Abstract
Event-related potentials (ERPs) are a powerful tool in understanding and evaluating cognitive, affective, motor, and sensory processing in both healthy and pathological samples. A typical ERP recording session takes considerable time but is designed to isolate only 1–2 components. Although this is appropriate for most basic science purposes, it is an inefficient approach for measuring the broad set of neurocognitive functions that may be disrupted in a neurological or psychiatric disease. The present study provides a framework for more efficiently evaluating multiple neural processes in a single experimental paradigm through the manipulation of functionally orthogonal dimensions. We describe the general MONSTER (Manipulation of Orthogonal Neural Systems Together in Electrophysiological Recordings) approach and explain how it can be adapted to investigate a variety of neurocognitive domains, ERP components, and neural processes of interest. We also demonstrate how this approach can be used to assess group differences by providing data from an implementation of the MONSTER approach in younger (18–30 y of age) and older (65–85 y of age) adult samples. This specific implementation of the MONSTER framework assesses 4 separate neural processes in the visual domain: (1) early sensory processing, using the C1 wave; (2) shifts of covert attention, with the N2pc component; (3) categorization, with the P3 component; and (4) self-monitoring, with the error-related negativity. Although the MONSTER approach is primarily described in the context of ERP experiments, it could also be adapted easily for use with functional magnetic resonance imaging.
Keywords: ERPs, neurocognitive dimensions, psychopathology
Noninvasive measures of brain activity, such as event-related potentials (ERPs) and functional magnetic resonance imaging (fMRI), hold great promise for providing biomarkers of impaired neural and cognitive processes in psychiatric disorders.1–3 One key limitation, however, is that ERP and fMRI paradigms that precisely isolate specific neural and cognitive systems, often require large numbers of trials to provide reliable measures, especially at the individual-patient level. This problem becomes especially acute when a dimensional approach is taken, as recommended by the recent Research Domain Criteria (RDoC) initiative,4–6 because multiple paradigms will typically be necessary to characterize a given individual along multiple dimensions. At a recent meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative, we proposed a possible solution to this problem in the context of ERP recordings. This solution involves identifying several manipulations that individually isolate specific neural or cognitive systems and factorially combining them into a single higher-level paradigm, allowing the different measures to be obtained in parallel (a single experimental session) rather than in serial (multiple experimental sessions). This approach, which could also be used with fMRI, is termed Manipulation of Orthogonal Neural Systems Together in Electrophysiological Recordings (the MONSTER approach). The present article will describe this approach and provide an example of how it can be used to assess, in parallel, sensory processing in primary visual cortex, shifts of visuospatial attention, stimulus categorization, and performance monitoring. This is just a single example and other neural/cognitive processes could also be isolated in parallel using this approach, as described below.
The importance of this approach is clear when the goals of the CNTRICS initiative are juxtaposed with the dimensional approach embodied by the RDoC initiative. The CNTRICS initiative seeks to develop state-of-the-art measures of neurocognitive processes that have a combination of high validity, specificity, and reliability, while being practical for use in clinical trials. However, measuring a specific neurocognitive processes with high reliability typically requires a long period of testing (often 20–60 min), and it would be impractical to measure several dimensions if each requires a separate testing period. The goal of the MONSTER approach is to provide a means of reliably measuring multiple dimensions in a single testing period.
The Importance of Difference Waves in Isolating ERP Components
The MONSTER approach is based on an assumption that is considered obvious in fMRI research and is common in basic science ERP research but is not as universal in clinical applications of ERPs. Specifically, we assume that the activity recorded at any given time point reflects the sum of several different neural processes, and isolating a specific neural process usually requires examining the difference in activity between different conditions. This is completely obvious in fMRI experiments focusing on the blood oxygen level-dependent signal because any individual image is mainly a structural image and cannot be used alone to measure neural activity. It is the difference between images that provides an index of neural activity. (Regression-based analytic approaches to fMRI analysis are just a more sophisticated generalization of the difference-based approach.). It is possible to obtain measures of absolute blood flow (eg, via positron emission tomography or arterial spin labeling), but it is widely recognized that these measures do not usually isolate specific neural processes. Instead, differences between experimental conditions are usually necessary to isolate specific processes. The same logic applies to ERP research, in which difference waves are used to isolate processes that differ across experimental conditions.
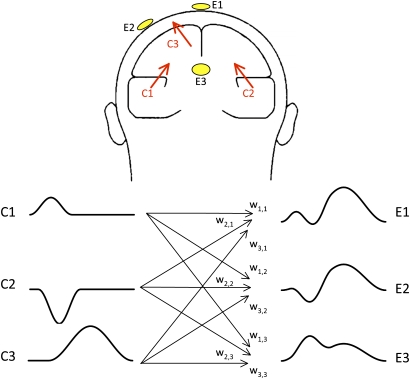
The importance of this is illustrated in figure 1, which shows how activity from multiple neural generator sources becomes combined at any individual electrode site. The voltage measured at a given time from a given electrode site reflects a weighted sum of the activity generated at every location in the brain. In other words, every ERP generator contributes voltage at every electrode site (except for a narrow belt of zero voltage at the transition between the positive and negative sides of the distribution). Techniques exist for reducing this problem, but none are both 100% effective and guaranteed to work.8–11 Moreover, any large patch of cortex is likely to be involved in multiple different processes, so localizing the activity does not isolate a specific neural process (just as mere localization, in the absence of an experimental manipulation, is usually insufficient in neuroimaging studies).
