Abstract
Objective: While all second-generation antipsychotics (SGAs) are promoted for having a low risk of extrapyramidal side effects (EPS), clinical observations suggest differences between the various agents. Nevertheless, this question has never been examined in a systematic review and meta-analysis of head-to-head comparisons. Methods: We searched the register of the Cochrane schizophrenia group (last search May 2007), supplemented by MEDLINE (last search July 2009) for randomized, blinded studies comparing the following SGAs in the treatment of schizophrenia or related disorders: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, and zotepine. At least 3 reviewers extracted the data independently. The primary outcome was “use of antiparkinson medication.” The results were combined in a meta-analysis. Results: We included 54 studies with 116 arms. Risperidone was associated with more use of antiparkinson medication than clozapine, olanzapine, quetiapine, and ziprasidone. Ziprasidone showed more use of antiparkinson medication than olanzapine and quetiapine and zotepine more than clozapine. There was no significant difference between amisulpride and its comparators (olanzapine, risperidone, or ziprasidone). Quetiapine showed significantly less use of antiparkinson medication than the 3 other SGAs it was compared with (olanzapine, risperidone, and ziprasidone). Scale-derived data (Barnes Akathisia Scale and Simpson Angus Scale) were limited. Conclusions: Our meta-analysis demonstrates that there are differences between the SGAs in their ability to induce EPS that clinicians consider warrant treatment with antimuscarinic drugs. Even though the differences were relatively small, they might be important for individual patients and should be taken into account in drug choice.
Keywords: antiparkinson medication, akathisia, classification of antipsychotics
Introduction
The introduction of “atypical” or second-generation antipsychotics (SGAs) was considered to be a milestone in the treatment of people with schizophrenia because the SGAs have a lower incidence of the “typical” extrapyramidal side effects (EPS) such as parkinsonism, dystonia, dyskinesia, and akathisia than high-potency first-generation antipsychotics (FGAs).1–6
Most pharmaceutical companies promote the SGAs as virtually free of EPS. In fact, meta-analyses of SGAs vs haloperidol might give such an impression.6 However, the common clinical impression is that at least some SGAs do produce EPS and that there are differences in the amount of these side effects among the SGAs. Within this field, there is a large amount of research with randomized controlled trials (RCTs); however, no meta-analysis exists that combines all these RCTs and compares the important EPS of SGAs head-to-head. We therefore conducted a systematic review of RCTs directly comparing the following SGAs with one another: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, and zotepine. This article focuses on the EPS of SGAs among themselves in treatment of schizophrenia or related disorders; the data on the efficacy of these medications in head-to-head comparison have been published elsewhere.7
Methods
Search Strategy
The register of the Cochrane Schizophrenia Group (CSG) was searched for randomized, blinded trials comparing orally administered SGAs (amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, and zotepine) head-to-head in the treatment of schizophrenia or related disorder (schizoaffective, schizophreniform, or delusional disorder, any diagnostic criteria). There were no language restrictions. The search terms used were all possible 36 combinations of the names of the SGAs including various trade names. The last search of the CSG register was done in May 2007; until July 2009, we searched MEDLINE for further randomized controlled studies fulfilling our inclusion criteria. The CSG register is compiled by regular methodical searches in numerous electronic databases (BIOSIS, CINAHL, Dissertation Abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsychINFO, RUSSMED, and Sociofile), supplemented by hand searching of relevant journals and conference proceedings (for details, see the description of the CSG).8 Only studies meeting the quality criteria A (adequate randomization) and B (primarily studies stated to be randomized without further details) according to the Cochrane handbook 2005 were included.9 All manufacturers of SGA were contacted for further details of published trials and asked for unpublished studies; first authors of included studies were contacted for missing information. Detailed methodology is published in the Cochrane library in systematic reviews of individual SGAs in comparison to other SGAs.10–17 Here we present a summary to show an overall description in terms of EPS of the SGAs.
Data Extraction and Outcome Parameters
All data were extracted independently by at least 3 reviewers (K.K., C.R., H.H., F.S., S.S., S.L.). The outcome “use of antiparkinson medication at least once” was chosen as the primary outcome because it is a global measure for EPS. The changes from baseline to endpoint in the Barnes Akathisia Scale (BAS) and Simpson Angus Scale (SAS) were chosen as secondary outcomes. Intention-to-treat results were used whenever possible.
Meta-analytic Calculations
Relative risks (RRs) were used as effect size measure for the dichotomous outcome use of antiparkinson medication. Number needed to treat/harm (NNT/NNH) was calculated as the inverse of the risk difference in case of statistical significance. The NNT is defined as the expected number of people who need to receive the experimental intervention for 1 additional person to avoid an event, such as eg, EPS, in a given time frame.18 The term NNH is used when an intervention leads to deterioration in the outcome. Continuous outcomes were analyzed using mean differences (MD) and their 95% CI because this preserves the original units, which are intuitively interpreted (eg, for the BAS, an MD of 0.5 means 0.5 points difference in the scale between the 2 groups).
