (see below).
Comparison With Preclinical Studies
Behavioral Studies
In rodents, systemic administration of NMDA antagonists induces hyperlocomotion and stereotypies, behaviors that are potentially related to positive symptoms of schizophrenia, and are often associated with elevated dopaminergic and serotonergic tones. NMDA receptor antagonists also produce disruptions in PPI and deficits in different domains of cognition, features that have relevance to impairment of cognitive symptoms consistently observed in schizophrenia. As mentioned above, NMDA receptor antagonist–induced deficits in PPI in rodents contrast with reports in humans in which NMDA antagonists fail to decrease PPI. In accordance with human studies, however, NMDA receptor antagonists block the generation of mismatch negativity in monkeys.11
Neurochemical and Neurophysiological Studies
Acute NMDA receptor antagonism induces an excessive prefrontal activity measured as increased firing rate of pyramidal neurons and expression of c-fos mRNA. As a consequence, there are also increases in the release of cortical transmitters, such as glutamate, dopamine, and serotonin (5-HT).12,13 These facilitatory effects likely result from enhanced excitatory glutamatergic inputs onto midbrain dopamine and 5-HT cell groups arising from the prefrontal cortex. Although no direct measure of brain glutamate release exists for the brain of schizophrenics, some studies have evidenced activation of prefrontal cortex in first-episode schizophrenia,14,15 and after ketamine administration,16 which is suggestive of increased glutamate release in humans. In contrast, hypofrontality seems to be prominent in chronic schizophrenia and has been consistently observed following long-term exposure to NMDA receptor antagonists.6
In line with the increased dopaminergic transmission in rodents, positron emission tomography (PET) imaging studies in humans have consistently evidenced an increased release of dopamine in striatum in schizophrenia. In addition, the administration of ketamine to healthy subjects has been shown to increase the release of dopamine in ventral striatum, which strongly correlated with the appearance of psychotic symptoms.17 No similar abundance of results has been reported for serotonergic systems. A few studies with unmedicated patients, however, have reported diminished density of 5-HT2A receptors in schizophrenia that could result from a compensatory response to an excessive release of endogenous 5-HT.18
At first sight, it may seem paradoxical that the blockade of an excitatory glutamatergic receptor results in a multitude of such facilitatory actions. Based fundamentally on an accumulating body of results using experimental animals, it has been proposed that the array of effects of NMDA antagonists arises from their preferential blockade of NMDA receptors localized to gamma amino butyric acid (GABA)ergic neurons of corticolimbic circuits, thus disrupting cortical inhibition and resulting in a disinhibition of glutamatergic (pyramidal) neurons, which is associated with excessive neuronal activity.19 In good accordance with this view, it has been demonstrated that 2 consecutive injections of ketamine reduced activity in cortical interneurons via activation of immune processes.20 Interestingly, the acute administration of NMDA antagonists results in a decrease of GABA release in the prefrontal cortex and the reduction of prefrontal GABA activity has been shown to induce cognitive, behavioral, and dopaminergic alterations that resemble those of schizophrenia.21 It is important to note that caution must be taken when using the terms hypoglutamatergia or hyperglutamatergia with reference to changes in glutamatergic transmission. Thus, a hypoglutamatergia, measured as a functional deficit of NMDA receptors, results in a hyperglutamatergia, measured as excitability of pyramidal neurons and excessive release of glutamate in cortical areas. As aforementioned, another issue to take into consideration is chronicity of the illness vs duration of treatment with NMDA antagonists. In this regard, although the nature of some changes is similar following acute or protracted treatment with NMDA antagonists and comparable with those occurring in early stages of schizophrenia, the duration of such changes after sustained administration seems to be more in accord with the persistence of clinical symptoms of the illness.6
Neuropathological Studies
In recent years, postmortem studies have consistently evidenced alterations in the GABAergic system of schizophrenia patients.22 These include (1) reduced levels of the mRNA encoding the 67-kDa isoform of glutamic acid decarboxylase (GAD67), the primary synthesizing enzyme for GABA; (2) reduction of the mRNA for the calcium-binding protein, parvalbumin (PV); (3) decreased immunoreactivity of the presynaptic GABA membrane transporter (GAT1) in chandelier axon cartridges; and (4) increased immunoreactivity for the GABAA α2 subunit in the postsynaptic axon initial segments of pyramidal neurons. PV interneurons regulate the oscillatory activity in the cerebral cortex, and disruption of cortical oscillatory dynamics is postulated to underlie many of the neurocognitive deficits of schizophrenia.