Fig. 1.
Relation between the underlying component waveforms and the observed scalp waveforms. In this example, 3 components are present (C1, C2, and C3), each of which has a waveform (shown at the bottom left) and a generator location (represented by the arrows in the head). The contribution of each component waveform to the observed waveform at a given electrode site is determined by a weighting factor that reflects the location and orientation of the generator relative to that electrode, along with the conductivity of the tissues that form the head. The observed waveform at a given electrode site (shown at the bottom right) is equal to the sum of each of the component waveforms, multiplied by the weighting factor between each component and that electrode site. The weights are indicated by the w’s on the arrows between the component waveforms and the observed waveforms (eg, w2,3 represents the weighting factor between component 2 and electrode 3). Reprinted by permission of Oxford University Press, Inc. from Kappenman and Luck,7 copyright 2011 by Kappenman and Luck,7 all rights reserved.
Consequently, isolating a specific neural or cognitive process with ERPs usually involves constructing difference waves in which the ERP waveform from one condition is subtracted from the waveform in a condition that is nearly identical except for the operation of a few processes.
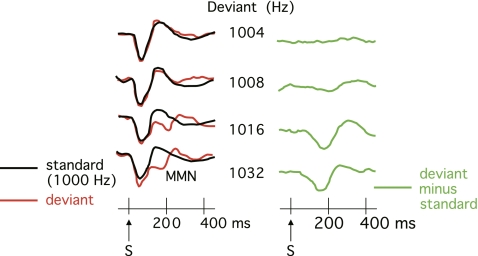
As an example, consider the mismatch negativity (MMN) study shown in figure 2. In this paradigm, subjects hear a sequence of tones consisting of a frequently occurring “standard” pitch and occasional “deviant” pitches; the tones are ignored, and the subject reads a book while the tones are presented. The deviant pitches elicit a negative-going potential from approximately 150–200 ms (the MMN), which sums with the other ERP activity that is present during this time interval. If one measured the voltage during this period from the waveforms elicited by the deviant stimuli, the resulting voltage would reflect both the MMN and the other nonspecific ERP activity. To isolate brain activity that is specifically associated with the detection of deviant tones, researchers typically construct deviant minus standard difference waves (right panel of figure 2) and measure the voltage of the MMN from these difference waves.
Fig. 2.
Example of the use of difference waves to isolate the mismatch negativity (MMN). While the subjects read a book, brief tones were presented at a rate of approximately 1 Hz, with 80% standard tones (1000 Hz) and 20% deviant tones (1004, 1008, 1016, or 1032 Hz). The MMN was isolated by differences waves (right column) in which the event-related potential (ERP) elicited by the standard stimulus was subtracted from the ERP elicited by the deviant stimulus. Reprinted by permission of Oxford University Press, Inc. from Näätänen and Kreegipuu,12 copyright 2011 by Näätänen and Kreegipuu,12 all rights reserved.
Although many ERP components can be seen in the waveforms in a given experiment, most of these components are difficult to measure independently, and most experiments are designed to create one difference wave that isolates only 1 or 2 components. For example, an auditory oddball paradigm that is optimized for measuring the P3 wave also leads to a variety of auditory sensory components, but most auditory oddball paradigms are not designed in a way that allows each of these sensory components to be isolated. The goal of the MONSTER approach is to permit the efficient creation of multiple difference waves in a given experiment, making it possible to isolate multiple components.
Isolating Multiple Components with Orthogonal Difference Waves
An important property of ERP components is that they simply sum together. That is, if 2 voltages are generated by neural activity in the brain, the voltage recorded on the scalp will simply be the sum of the 2 voltages (weighted according to the location and orientation of the generator sites relative to the recording electrode; see ref. 7 for a description). Consequently, if an experimental manipulation causes a change in the magnitude of the activity of one generator, yielding a 2 μV change at the scalp, the size of this effect will be independent of the voltage produced by the other generator. In other words, independent changes in different ERP generators will lead to additive changes in the activity measured at the scalp. Interactive effects at the scalp will be observed only if there are true interactions between the generator sources. These facts (which arise from the physics of voltage conduction) make it possible to use very simple subtraction approaches to isolate the effects of factorially combined experimental manipulations.