The studies were pooled with the random effects model of Der-Simonian and Laird,19 which takes into account certain differences between studies even if there is no statistically significant heterogeneity.18 In addition, a fixed effects model was used for the primary outcome to verify the results under this assumption. We explored study heterogeneity by a chi-square test of homogeneity and the I-squared statistic assuming that I2 values greater than 50% suggested considerable heterogeneity. Meta-regressions were planned to possibly explain heterogeneity in case of heterogenous results and to explore the influence of sponsorship and washout period.
We assessed the methodological quality of included trials in this review using the Cochrane Collaboration's risk of bias tool with the domains sequence generation, allocation concealment, blinding, selective outcome reporting, and other sources of bias.18
In sensitivity analyses, we excluded studies with doses lower or higher than the following ranges (amisulpride 400–800 mg/d [for predominantly negative symptoms, optimum dose is 50–300 mg/d], aripiprazole 10–30 mg/d, clozapine 300–800 mg/d, olanzapine 10–20 mg/d, quetiapine 250–750 mg/d, risperidone 4–6 mg/d, sertindole 16–24 mg/d, ziprasidone 120–160 mg/d, and zotepine 75–450 mg/d).6,20 However, for higher doses, this was only the case for some olanzapine and risperidone studies and for lower doses for some risperidone and ziprasidone studies.
The calculations were done with Review Manager Version 5.0, the meta-analytic standard software used by the Cochrane Collaboration, and with STATA Version 7. All analyses were 2-tailed with alpha set to 0.05, except for the heterogeneity test (alpha set to 0.1).
Results
The Search
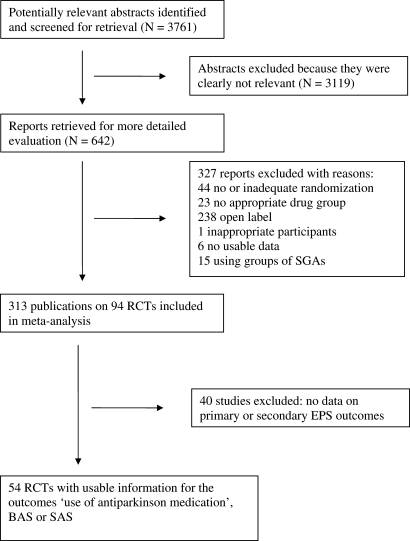
The search strategy identified 3761 citations. Of these, 642 articles were ordered and inspected and 327 studies were excluded21 (for quality of reports of meta-analysis flow diagram, see figure 1). Three hundred thirteen publications on 94 studies were included; however, of these 94 studies, 54 studies with 116 relevant arms reported usable data on at least one of the outcomes “use of antiparkinson medication,” BAS or SAS; 40 studies did not report such data and were therefore excluded. Of these 54 studies, 6 studies included amisulpride, 6 aripiprazole, 15 clozapine, 34 olanzapine, 11 quetiapine, 32 risperidone, 2 sertindole, 8 ziprasidone, and 1 zotepine.
Fig. 1.
Quality of Reports of Meta-analysis (QUOROM) Flow Diagram Describing the Search Process.
The participants were relatively chronic with mean ages in the mid-30s. The diagnostic criteria were mainly Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV/DSM-III-R or ICD-10 (see detailed Supplementary table I on the journal's website).
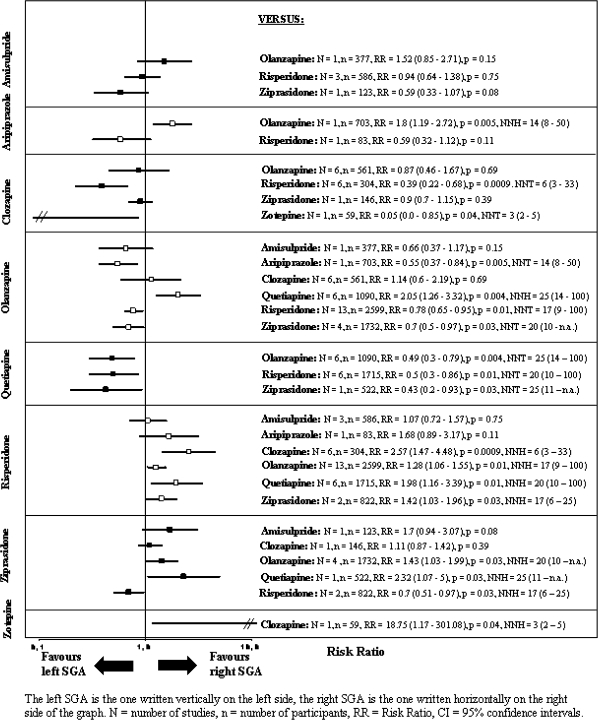
The pooled effect sizes of each SGA vs every other SGA are shown in figure 2. It is noted that all results are shown twice. For example, the comparison between amisulpride and olanzapine is described under “amisulpride vs other SGA” and under “olanzapine vs other SGA.” Despite the redundancy, the results are easier to understand. Otherwise, if the reader is interested in a given drug, he would have to look up the findings in different sections, making it difficult to see the gestalt.
