Abstract
The sixth meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) focused on selecting promising imaging paradigms for each of the cognitive constructs selected in the first CNTRICS meeting. In the domain of perception, the 2 constructs of interest were “gain control” and “visual integration.” CNTRICS received 6 task nominations for imaging paradigms for gain control and 3 task nominations for integration. The breakout group for perception evaluated the degree to which each of these tasks met prespecified criteria. For gain control, the breakout group believed that one task (mismatch negativity) was already mature and was being incorporated into multisite clinical trials. The breakout group recommended that 1 visual task (steady-state visual evoked potentials to magnocellular- vs parvocellular-biased stimuli) and 2 auditory measures (an event-related potential (ERP) measure of corollary discharge and a functional magnetic resonance imaging (fMRI) version of prepulse inhibition of startle) be adapted for use in clinical trials in schizophrenia research. For visual integration, the breakout group recommended that fMRI and ERP versions of a contour integration test and an fMRI version of a coherent motion test be adapted for use in clinical trials. This manuscript describes the ways in which each of these tasks met the criteria used in the breakout group to evaluate and recommend tasks for further development.
Keywords: perception, CNTRICS, sensory processes, gain control, integration
Introduction
Perception was identified as one of the key domains for development of measures that could be used in clinical trials in schizophrenia at the first Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) meeting (for review see1). Within the domain of perception, 2 constructs were identified as being useful in clinical trials of cognition enhancers: gain control and integration.2 Gain control refers to processes that allow sensory systems to adapt and optimize their response levels to take into account their immediate context, to make best use of a limited dynamic signaling range. Gain control mechanisms are thought to reflect both intrinsic neuronal properties and lateral interactions between neurons. These interactions amplify or attenuate the signal and thus affect integrity of sensory registration. Integration is defined as the processes linking the output of neurons—that individually code local (typically small) attributes of a scene—into a global (typically larger) complex structure more suitable for guidance of behavior. As previously described,3 gain control and integration have a number of features that make them appropriate for clinical trials in schizophrenia including: they can be measured in humans and nonhuman animals, there is evidence of impairment in schizophrenia, and there are links to neural circuitry and pharmacology, to name a few. At the third CNTRICS meeting, paradigms that were best suited to examine the constructs of gain control and integration based on specific criteria including construct validity and psychometric characteristics were discussed and reported.3
The current article reports on the results of the discussion from the perception breakout group from the sixth CNTRICS meeting, which, as described in the introduction to this theme,1 focused on identification of promising imaging paradigms. As mentioned in that introductory article, the criteria for evaluating paradigms were derived from a survey administered to industry and academia and resulted in the following: (1) neural construct validity; (2) relationship between individual differences in brain activity and performance; (3) sensitivity to manipulation by pharmacological or psychological manipulations; (4) linked to functional outcome in schizophrenia; (5) can identify impairments in neural systems associated with the construct in schizophrenia; (6) good psychometric characteristics; and (7) good practicability and tolerability. The meeting included presentations by basic cognitive neuroscientists that revisited the individual constructs and data about the neural systems supporting them. Breakout groups consisting of scientists from academia and industry then focused on measures from individualized domains, in this case perception. They evaluated and ranked each measure using the criteria described above and selected, where possible, the top 2 or 3 measures per method (functional magnetic resonance imaging [fMRI] and electroencephalography [EEG]) to be recommended for further development. This article describes the perception tasks that were determined to best fit the criteria (table 1), as well as other tasks that were nominated but did not fit the criteria as well.
Table 1.
Perception in Schizophrenia: Neuroimaging and Neurophysiological Tasks
| Gain control: processes that allow sensory systems to adapt and optimize their response levels to take into account their immediate context, to make best use of a limited dynamic signaling range. |
| Tasks recommended for adaptation for use in clinical trial contexts |
| Steady-state visual evoked potentials to magnocellular- vs parvocellular-biased stimuli |
| Efference copy/corollary discharge: ERP paradigm |
| Prepulse inhibition of startle: fMRI paradigm |
| Already mature task |
| Mismatch negativity: ERP paradigm |
| Integration: the processes linking the output of neurons that code local attributes of a scene into global complex structure |
| Tasks recommended for adaptation for use in clinical trial contexts |
| Contour integration task: fMRI and ERP paradigms |
| Coherent motion detection task: fMRI paradigm |
Note: ERP, event-related potential; fMRI, functional magnetic resonance imaging.