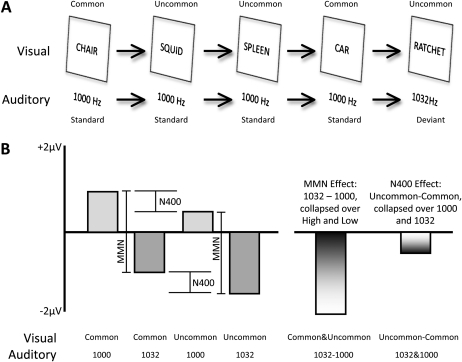
As an example, consider a hypothetical experiment in which auditory and visual stimuli are presented simultaneously, with one auditory-visual pair per second (see figure 3A). The auditory component of each pair is a 1000-Hz standard tone on 80% of trials and a 1032-Hz deviant tone on 20% of trials, but subjects are instructed to ignore the auditory component. The MMN is present even when tones are ignored,12 so it should be visible in a deviant-minus-standard difference wave. The visual component of each auditory-visual pair is a common word (eg, “chair”, “car”, “flower”) on 50% of trials and an uncommon word (eg, “squid”, “spleen”, “ratchet”) on the other 50%. The words are presented visually at the center of a video monitor. The N400 component is larger for uncommon words than for common words,13 so it should be visible in an uncommon-minus-common difference wave.
Fig. 3.
Hypothetical implementation of the MONSTER approach. (A) Example portion of stimulus sequence. Each stimulus consists of a word and a tone. The words are common (P = .5) or uncommon (P = .5), and the tones are 1000 Hz (standards, P = .8) or 1032 Hz (deviants, P = .2). (B) Hypothetical amplitude of the event-related potential waveform (in a time window that includes both the mismatch negativity [MMN] and the N400). In this example, the uncommon minus common N400 effect is independent of the deviant-minus-standard MMN effect. The right portion of the panel shows each of these differences, collapsed across the other dimension.
These 2 factors (standard vs deviant tone and common vs uncommon word) are factorially combined, with a standard tone accompanying 80% of common words and 80% of uncommon words and a deviant tone accompanying 20% of common and 20% of uncommon words.
Figure 3B shows hypothetical results in which the MMN and N400 effects are independent, such that the size of the MMN effect is equivalent for tones that accompany common and uncommon words and the size of the N400 effect is equivalent for words that accompany standard and deviant tones. When the effects are independent, one can combine the waveforms along one dimension prior to measuring the difference between the waveforms in the other dimension. For example, to measure the MMN effect, one would create a waveform for the standard-tone trials, collapsed across trials in which these tones were accompanied by common and uncommon words, and subtract this from the waveform for the deviant-tone trials, again collapsed across common and uncommon words. Similarly, to measure the N400 effect, one would create a waveform for the common-word trials, collapsed across trials in which the words were accompanied by standard and deviant tones, and subtract this from the waveform for the uncommon-word trials, again collapsed across standard and deviant tones (see difference values at the right of figure 3B).
The key advantage of this approach is that a single set of trials can be divided in 2 different ways to isolate 2 different ERP components, allowing the 2 components to be isolated in the same amount of time that would be required to isolate a single component in a conventional approach. Imagine, for example, that each subject received a total of 200 trials (80 common + standard, 20 common + deviant, 80 uncommon + standard, and 20 uncommon + deviant). After collapsing across common and uncommon words, averaged ERP waveforms based on 160 standard tones and 40 deviant tones would be used to measure the deviant-minus-standard MMN effect. After collapsing across standard and deviant tones, averaged ERP waveforms based on 100 common words and 100 uncommon words would be used to measure the uncommon-minus-common N400 effect. The conventional approach to ERP recordings, in contrast, would require 200 trials to measure one effect followed by another 200 trials to measure the other effect, requiring 400 total trials to achieve the same statistical power obtained with 200 total trials using the MONSTER approach. As the next example will show, the MONSTER approach becomes progressively more advantageous as more manipulations are combined to isolate more components. In this example, 4 components are isolated by means of 4 orthogonal difference waves, with 1600 trials contributing to the measurement of each component, whereas the conventional approach would require 6400 trials to isolate these same 4 components.
Potential Limitations of the MONSTER Approach
Two important issues must be considered in evaluating this approach. First, it is important to ask whether the presence of a component in the dimension being collapsed adds noise to the measurement of a component in the dimension being isolated. In the MMN + N400 example, the MMN and N400 are actually measured during different time windows and would be expected to have little or no temporal overlap, so trial-to-trial or subject-to-subject variation in one component would not be expected to increase the variance in the measure of the other component. In many cases, however, one would want to isolate components that overlap in time (eg, the P3 and the error-related negativity [ERN]). Fortunately, the presence of the other component is still unlikely to add much variance to the component being measured. The reason for this is that spontaneous variation in the electroencephalographic (EEG) waveform is typically much larger than spontaneous variation in the amplitude of a stimulus-elicited ERP component. For example, trial-to-trial variations in the amplitude of the N400 component elicited by an uncommon word is unlikely to be more than ±5 μV given that the overall amplitude of the N400 is usually well under 10 μV,13 whereas the single-trial EEG typically varies over a range of ±50 μV or more.