Fig. 2.
Use of Antiparkinson Medication.
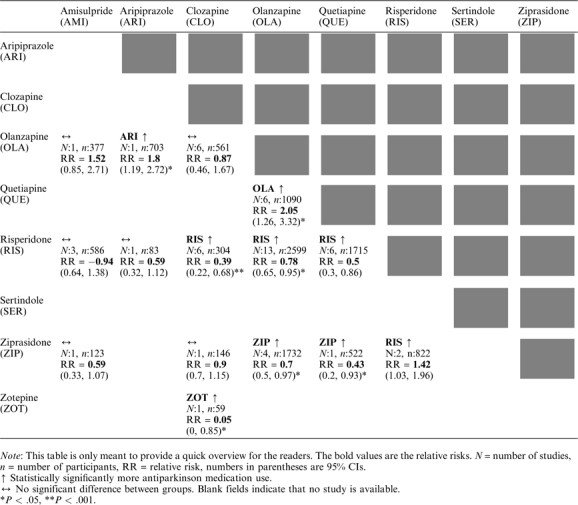
In the text, we present the number of studies (N), the number of participants (n), the relative risk, the CI, the P value, and, for statistically significant results, the NNT/NNH with CI. A brief summary of the results on the use of antiparkinson medication is presented in table 1 to provide a quick overview.
Table 1.
Summary of the Results of the Primary Outcome “Use of Antiparkinson Medication”
 |
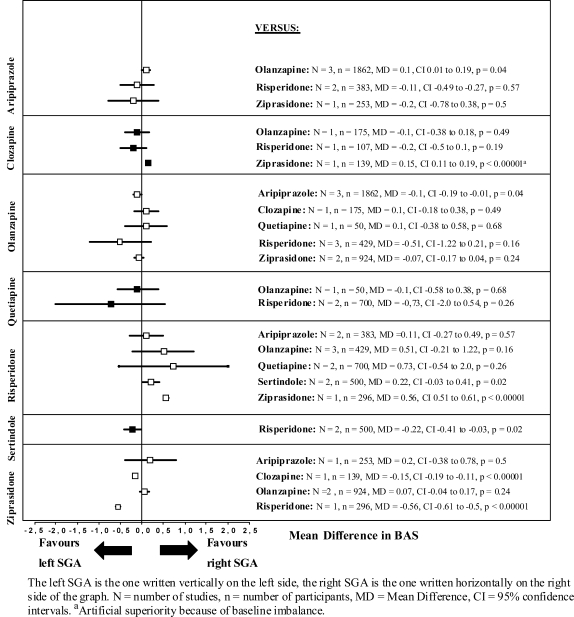
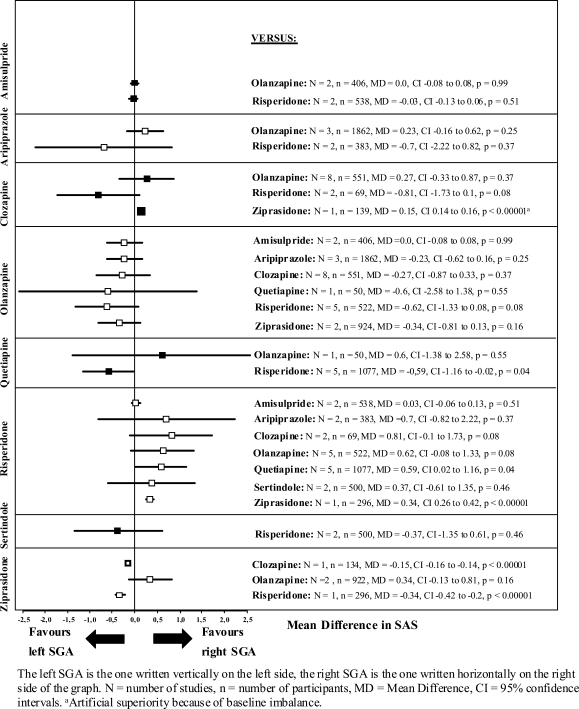
The results on the secondary outcomes and the differences in means of the BAS and SAS are presented in figures 3 and 4.
Fig. 3.
Barnes Akathisia Scale (BAS) Change From Baseline to Endpoint.
Fig. 4.
Simpson Angus Scale (SAS) Change From Baseline to Endpoint.
Further data, such as information on the single studies, can be found on the journal's website.
Outcome Results
Primary Outcome—Use of Antiparkinson Medication.
The following results are ordered in the way that the SGA with the most significant results on the use of antiparkinson medication is displayed first and the SGA with the least use of antiparkinson medication is displayed at the end (see also figure 2).