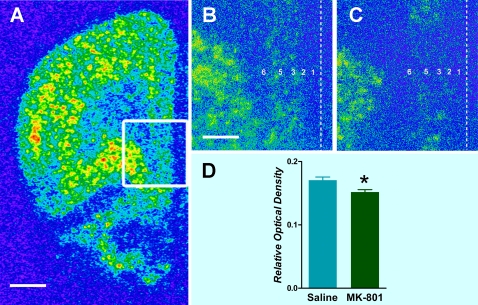
Less attention has been paid to the presence of such changes after administration with NMDA receptor antagonists. In the animal setting, it is generally considered that sustained exposure to NMDA receptor antagonists is needed to produce a robust decrease of the expression of PV in the rat. In a recent study, however, we have found a reduction of PV mRNA expression in discrete corticolimbic regions (prefrontal cortex, hippocampal formation, and amygdala)23 that have been implicated in schizophrenia. Further, the decrease of PV mRNA in the prefrontal cortex is limited to layers 3 and 5 (figure 1), similar to what has been found in postmortem tissue of schizophrenia brain.22 However, the lack of changes in GAD67 expression after acute NMDA blockade23 are at variance with human postmortem studies22 and would suggest that this enzyme isoform is rather involved in adaptive changes in development and maturation of GABA neurons. In fact, alterations in GAD67 have been observed only when NMDA antagonists were administered early after birth or after repeated exposure. Overall, the similarity of changes in PV expression in schizophrenia and after NMDA administration adds construct validity to this animal model.
Fig. 1.
Expression of parvalbumin (PV) mRNA in the medial prefrontal cortex of the rat. (A) Low magnification pseudocolored picture showing the distribution pattern of PV in a saline-injected rat. (B) High magnification photomicrograph of the area delimited in (A) showing the predominant distribution of PV within layers 3 and 5 in the infralimbic region of the medial prefrontal cortex. (C) Reduced level of PV mRNA after 1 mg/kg of MK-801. (D) Bar graphs showing the mean ± standard error of the mean of PV mRNA of rats injected with saline or MK-801; *P < .05. Dashed line shows the localization of cerebral midline, and Arabic numbers indicate the different cortical layers (note the absence of layer 4 in the medial prefrontal cortex of the rat). Scale bars: 2 mm in (A) and 600 μm in (B) and (C).
Disconnectivity in the NMDA Hypofunction Model
Over the last 30 years, evidence has emerged that schizophrenia can result from impairment in the connectivity between different areas of the brain rather than damage of particular areas. This would lead to a wrong processing of the information. Diffusion tensor imaging studies have found abnormalities in white matter in frontal and temporal lobes in schizophrenia that correlated with the severity of psychotic symptoms.24 These alterations are compatible with demyelinating processes. Interestingly, the myelination of white matter in humans starts postnatally and is completed in the adolescence/early adulthood, just the period of life when the first symptoms of schizophrenia usually appear. The key role in the myelination process is played by oligodendrocytes, and postmortem studies have reported a decrease in the number of oligodendrocytes in prefrontal cortex of subjects with schizophrenia.25
Although an association between NMDA hypofunction and impairment of the corpus callosum in schizophrenia has not been established unambiguously, a recent study has described that chronic ketamine consumption lead to abnormalities in frontal callosal fibers, similar to those present in schizophrenia patients.26 Further research is needed to extend the knowledge of the effects of NMDA antagonists on the integrity of callosal fibers in the animal setting. In this regard, we have recently described that NMDA antagonists need to block NMDA receptors in both prefrontal cortices to produce the changes in prefrontal serotonergic and glutamatergic transmission.27 Thus, our results support the hypothesis that a bilateral impairment of cortical inhibition in the medial prefrontal cortex is needed for noncompetitive NMDA antagonists to induce the state of cortical hyperactivity. It is possible that some of the alterations after NMDA antagonism observed in rodents (increase of cortical excitability and stereotypies) and in schizophrenia patients (distortion of reality, hallucinations, and thought disorder) are caused by a disrupted corticocortical communication and the subsequent alterations in the processing of the information between hemispheres.