A second and more significant issue is that the MONSTER approach assumes that the orthogonal manipulations lead to independent, additive, and noninteracting effects. In our MMN + N400 example, this is likely to be a good assumption. That is, the N400 effect is unlikely to be influenced by whether a concurrent task-irrelevant tone is a standard or a deviant, and the MMN effect is unlikely to be influenced by whether an accompanying word is common or uncommon. However, there are many cases in which the effects of one manipulation will vary across the conditions of the other manipulation.
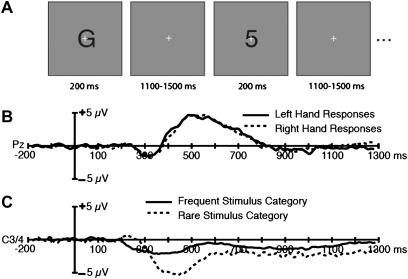
Consider, for example, the study illustrated in figure 4.14 This experiment contained 2 factorial manipulations to isolate the P3 component and the lateralized readiness potential (LRP). The P3 is well known to be larger for infrequently occurring stimulus categories than for frequently occurring stimulus categories, and it is typically isolated by comparing rare “target” items and frequent “standard” items in the oddball paradigm.15 The LRP reflects activation of motor cortex, and it is typically isolated by comparing the hemispheres contralateral and ipsilateral to the response. In particular, the LRP is larger (more negative) over the hemisphere contralateral to the responding hand, and it can therefore be isolated by a contralateral-minus-ipsilateral difference wave.16 In the study shown in figure 4, alphanumeric characters were presented in the center of the screen at a rate of one stimulus every 1500 ± 200 ms. Subjects were instructed to make a left-hand button press for letters and a right-hand button press for digits or vice versa. In addition, 1 of these 2 categories was rare (P = .2) and the other was frequent (P = .8). The assignment of hands to stimulus categories was varied orthogonally with the probability of the 2 stimulus categories (across blocks of trials), leading to the following combinations: frequent category with left-hand response, rare category with right-hand response, frequent category with right-hand response, and rare category with left-hand response.
Fig. 4.
Task and results from the study of Luck et al14. (A) Example stimuli. A sequence of letters and digits was presented in the center of the screen, with one category being frequent (0.8) and the other rare (0.2). Subjects made a left-hand button press for one category and a right-hand button press for the other. (B) Grand average P3 difference waveforms (rare minus frequent) from healthy control subjects at electrode site Pz. Note that the amplitude of the P3 did not differ between left-hand and right-hand responses. (C) Grand average lateralized readiness potential difference waveforms (contralateral minus ipsilateral, defined relative to the response hand) from healthy control subjects, collapsed across the C3 and C4 electrode sites. Note that the amplitude was substantially larger for the rare category than for the frequent category, showing an interaction between the 2 main factors (probability and response hand).
The P3 was larger for the rare category than for the frequent category, and this effect was the same for left-hand and right-hand responses (figure 4B). However, the difference in amplitude between electrodes contralateral and ipsilateral to the responding hand—the LRP component—was larger when the stimulus that elicited the response belonged to the rare category than when it belonged to the frequent category (figure 4C). In this particular experiment, the LRP was reduced in schizophrenia patients by approximately 50% relative to control subjects for both the rare and frequent stimulus categories, so the interpretation of the LRP results is the same for the rare and frequent stimulus categories.
However, it is easy to imagine a scenario in which patients might differ from controls at one level of the other variable but not the other level (eg, a reduced LRP for the rare category but not for the frequent category). In such a scenario, it could be problematic to collapse across the 2 levels of the other category. For example, if we collapsed across the rare and frequent stimulus categories before measuring the LRP and the patient reduction was confined to the rare stimulus category, the patient impairment might be relatively small in the collapsed waveforms. Moreover, if only the collapsed data are analyzed, one might assume that a difference between patients and controls for one manipulation was present for all combinations of the other manipulation even if this is not true. This may or may not be an important issue, depending on the nature of the question being asked.
This possibility can be addressed in several ways. First, one can choose manipulations that are likely to produce independent effects (as in the MMN + N400 example). Second, one can conduct preliminary experiments in convenience populations (eg, college students) to determine whether the effects are in fact independent (although this may not necessarily generalize to patients and matched control subjects). Third, one can examine the uncollapsed data for signs of interactions prior to collapsing (see below for an example of this). There may not be enough trials to assess the significance of any interactions, but the presence of similar effects across conditions will usually be enough to be confident that any interactive effects are modest. Finally, if a given combination of manipulations is ultimately used in a large-scale clinical trial or in a physician’s office, then intensive studies with modest sample sizes would presumably be conducted first to establish the validity of that specific implementation of the MONSTER approach. Moreover, if the goal is to measure several independent dimensions of illness, then one would want to combine manipulations that lead to largely independent effects. Thus, it is important to assess the independence of the effects of the manipulations in a given implementation of the MONSTER approach, but this is not a significant limitation on the approach in general.