Risperidone was associated with more use of antiparkinson medication than all other SGAs (vs clozapine: N = 6, n = 304, RR = 2.57, CI = 1.47–4.48, P = .0009, NNH = 6, CI = 3–33; vs olanzapine: N = 13, n = 2599, RR = 1.28, CI = 1.06–1.55, P = .01, NNH = 17, CI = 9–100; vs quetiapine: N = 6, n = 1715, RR = 1.98, CI = 1.16–3.39, P = .01, NNH = 20, CI = 10–100; vs ziprasidone: N = 2, n = 822, RR = 1.42, CI = 1.03–1.96, P = .03, NNH = 17, CI = 6–25), except for amisulpride (N = 3, n = 586, RR = 1.07, CI = 0.72–1.57, P = .75) and aripiprazole (N = 1, n = 83, RR = 1.68, CI = 0.89–3.17, P = .11) where no significant differences were found.
Ziprasidone was associated with statistically significantly more use of antiparkinson medication than olanzapine (N = 4, n = 1732, RR = 1.43, CI = 1.03–1.99, P = .03, NNH = 20, CI = 10–not applicable [n.a.]) and quetiapine (N = 1, n = 522, RR = 2.32, CI = 1.07–5, P = .03, NNH = 25, CI = 11–n.a.). No difference was found between ziprasidone and amisulpride (N = 1, n = 123, RR = 1.7, CI = 0.94–3.07, P = .08) and ziprasidone and clozapine (N = 1, n = 146, RR = 1.11, CI = 0.87–1.42, P = .39).
Zotepine was associated with more use of antiparkinson medication than clozapine (N = 1, n = 59, RR = 18.75, CI = 1.17–301.08, P = .04, NNH = 3, CI = 2–5).
Aripiprazole was associated with more use of antiparkinson medication than olanzapine (N = 1, n = 703, RR = 1.8, CI = 1.19–2.72, P = .005, NNH = 14, CI = 8–50); no difference was found with risperidone (N = 1, n = 83, RR = 0.59, CI = 0.32–1.12, P = .11).
There was no significant difference between amisulpride and any of its comparators (vs olanzapine: N = 1, n = 377, RR = 1.52, CI = 0.85–2.71, P = .15; vs risperidone: N = 3, n = 586, RR = 0.94, CI = 0.64–1.38, P = .75; vs ziprasidone: N = 1, n = 123, RR = 0.59, CI = 0.33–1.07, P = .08).
Clozapine had significantly less use of antiparkinson medication than risperidone (N = 6, n = 304, RR = 0.39, CI = 0.22–0.68, P = .0009, NNT = 6, CI = 3–33) and zotepine (N = 1, n = 59, RR = 0.05, CI = 0–0.85, P = .04, NNT = 3, CI = 2–5); no difference was found compared with olanzapine (N = 6, n = 561, RR = 0.87, CI = 0.46–1.67, P = .69) and ziprasidone (N = 1, n = 146, RR = 0.9, CI = 0.7–1.15, P = .39).
Participants treated with olanzapine received significantly less antiparkinson medication than those receiving aripiprazole (N = 1, n = 703, RR = 0.55, CI = 0.37–0.84, P = .005, NNT = 14, CI = 8–50), risperidone (N = 13, n = 2599, RR = 0.78, CI = 0.65–0.95, P = .01, NNT = 17, CI = 9–100), and ziprasidone (N = 4, n = 1732, RR = 0.7, CI = 0.5–0.97, P = .03, NNT = 20, CI = 10–n.a.). There was no significant difference compared with amisulpride (N = 1, n = 377, RR = 0.66, CI = 0.37–1.17, P = .15) and clozapine (N = 6, n = 561, RR = 1.14, CI = 0.6–2.19, P = .69). However, olanzapine produced more EPS than quetiapine (N = 6, n = 1090, RR = 2.05, CI = 1.26–3.32, P = .004, NNH = 25, CI = 14–100).
Quetiapine showed less use of antiparkinson medication than all 3 other SGAs for which comparisons were available (vs olanzapine: N = 6, n = 1090, RR = 0.49, CI = 0.3–0.79, P = .004, NNT = 25, CI = 14–100; vs risperidone: N = 6, n = 1715, RR = 0.5, CI = 0.3–0.86, P = .01, NNT = 20, CI = 10–100; vs ziprasidone: N = 1, n = 522, RR = 0.43, CI = 0.2–0.93, P = .03, NNT = 25, CI = 11–n.a.).
No differences in these results were found with the fixed effects model.
Secondary Outcomes.
The few comparisons that reached statistical significance in the BAS and the SAS are summarized below (see also figures 3 and 4).
BAS
Aripiprazole produced more akathisia than olanzapine (N = 3, n = 1862, MD = 0.1, P = .04) and clozapine more than ziprasidone (N = 1, n = 139, MD = 0.15, P < .0001). Risperidone was associated with more akathisia than sertindole (N = 2, n = 500, MD = 0.22, P = .02) and ziprasidone (N = 1, n = 296, MD = 0.56, P < .00001).