Predictive Validity of NMDA Antagonism
Predictive validity refers to the potential of a model to make adequate predictions about some measure. Nevertheless, in psychiatry, the term predictive validity is often limited to indicate the ability of the model to predict the therapeutic efficacy of new drugs in the human condition. In the clinical setting, psychosis caused by the administration of NMDA receptor antagonists has been shown to be attenuated by atypical (clozapine) rather than typical (haloperidol) antipsychotic drugs.6 In line with the lack of effect of typical antipsychotics on cognitive functions, it was observed that negative symptoms and cognitive deficits present in schizophrenia do not seem to result from an excessive dopaminergic transmission at D2 receptors.
In the animal setting, the effects of typical and atypical antipsychotic drugs may vary with regard to the experimental parameters measured. For instance, the NMDA receptor antagonist–induced hyperlocomotion and stereotypy is regarded as a surrogate for positive (psychotic) symptoms of schizophrenia and has been associated with stimulation of dopamine transmission in striatum and nucleus accumbens. In contrast, deficits in executive and social functions as well as in working memory seem to rather model cognitive impairment in this illness. Both typical and atypical antipsychotic drugs are able to block the hyperlocomotion and stereotypies elicited by NMDA receptor antagonists. In a similar way, the expression of c-fos as well as the increase in pyramidal cell firing in prefrontal cortex evoked by NMDA receptor antagonists is also prevented by both clozapine and haloperidol. However, other experimental measures in preclinical studies appear to respond preferentially to atypical antipsychotic drugs. Thus, clozapine, but not haloperidol, is able to attenuate the activation of glucose metabolism and the disruption of PPI induced by NMDA receptor antagonists.28
Given that NMDA receptor antagonists cause a marked activation of the prefrontal cortex, it would be expected that drugs that reduce or prevent excessive prefrontal activation might be useful for treating positive and negative symptoms of schizophrenia.12 In this regard, activation of mGlu2/3 receptors has been shown to produce improvements in both positive and negative symptoms of schizophrenia.29 On the other hand, it appears that the excessive glutamatergic transmission after blockade of NMDA receptors is strongly dependent on dopamine D2 receptor activation, thereby being responsive to classical as well as atypical antipsychotics.13
In our lab, we have found that NMDA receptor antagonists–induced increase in 5-HT transmission in the prefrontal cortex is prevented by local or systemic administration of atypical, but not typical, antipsychotic drugs.13,30 Based on this observation, we have hypothesized that an excessive prefrontal 5-HT transmission may be implicated in the negative symptoms and cognitive impairment in schizophrenia, for which atypical drugs seem to display superior efficacy. Although the number of drugs tested is low, our results suggest that increased prefrontal 5-HT release might be predictive of a better response to atypical antipsychotics. Antagonism at 5-HT2A and α1-adrenergic receptors as well as agonism at 5-HT1A receptors seem to play an important role in these effects.25 Further research is needed to validate such prediction unequivocally.