An Example of the MONSTER Approach
To illustrate how the MONSTER approach can be scaled up to several orthogonal manipulations, we conducted a small proof-of-concept study with 6 healthy young adults (18–30 y of age) and 6 healthy older adults (65–85 y of age). This implementation focused on the visual domain and evaluated both early and late stages of processing. Specifically, this paradigm examined 4 neural processes: (1) early sensory processing, using the C1 wave; (2) shifts of covert visual attention, with the N2pc component; (3) categorization, with the P3 component; and (4) performance monitoring, with the ERN.
The C1 reflects the initial feedforward wave of sensory activity in primary visual cortex. In humans, primary visual cortex is folded around the calcarine fissure, and the retinotopic mapping of this area leads to opposite polarities at the scalp for stimuli presented in the upper and lower visual fields.17 Thus, the C1 can be isolated from other visual components by a difference wave that contrasts the responses to upper and lower field stimuli. The N2pc (N2-posterior-contralateral) component reflects the focusing of attention onto a lateralized stimulus in the presence of distractors.18 It is largest over posterior electrode sites contralateral to the stimulus being attended and arises from intermediate and high levels of the ventral visual pathway.19 It can be isolated from other components by presenting targets unpredictably to the left and right visual fields, accompanied by distractors, and examining the difference in voltage between the electrode sites contralateral and ipsilateral to the target. The P3 component can be used to track stimulus categorization processes because it is larger for rare stimulus categories than for frequent stimulus categories; a difference between rare and frequent categories is not possible until the brain has begun to determine whether a given stimulus belongs to the rare category or the frequent category.10 The ERN can be used to assess performance monitoring because it is larger (more negative) for error trials than for correct trials and is isolated by examining the activity time locked to the response.20
Task Design
An example stimulus display is presented in figure 5. In this task, a letter (either “X” or “O”) was presented on one side of a central fixation cross and a digit (either “1” or “2”) was presented on the other side. In one half of the experiment, letters were targets, and participants pressed one button for an “X” and another button for an “O,” ignoring the “1” or “2.” In the other half of the experiment, digits were targets, and participants pressed one button for a “1” and another button for a “2,” ignoring the “X” or “O.” Responses were made using the index and middle fingers of the right hand.
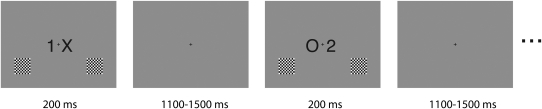
Fig. 5.
Example stimulus display from an implementation of the MONSTER task. In this task, one letter (either “X” or “O”) and one number (either “1” or “2”) were presented in black to the left and right of a central fixation cross on a light grey background. Additionally, 2 black-and-white distractor checkerboards were presented in either the upper or lower corners of the display on each trial. Stimulus identity and location were randomized across trials. All stimuli were presented simultaneously for 200 ms, followed by a variable interstimulus interval of 1100–1500 ms (rectangular distribution). Participants responded on the basis of either the letter category or the number category (counterbalanced across blocks) with a Logitech game pad using the index and middle fingers of the right hand.
The target stimulus appeared unpredictably on the left or right side of fixation, requiring a shift of spatial attention to the left or right side and making it possible to isolate the N2pc component. In each block, one of the 2 instances of the target set occurred on 80% of the trials (eg, “X”) and the other instance occurred on 20% of the trials (eg, “O”), with stimulus probability assignment counterbalanced across blocks. This made it possible to isolate the P3 wave by comparing the ERPs elicited by frequent (80%) and rare (20%) stimuli. In addition, task-irrelevant checkerboards appeared in either the upper or lower halves of the display on each trial, making it possible to isolate the C1 wave. The experiment was also designed to allow a comparison of correct trials and error trials to isolate the ERN. To ensure an adequate number of error trials, feedback was provided after every 40 trials (“Try to respond a bit faster” if the error rate dipped below 5% or “Try to respond more accurately” if the error rate exceeded 15%).
The experiment consisted of four 10-minute blocks of 400 trials each, yielding a total of 1600 trials (this could easily be cut in half, which would reduce the signal-to-noise ratio by only 29% [The signal-to-noise ratio of an averaged ERP is proportional to the square root of the number of trials in the average. Consequently, anything that reduces the number of trials by proportion X will reduce the signal-to-noise ratio by a proportion of (1 − sqrt(1 − X)), assuming that the size of the signal itself has not changed. For example, a 0.5 reduction in the number of trials will cause a reduction in the signal-to-noise ratio of (1 − sqrt(1 − 0.5)) = 0.293. A 0.2 reduction in the number of trials will cause a reduction in the signal-to-noise ratio of (1 − sqrt(1 − 0.2)) = 0.106]). The stimulus factors were factorially combined with independent probabilities, so that the 1600 trials could be divided in 4 different ways, collapsing across the other factors: 800 trials with a left-side target and 800 trials with a right-side target (isolating N2pc), 800 trials with an upper field checkerboard and 800 trials with a lower field checkerboard (isolating C1), 1280 trials with a standard target and 320 trials with an oddball target (isolating P3), and approximately 1440 correct trials and 160 error trials (isolating ERN).