Simpson Angus Scale
Risperidone produced more extrapyramidal symptoms according to the SAS than quetiapine (N = 5, n = 1077, MD = 0.59, CI = 0.02–1.16, P = .04) and ziprasidone (N = 1, n = 296, MD = 0.34, CI = 0.26–0.42, P < .00001). It should be noted here that the SAS focuses on parkinsonism.
Heterogeneity and Risk of Bias Assessment
Significant heterogeneity of studies, which might complicate their meta-analytic pooling, was not found for the comparisons of the outcome use of antiparkinson medication. The meta-regressions for the moderator washout period were only statistically significant for the comparison of olanzapine vs ziprasidone (the longer the washout period the higher the superiority of olanzapine relative to the use of antiparkinson medication). For the moderator sponsorship, there were no statistically significant results (see Supplementary table II).
In the assessment of the methodological quality of inclusive trials with the risk of bias tool, we found that the sequence generation and the allocation concealment were frequently not clearly reported. All studies were double-blind trials. Concerning the domain “incomplete outcome data,” dropout rates were often high and usually imperfect last observation carried forward procedures were used to account for attrition. In the domain “selective reporting,” 13 studies did not report the use of antiparkinson medication (selective reporting). Some studies had other risks of bias such as baseline imbalance between groups (also see Supplementary figure I).
Sensitivity Analyses
Excluding Studies With Higher Than the Recommended Doses.
Excluding studies with risperidone mean daily doses over 6 mg displayed that the results of the primary outcome remained the same in comparison to aripiprazole (no significant difference: N = 1, n = 83, RR = 1.68 CI = 0.89, 3.17, P = .11), to clozapine (significant difference: N = 1, n = 41, RR = 4.67 CI = 1.23, 17.68, P = .02), and to quetiapine (significant difference: N = 5, n = 1684, RR = 1.9 CI = 1.09, 3.31, P = .02). However, the differences between risperidone and olanzapine (N = 9, n = 2087, RR = 1.15 CI = 0.97, 1.37, P = 11) and risperidone and ziprasidone (N = 1, n = 526, RR = 1.24 CI = 0.68, 2.26, P = .48) were no longer statistically significant.
Excluding studies with olanzapine mean daily doses over 20 mg showed that the results remained the same for the comparisons of olanzapine and clozapine (no significant difference: N = 3, n = 289, RR = 2.12 CI = 0.83–5.42, P = .11), olanzapine and risperidone (significant difference: N = 11, n = 842, RR = 0.80 CI = 0.65–0.99, P = .04), and olanzapine and ziprasidone (N = 3, n = 1211, RR = 0.64 CI = 0.44–0.94, P = .02). However, the difference between olanzapine and quetiapine was no longer statistically significant (N = 5, n = 417, RR = 1.85 CI = 0.92–3.72, P = .08).
Excluding Studies With Lower Than the Recommended Doses.
Excluding studies with mean daily risperidone doses lower than 4 mg showed that the results remained the same for the comparisons of olanzapine and risperidone (significant difference: N = 9, n = 1360, RR = 0.75 CI = 0.59–0.95, P = .02) but not for the comparison of quetiapine and risperidone (no significant difference anymore: N = 4, n = 770, RR = 0.6 CI = 0.25–1.45, P = .25). Excluding studies with ziprasidone mean daily doses lower than 120 mg showed that between olanzapine and ziprasidone, there was no significant difference anymore (N = 2, n = 663, RR = 0.75 CI = 0.52–1.09, P = .13).
Publication Bias
The test for funnel plot asymmetry was used only for the comparison of olanzapine vs risperidone because the test is considered reasonable only if 10 or more studies are available.18 The funnel plot did not suggest a relevant publication bias (Egger’s test22 df = 11, P = .41) (see Supplementary figure II).
Discussion
We present the first meta-analysis comparing EPS of SGAs head-to-head. We found significant differences between the SGAs in their ability to induce EPS that clinicians warrant treatment with antiparkinson medication. The main result is that risperidone resulted in more use of antiparkinson medication and thus showed more EPS than clozapine, olanzapine, quetiapine, and ziprasidone; no significant differences were found with amisulpride and aripiprazole. The comparisons of risperidone and olanzapine and risperidone and ziprasidone did not remain significant when we excluded all risperidone studies with a mean daily dose over 6 mg. This confirmed the dose effect for the EPS with risperidone. In pivotal studies, risperidone with a mean daily dose over 6 mg did not lead to better efficacy but to more EPS.23 Ziprasidone caused more use of antiparkinson medication than olanzapine and quetiapine; no significant differences were found with amisulpride and clozapine (although the data on the ziprasidone-clozapine comparison were very limited). Quetiapine, however, caused significantly less use of antiparkinson medication in all available comparisons (olanzapine, risperidone, and ziprasidone).