Conclusions
Given the difficulty of modeling schizophrenia as a whole, most recent approaches tend to recreate only specific symptoms associated with the illness. In this review, we have discussed some of the similarities and differences between NMDA receptor hypofunction and schizophrenia that have relevance to the human condition. Notably, the circuitry governing the different variables (cortical and subcortical neurotransmitter systems, stereotypical behavior) is distinct, although overlapping. In the animal model, face validity rely on hyperlocomotion and stereotypies, a behavioral endpoint, which is not generated as a consequence of prefrontal function, and is regarded as a surrogate for the positive symptoms of schizophrenia. Construct validity of the animal model has remained elusive until one the most compelling findings in schizophrenia, ie, a postmortem reduction of the cortical expression of PV, has been reproduced in rodents, given acute or chronic NMDA receptor antagonists. However, the most difficult feature of NMDA hypofunction to model in rodents is transient nature of the drug effects, which contrasts with the neurodevelopmental nature of schizophrenia. It would be conceivable that acute administration of NMDA receptor antagonists would lead to a reversible malfunctioning of PV-containing interneurons, whereas in schizophrenia, a damage of these neurons takes place at early stages of neurodevelopment. In any event, the resulting abnormalities in central neurotransmitter systems and behavioral responses could bear some resemblance.
Finally, blockade of noncompetitive NMDA receptor antagonist–induced increases in locomotor activity is a key feature used in drug companies to discover novel antipsychotic drugs. In addition, we have proposed that blockade of the increase in cortical 5-HT transmission may be predictive of the “atypicality” of antipsychotic drugs. In summary, a better knowledge of the circuitry involved in mediating the various behavioral and neurochemical effects of NMDA receptor antagonists would likely increase the predictive utility of this model.
Funding
Spanish Ministry of Health (FIS PI070111); Proyecto Intramural Especial CSIC 201020E046; Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM. X.L.-G. and L.J.-S. are recipients of a postdoctoral fellowship from the Consejo Superior de Investigaciones Científicas (CSIC) and a predoctoral fellowship from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), respectively.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S, Sucher NJ, Bradley D, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilowsky LS, Bressan RA, Stone JM, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 5.Stone JM, Erlandsson K, Arstad E, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [123I]CNS-1261 SPET study. Psychopharmacology (Berl) 2008;197:401–408. doi: 10.1007/s00213-007-1047-x. [DOI] [PubMed] [Google Scholar]
- 6.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 7.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 8.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 9.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 10.Duncan EJ, Madonick SH, Parwani A, et al. Clinical and sensorimotor gating effects of ketamine in normals. Neuropsychopharmacology. 2001;25:72–83. doi: 10.1016/S0893-133X(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 11.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 13.López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- 14.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 15.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 16.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 17.Vollenweider FX, Vontobel P, Oye I, Leenders KL, Hell D. Effects of S-ketamine on striatal dopamine release: a [11C] raclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 18.Ngan ET, Yatham LN, Ruth TJ, Liddle PF. Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: a PET study using [18F]setoperone. Am J Psychiatry. 2000;157:1016–1018. doi: 10.1176/appi.ajp.157.6.1016. [DOI] [PubMed] [Google Scholar]
- 19.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 20.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 23.Romón T, Mengod G, Adell A. Expression of parvalbumin and glutamic acid decarboxylase-67 after acute administration of MK-801. Implications for the NMDA hypofunction model of schizophrenia. Psychopharmacology (Berl) 2011;217:231–238. doi: 10.1007/s00213-011-2268-6. [DOI] [PubMed] [Google Scholar]
- 24.Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- 25.Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry. 2004;161:882–888. doi: 10.1176/appi.ajp.161.5.882. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Tang J, Mingdong M, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133:2115–2122. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- 27.López-Gil X, Jiménez-Sánchez L, Romón T, Campa L, Artigas F, Adell A. Importance of interhemispheric prefrontal connection in the effects of noncompetitive NMDA receptor antagonists. Int J Neuropsychopharmacol. doi: 10.1017/S1461145711001064. In press. doi:10.1017/S1461145711001064. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman JA, Bymaster FP, Meltzer HY, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil ST, Zhang L, Martenyi F. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 30.López-Gil X, Artigas F, Adell A. Unraveling monoamine receptors involved in the action of typical and atypical antipsychotics on glutamatergic and serotonergic transmission in prefrontal cortex. Curr Pharm Des. 2010;16:502–515. doi: 10.2174/138161210790361416. [DOI] [PubMed] [Google Scholar]