Isolating Orthogonal Components
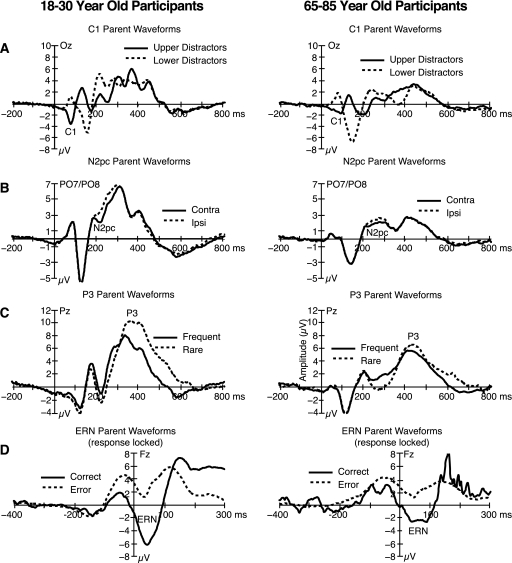
Figure 6 shows the 4 pairs of waveforms created by dividing the 1600 trials into 2 subsets along each of these 4 dimensions. The C1 can be seen in figure 6A as a difference (ca. 40–100 ms) between trials in which the task-irrelevant checkerboards were in the upper field vs the lower field. The N2pc component is defined as a negativity over the hemisphere contralateral to an attended target, and it was isolated in 2 steps. First, the trials were divided based on whether the target item was on the left or right side; second, the activity at electrode sites contralateral and ipsilateral to the target were combined for the left and right targets, yielding a contralateral average and an ipsilateral average, relative to the location of the target (see ref. 18 for a detailed discussion). The N2pc can be seen in figure 6B as the difference (ca. 200–300 ms) between the contralateral and ipsilateral waveforms. The overall voltage in this time range is positive, but the presence of the N2pc component in the contralateral waveform makes this waveform more negative (less positive) than the ipsilateral waveform from 200 to 300 ms. The P3 wave can be seen in figure 6C as a difference (ca. 300–600 ms) between the rare (0.2) and frequent (0.8) targets. Finally, the ERN can be seen in figure 6D as a more negative response (ca. −100 to 100 ms relative to the button press) for error trials compared with correct trials. Note that the ERN is typically assessed in waveforms that are time locked to the response, whereas the other components are typically assessed in stimulus-locked waveforms.
Fig. 6.
Grand average event-related potential waveforms for young adult (left) and older adult (right) participants for the comparisons of stimuli with upper vs lower field checkerboards (A), contralateral vs ipsilateral targets, relative to the electrode location (B), rare and frequent targets (C), and trials with correct and incorrect responses (D). All waveforms were time locked to stimulus onset, except that the waveforms in (D) were time locked to the button-press response. Note that each component is plotted with a different voltage scale.
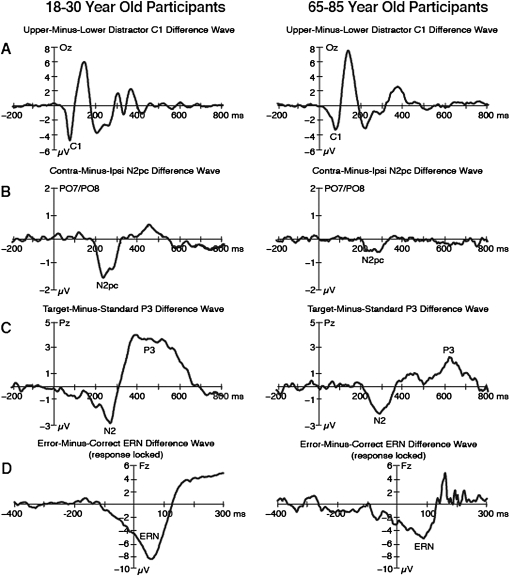
Any brain activity that is unrelated to a given factor will be equal in the ERPs for a given pair of waveforms in figure 6. Thus, brain activity that is related to a given factor can be isolated by creating a difference wave between the 2 waveforms in a pair, as shown in figure 7. Much of the brain activity is eliminated in these difference waves. For example, the overall positive voltage between 200 and 300 ms is subtracted away in the contralateral minus ipsilateral difference wave (figure 7B), making it possible to see the N2pc component as a negative-going deflection.
Fig. 7.