The clinical observation that there are differences in the amount of EPS within the group of the SGAs and that the SGAs are not a homogenous group of antipsychotics with regards to EPS has now been documented for the first time with this meta-analysis by demonstrating differences in the use of antiparkinson medication. In an earlier meta-analysis comparing the SGAs with placebo, none of the SGAs induced significantly more EPS than placebo—however, risperidone was almost significant—showing that the EPS risks from the SGAs are generally low but not that there are differences in the amount of EPS among the SGAs.24 A reason for not finding such differences compared with placebo might have been that washout periods usually only last a few days in current schizophrenia trials and therefore carryover effects of previously taken antipsychotic medication can influence outcomes such as EPS. But when looking at the raw frequency of use of antiparkinson medication (ie, unadjusted for the difference compared with placebo) in the aforementioned meta-analysis, there were differences between the SGAs, similar to the results of our current meta-analysis: when compared with placebo, the most use of antiparkinson medication was found for risperidone (in 32%) and ziprasidone (in 21%), whereas the least use was seen with amisulpride (in 2%, however, in low doses up to 300 mg/d) and quetiapine (in 10%).24
Our analysis also supports the notion that the outcome use of antiparkinson medication at least once is a useful proxy variable for the emergence of EPS. However, it may not necessarily be a global EPS measure for 2 main reasons: first, EPS cover different types of symptoms, such as parkinsonism, acute and tardive dystonia, acute and chronic akathisia, and tardive dyskinesia, which can partly be treated with anticholinergics but also in a different manner; eg, β-blockers may also be used for akathisia and tardive dyskinesia may not be treated with anticholinergic medication. Second, cholinergic rebound in the early stage of the study of patients being switched from their previous medication to the study medication could be a problem, although this would be pertinent for both comparison groups. On the other hand, specific EPS are not always reported because a rater can usually decide whether he mentions an event or not. The single EPS are often classified differently among different studies. Furthermore, the results of EPS scales such as the SAS or BAS are frequently skewed, which is problematic for meta-analyses. Dichotomous data such as use of antiparkinson medication are preferred in evidence-based medicine due to better clinical interpretation. Therefore, at least for systematic reviews, the use of antiparkinson medication is a useful proxy measure for EPS.
The NNT/NNH for the main outcome ranged from 3 to 6 for clozapine but were higher for the other SGAs: for olanzapine and quetiapine, they ranged from 14 to 25. A NNT of 25, eg, means that 25 patients have to be treated with the studied SGA to avoid the use of antiparkinson medication, ie, EPS, in 1 patient. These numbers are relatively high, showing that the differences between most SGAs are rather small; perhaps, even smaller than might have been expected from clinical observation. In routine care, polypharmacy is frequently used and increases the risk for EPS.25 In clinical trials, carryover effects and short washout periods might have contributed to the rather small differences in EPS of the SGAs. Only one meta-regression found a significant association between the duration of washout and the EPS superiority (olanzapine compared with ziprasidone). However, washout periods were generally short (ie, median 2 d). Possibly, with longer washout phases, more differences in the use of antiparkinson medication could be detected. In more sensitive populations, such as adolescents with schizophrenia,26 patients with bipolar disorder,27 and patients with dementia,28 differences in EPS of SGAs vs placebo could be seen more clearly because these patients usually had much less exposure to antipsychotics in the past than most participants in schizophrenia trials.
The differences in EPS of the SGAs are important for 2 main reasons: First, especially EPS were shown to have a strong association with medication noncompliance29 or may even lead to patient medication noncompliance30—one of the major issues in the treatment of people with schizophrenia.31 Second, having a lower EPS liability is important for patients not only for motor side effects but also for the impact on cognitive function32,33 and potential tardive dyskinesia risk,34,35 even though SGAs have been reported to be associated with a lower risk of tardive dyskinesia compared with FGAs.36,37 We found that SGAs produce fewer EPS than the high-potency antipsychotic drug haloperidol (even in low doses), but a superiority compared with low-potency FGAs has only been shown for a few SGAs.6 However, the results of the recent effectiveness trials were contradictory: the EPS analysis of Clinical Antipsychotic Trials of Intervention Effectiveness showed no major difference,38 nor did the Veteran's affairs study on haloperidol combined with benztropine vs olanzapine.39 In contrast, in the open, randomized European First Episode Schizophrenia Trial study, low-dose haloperidol (1–4 mg/d) was associated with most EPS.40
However, the differences in EPS between the SGAs need to be weighed against other side effects such as metabolic (weight gain or glucose insensitivity) or sexual problems and also against possible efficacy differences of the compounds.7 That means that the relative risk of EPS needs to be seen in the context of the relative benefit of a specific drug and a specific dosage. If a low dose of drug A is associated with fewer EPS, but also a lower likelihood of optimum response, this needs to be part of the equation. In addition, the advantages of some antipsychotics on EPS liability might in part be offset, eg, by metabolic side effects. But because patients with schizophrenia substantially differ in which side effects they have, with what severity, and also in the clinical response, the treatment with SGAs, which are not a homogenous group of antipsychotics themselves, needs to be individualized for every patient.