Grand average event-related potential difference waveforms for young (left panel) and older adult (right panel) participants for the C1 wave (A), the N2pc component (B), the P3 (C), and the error-related negativity (D). The difference waveforms represent the subtraction between the waveforms overlaid in figure 6. Note that each component is plotted with a different voltage scale.
However, a given difference wave may still contain multiple ERP components if multiple brain processes differ between the 2 parent waveforms used in the creation of the difference wave. For example, the rare-minus-frequent difference wave eliminated all of the sensory responses, which were equivalent for the rare and frequent targets, but there was also an N2 prior to the P3 in the difference wave (figure 7C) because the N2 is also sensitive to target probability.21 Thus, although the difference wave approach can be useful in isolating a component of interest, it is important to keep in mind that overlapping components may still be present in the difference wave (for further discussion, see ref. 7,10).
Assessing Interactions Between Dimensions
To determine whether the manipulations used in the present implementation of the MONSTER approach were in fact functionally orthogonal, we examined the waveforms for each component separately as a function of each of the other factors in the experiment. For the C1 wave, we compared the upper-minus-lower difference wave separately for left- and right-side targets, rare and frequent target stimuli, and correct response and error trials. For the N2pc, we compared the contralateral-minus-ipsilateral difference wave for upper and lower field distractors, rare and frequent target stimuli, and correct response and error trials. For the P3 wave, we compared the rare-minus-frequent difference wave for upper and lower field distractors, left- and right-side targets, and correct response and error trials. Finally, for the ERN, we compared the error-minus-correct difference wave for upper and lower field distractors, left- and right-side targets, and rare and frequent target stimuli.
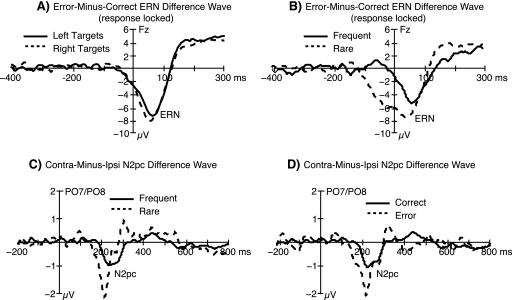
A subset of the comparisons for the ERN (upper panel) and N2pc (lower panel) are shown in figure 8. The ERN was virtually identical for the left- and right-side targets (figure 8A), indicating that the ERN was orthogonal with respect to the target side manipulation. By contrast, the error-minus-correct difference wave was substantially larger on trials with rare targets compared with trials with frequent targets (figure 8B). Examination of the correct and error waveforms separately for the rare and frequent targets revealed that this effect was driven by the P3, which occurred around the time of the response (causing it to overlap with the ERN in the response-locked averages) and was much larger for rare than for frequent target stimuli. If an individual fails to generate a P3 when making an error for a rare target, the error-minus-correct difference will reflect both the lack of a P3 on the error trials and the presence of an ERN. The coincident timing of the P3 and ERN in the present case is not surprising given the nature of the task, but the potential impact of the P3 on ERN amplitude has received scant attention in the literature.22 This is clearly an issue that deserves more careful consideration.
Fig. 8.
Grand average event-related potential difference waveforms from the young adult subjects, showing a subset of the comparisons used to assess possible interactions between dimensions in the present implementation of the MONSTER framework. The upper panel shows the error-related negativity (error minus correct difference) as a function of target side (A) and probability (B), and the lower panel shows the N2pc (contralateral minus ipsilateral difference) as a function of probability (C) and accuracy (D).
This interaction will complicate the evaluation of the ERN if the amplitude or timing of the P3 wave differs across conditions or groups of subjects. However, there is a viable solution for working around the interaction in the present case, which is to examine the ERN only on trials with frequent target stimuli, ignoring the rare target stimuli. Because the P3 to frequent target stimuli is very small at fronto-central sites, where the ERN is largest, the ERN will be uncontaminated by the P3 on frequent target trials. This reduces the number of trials contributing to the ERN difference wave but only by 20% (The exact effect of this on the signal-to-noise ratio will depend on the relative proportions of errors for the rare and frequent stimulus categories).
The differential effect of target probability can also be seen for the N2pc, which is larger for rare than for frequent target stimuli (figure 8C). This may reflect an increased allocation of attention to rare stimuli. We can also see the shared effect of target probability and errors on the N2pc by examining the N2pc separately for error and correct responses (figure 8D). Here, the N2pc is larger for error than correct response trials, but this is simply a consequence of the increased N2pc for rare target stimuli and the larger proportion of errors for rare compared with frequent target stimuli. As in the ERN, these interactions can be avoided by examining the N2pc only for frequent target stimuli.