The main limitation of this meta-analysis is that there are no or only few data available for some comparisons. Not all included studies published the use of antiparkinson medication, even though the information would have been available because the use of all concomitant medication has to be documented during a medication trial. Therefore, even though one can already see a gestalt on the situation of the EPS in the SGAs from the current available studies, more head-to-head studies with the concomitant medication being published are needed to see the differences in extrapyramidal symptoms for all SGAs.
In conclusion, even with regard to EPS, the SGAs are not a homogenous class and are definitely not all “free” from the extrapyramidal symptoms. “Inducing EPS or not” therefore is not a good classification basis for atypical antipsychotics. Consequently, a more adequate classification system for SGAs—as have been called for in the recent years6,41–43—seems to be justified.
Funding
Technische Universität München (HWP II) (C.R.); German Federal Ministry of Education and Research (no. FKZ: 01KG 0606, GZ: GFKG01100506 to S.L.); National Institute of Mental Health, Advanced Center for Intervention and Services Research Center (MH-68580); (1 P01MH68580-01 CFDA #93.242); Maryland Psychiatric Research Center (J.M.D.); EliLilly; SanofiAventis to S.L.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org/.
Acknowledgments
We thank the following authors and pharmaceutical companies for providing additional information on their studies: D. Addington, I. Bitter, R. Conley, D. Daniel, L. De Haan, S. Dollfus, O. Fleurot, H. Heinrich, T. Hwang, D. Jeste, W. Kadus, J. Kane, R. Keefe, D. Kelly, M. Krakowski, M. Kluge, Y. Liu, S. McGurk, K. Mori, A. Mortimer, D. Naber, H. Ozguven, J. Peuskens, B. Quednow, Q. Ren, M. Riedel, N. Schooler, D. Sechter, J. Švestka, J. Volavka, M. Wagner, and K. Zhong and AstraZeneca, EliLilly, Lundbeck, Pfizer, and SanofiAventis. This review was conducted with the help of the Cochrane Schizophrenia Group to which we are greatly indebted. The funding source had no involvement in study design, collection, analysis and interpretation of data, writing the report and in the decision to submit the article for publication. Contributors: C.R., K.K., and S.L. contributed to designing the study, data extraction, statistical analysis, and writing the report. S.S., H.H., and F.S. contributed to data extraction and writing of the report. W.K. and J.M.D. contributed to designing the study and writing the report. All authors contributed to and have approved the final manuscript. Conflict of interest: Christine Rummel-Kluge has received speaker honoraria or travel grants to attend scientific meetings from Janssen-Cilag, EliLilly, and Pfizer. Stefan Leucht has received speaker and/or consultancy honoraria from SanofiAventis, BMS, EliLilly, Essex, GlaxoSmithKline, Janssen/Johnson and Johnson, Lundbeck, and Pfizer. Werner Kissling has received speaker or consultancy honoraria from SanofiAventis, BMS, Lilly, Janssen, Lundbeck, Bayer, and Pfizer. Katja Komossa, Heike Hunger, Franziska Schmid, and John M. Davis: none to declare.
References
- 1.Lieberman JA, Kane JM, Johns CA. Clozapine: guidelines for clinical management. J Clin Psychiatry. 1989;50:329–338. [PubMed] [Google Scholar]
- 2.Ereshefsky L, Watanabe MD, Tran-Johnson TK. Clozapine: an atypical antipsychotic agent. Clin Pharmacol. 1989;8:691–709. [PubMed] [Google Scholar]
- 3.Barnes TR, McPhillips MA. Novel antipsychotics, extrapyramidal side effects and tardive dyskinesia. Int Clin Psychopharmacol. 1998;13(suppl 3):S49–S57. doi: 10.1097/00004850-199803003-00009. [DOI] [PubMed] [Google Scholar]
- 4.Miller CH, Mohr F, Umbricht D, Woerner M, Fleischhacker WW, Lieberman JA. The prevalence of acute extrapyramidal signs and symptoms in patients treated with clozapine, risperidone, and conventional antipsychotics. J Clin Psychiatry. 1998;59:69–75. doi: 10.4088/jcp.v59n0205. [DOI] [PubMed] [Google Scholar]
- 5.Kane JM, Fleischhacker WW, Hansen L, Perlis R, Pikalov A, III, Assunc¸ão-Talbott S. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70:627–643. doi: 10.4088/JCP.08r04210. [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 7.Leucht S, Komossa K, Rummel-Kluge C, et al. et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–163. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 8.Adams CE, Coutinho E, Davis JM, et al. Cochrane Schizophrenia Group in the Cochrane Library Chichester. Chichester, UK: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5, in The Cochrane Library. Issue 3. Chichester, UK: Wiley & Sons Ltd; 2005. [Google Scholar]