No other interactions among dimensions in this implementation of the MONSTER approach were found, indicating that the majority of dimensions examined in the present implementation were indeed orthogonal. Determining whether interactions exist among the dimensions used in the paradigm is an important step toward establishing the true orthogonality of the comparisons and identifying potential solutions for accounting for interactions among dimensions. In other words, although true orthogonality among all dimensions is ideal, this is not always achievable or necessary to examine dimensions using the MONSTER approach, provided that interactions are taken into account in making comparisons between conditions or groups.
In our experience, target probability commonly interacts with other cognitive factors. However, because the other factors can easily be examined excluding the rare target trials, with only a slight reduction in signal-to-noise ratio, this does not preclude the use of a target probability factor in implementations of the MONSTER approach.
Assessing Group Differences
The MONSTER approach can be readily applied to assess processing in a variety of special populations, including children, older adults, and clinical samples. This can be seen from the present application of the MONSTER approach to older adults, shown in the right panels of figures 6 and 7. The older adults in this proof-of-concept study had no trouble tolerating this 40-minute task, and the components isolated by the 4 difference waves showed substantial and sensible differences between the younger and older subjects (shown in the left and right panels of figure 7, respectively). Although this experiment included only 6 subjects in each group, clear age differences were found in the amplitude of each of the 4 ERP components. First, the C1 wave—reflecting early sensory processing through primary visual cortex—was somewhat reduced in amplitude in the older adults compared with the young subjects. Second, the N2pc component—reflecting the shift of covert visual attention to the target stimulus—was attenuated and delayed in the older subjects. Third, the P3 wave was reduced in amplitude and delayed in latency in the older subjects, indicating an impairment in categorizing the targets. Fourth, the error minus correct difference wave showed a diminution and slowing of the ERN effect. Similar effects have been observed in previous experiments that focused on a single component.23,24
The advantage of the MONSTER approach is that all of these previous experiments looked at only one of these components, but we were able to isolate all 4 components in a single experiment, with a substantial number of trials per waveform. This makes it realistic to characterize individuals along multiple cognitive dimensions, which is likely to prove important in the development, assessment, and implementation of new treatments. This may prove especially useful in the study of schizophrenia, which is characterized by abnormalities in a number of ERP components, including the ERN and P3 examined in the present implementation of the MONSTER approach. Therefore, applying the present design or other implementations of the MONSTER approach both to schizophrenia and to psychiatric disorders more broadly is an important step for future research.
Funding
National Institutes of Health (R01MH076226, R01MH065034); National Science Foundation graduate fellowship (to E.S.K.).
Acknowledgments
We would like to thank Valerie Beck, Zachary Davis, Bill Gehring, Greg Hajcak, Johanna Kreither and Elsie Spilman for helpful advice and assistance. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. doi: 10.1016/j.biopsych.2010.09.021. Luck SJ, Mathalon DH, O'Donnell BF, et al. A roadmap for the development and validation of ERP biomarkers. Biol Psychiatry. 2011;70:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritsner MS, editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. Vol I. Dordrecht, The Netherlands: Springer; 2009. [Google Scholar]
- 4.Sanislow CA, Pine DS, Quinn KJ, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel TR, Cuthbert BN, Garvey MA, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 7.Kappenman ES, Luck SJ. ERP components: the ups and downs of brainwave recordings. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 8.Donchin E, Heffley EF., III . Multivariate analysis of event-related potential data: a tutorial review. In: Otto D, editor. Multidisciplinary Perspectives in Event-Related Brain Potential Research. Washington, DC: U.S. Government Printing Office; 1978. pp. 555–572. [Google Scholar]
- 9.Makeig S, Onton J. ERP features and EEG dynamics: an ICA perspective. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford; In press. [Google Scholar]
- 10.Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 11.Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci U S A. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Näätänen R, Kreegipuu K. The mismatch negativity (MMN) In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press.; In press. [Google Scholar]
- 13.Swaab TY, Ledoux K, Camblin CC, Boudewyn M. Language-related ERP components. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 14.Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, Gold JM. Impaired response selection in schizophrenia: evidence from the P3 wave and the lateralized readiness potential. Psychophysiology. 2009;46:776–786. doi: 10.1111/j.1469-8986.2009.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polich J. Neuropsychology of P300. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 16.Smulders FTY, Miller JO. Lateralized readiness potential. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 17.Clark VP, Fan S, Hillyard SA. Identification of early visually evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp. 1994;2:170–187. [Google Scholar]
- 18.Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 19.Hopf J-M, Luck SJ, Boelmans K, et al. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 21.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajcak G, Vidal F, Simons RF. Difficulties with easy tasks: ERN/Ne and stimulus component overlap. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Leipzig, Germany: MPI of Cognitive Neuroscience; 2004. pp. 204–211. [Google Scholar]
- 23.Lorenzo-Lopez L, Amenedo E, Cadaveira F. Feature processing during visual search in normal aging: electrophysiological evidence. Neurobiol Aging. 2008;29:1101–1110. doi: 10.1016/j.neurobiolaging.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Friedman D. The components of aging. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; In press. [Google Scholar]