- 10.Komossa K, Rummel-Kluge C, Hunger H, et al. Cochrane Database Syst. Amisulpride versus other atypical antipsychotics for schizophrenia. Rev. 2010;1:CD006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komossa K, Rummel-Kluge C, Hunger H, et al. Cochrane Database Syst. Olanzapine versus other atypical antipsychotics for schizophrenia. Rev. 2010;2:CD006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komossa K, Hunger H, Schmidt F, et al. Cochrane Database Syst. Quetiapine versus other atypical antipsychotics for schizophrenia. Rev. 2010;1:CD006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komossa K, Hunger H, Schmidt F, Schwarz S, Leucht S, Rummel-Kluge C. The Cochrane Library. Chichester, UK: Wiley & Sons Ltd; 2007. Risperidone versus other atypical antipsychotics for schizophrenia (protocol) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komossa K, Rummel-Kluge C, Hunger H, et al. Cochrane Database Syst Rev. Zotepine versus other atypical antipsychotics for schizophrenia. 2010; 1:CD006628. [DOI] [PubMed] [Google Scholar]
- 15.Komossa K, Rummel-Kluge C, Hunger H, et al. Sertindole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009:2:CD006752. doi: 10.1002/14651858.CD006752.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komossa K, Rummel-Kluge C, Hunger H, et al. Zotepine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009;4:CD006627. doi: 10.1002/14651858.CD006627.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komossa K, Rummel-Kluge C, Schmid F, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009;4:CD006569. doi: 10.1002/14651858.CD006569.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green S, Higgins JPT. Cochrane Handbook of Interventions. Chichester, UK: Wiley&Sons; 2008. [Google Scholar]
- 19.Der-Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: long-term treatment of schizophrenia. World J Biol Psychiatry. 2006;7:5–40. doi: 10.1080/15622970500483177. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 24.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 25.De Hert M, Wampers M, van WR, Peuskens J. Anticholinergic use in hospitalised schizophrenic patients in Belgium. Psychiatry Res. 2007;152:165–172. doi: 10.1016/j.psychres.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- 27.Scherk H, Pajonk FG, Leucht S. Second generation antipsychotics in the treatment of acute mania: a systematic review and meta-analysis of randomized, controlled trials. Arch Gen Psychiatry. 2007;64:442–455. doi: 10.1001/archpsyc.64.4.442. [DOI] [PubMed] [Google Scholar]
- 28.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 29.Karow A, Czekalla J, Dittmann RW, et al. Association of subjective well-being, symptoms, and side effects with compliance after 12 months of treatment in schizophrenia. J Clin Psychiatry. 2007;68:75–80. doi: 10.4088/jcp.v68n0110. [DOI] [PubMed] [Google Scholar]
- 30.Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23:637–651. doi: 10.1093/schbul/23.4.637. [DOI] [PubMed] [Google Scholar]
- 31.Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1–46. [PubMed] [Google Scholar]
- 32.Van Putten T, May PR. Subjective response as a predictor of outcome in pharmacotherapy: the consumer has a point. Arch Gen Psychiatry. 1978;35:477–480. doi: 10.1001/archpsyc.1978.01770280087009. [DOI] [PubMed] [Google Scholar]
- 33.Gervin M, Browne S, Garavan J, Roe M, Larkin C, O'Callaghan E. Dysphoric subjective response to neuroleptics in schizophrenia: relationship to extrapyramidal side effects and symptomatology. Eur Psychiatry. 1999;14:405–409. doi: 10.1016/s0924-9338(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 34.Tenback DE, van Harten PN, Slooff CJ, van OJ. Evidence that early extrapyramidal symptoms predict later tardive dyskinesia: a prospective analysis of 10,000 patients in the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Am J Psychiatry. 2006;163:1438–1440. doi: 10.1176/ajp.2006.163.8.1438. [DOI] [PubMed] [Google Scholar]
- 35.Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17:647–656. doi: 10.1089/cap.2006.0117. [DOI] [PubMed] [Google Scholar]
- 36.Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of one-year studies. Am J Psychiatry. 2004;161:414–415. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- 37.Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21:151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- 38.Miller DD, Caroff SN, Davis SM, et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008;193:279–288. doi: 10.1192/bjp.bp.108.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia—a randomized controlled trial. JAMA. 2003;290:2693–2702. doi: 10.1001/jama.290.20.2693. [DOI] [PubMed] [Google Scholar]
- 40.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 41.Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009;39:1591–1602. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- 42.Grunder G, Hippius H, Carlsson A. The 'atypicality' of antipsychotics: a concept re-examined and re-defined. Nat Rev Drug Discov. 2009;8:197–202. doi: 10.1038/nrd2806. [DOI] [PubMed] [Google Scholar]
- 43.Jindal RD, Keshavan MS. Classifying antipsychotic agents: need for new terminology. CNS Drugs. 2008;22:1047–1059. doi: 10.2165/0023210-200822120-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.