For gain control, there was discussion among members of the breakout group about including paradigms, such as auditory tasks, in which there is sequential rather than simultaneous presentation of information. Examples of gain control in the first CNTRICS article on perception focused on vision,2 including the “contrast-contrast effect” which involved simultaneous presentation of stimuli. The perceived contrast of a central disk is altered when it is surrounded by a high-contrast annulus.2,3 The group agreed that tasks that rely on sequential presentation of stimuli fulfill the criteria for gain control because the sequential presentation of sound also makes use of immediate context, and responses are altered according to the sensory context.4,5
Three measures of gain control were recommended for immediate development. These were steady-state visual evoked potentials (ssVEPs) to magnocellular (M) - vs parvocellular (P) -biased stimuli and 2 auditory measures: efference copy/corollary discharge and an fMRI version of prepulse inhibition of startle (PPI) (table 1). It should be noted that the ssVEP paradigm was also chosen for immediate development in the previous CNTRICS meeting that was devoted mostly to behavioral paradigms. In the current meeting, all 3 tasks scored strongly across many of the criteria, though psychometric properties and links to functional outcome need more work. In addition to these 3 measures, the group thought that mismatch negativity (MMN) met the criteria well, but noted that this paradigm is already well established in the schizophrenia research literature and has been used in multisite trials. Hence, MMN was recommended as an already mature task (table 1). Because both the ssVEP task and MMN were described in a previous CNTRICS article,3 they will only be described briefly in this article.
Several paradigms were nominated as measures of gain control but were not recommended for immediate development. One was an fMRI version of the contrast-contrast effect paradigm. The behavioral version of this task was previously thought to have promise,3 but the group thought more work needs to be done to refine the task for fMRI. Another paradigm that was nominated but not recommended for immediate development was P50 gating. P50 gating needs magnetoencephalography (MEG) for best implementation and, thus, was not considered to be practical.6
For the construct of integration, 2 measures were recommended for immediate development. Both measures (contour integration task and coherent motion task) were previously recommended in their behavioral forms for immediate development.3 Contour integration was recommended for development as both an fMRI and an event-related potential (ERP) task and coherent motion was recommended for development as an fMRI task. It should be noted that more work has been done on these tasks in their behavioral forms and that the electrophysiology/fMRI forms are relatively new tasks. For both paradigms, work has just begun exploring the relationships between electrophysiology/fMRI data collected during task performance and functional outcomes. A visual task was nominated that investigated perception of illusory contours (Kanisza squares) vs perception of the inducing “pac-men” in the absence of illusory contours.7 This is a valuable task that allows for investigation of the degree of competition between within-group feature binding and between-element suppression—an important and relatively unexplored issue in vision science. While this task has promise for clarifying neural mechanisms in schizophrenia, it was not considered to have “clinical trial readiness” for schizophrenia research yet because: (1) there is conflicting behavioral data in the schizophrenia literature regarding whether perception of illusory shapes is intact or impaired and (2) the issue of competition targeted by the task has never been studied in schizophrenia and so it is unclear whether patients differ from normals in this regard. In the sections below, descriptions are provided of each task and how it fits the criteria. It should be noted that 2 largely unresolved issues for many of these paradigms include diagnostic specificity and standardization across sites. The latter is particularly important for clinical trials and needs to be evaluated and established for many of these paradigms.
Gain Control
Steady-State Visual Evoked Potentials to M- and P-Biased Stimuli
As mentioned above, the ssVEP task was described in the previous CNTRICS perception article 3 and so will be only briefly summarized here. The task assesses responses to stimuli designed to bias responding toward the M vs P visual pathways and was developed by Zemon and Gordon.8 The M pathway shows a steeply rising increase in response to increases in low contrast and then nearly saturates at higher contrast, whereas the P pathway does not respond until about 10% contrast and has a linear increase in response throughout the contrast range (eg, see ref.9). The nonlinear response to contrast with steep initial slope and plateau at higher contrast was first described in cat retinal ganglion cells and termed “contrast gain control.”9 Contrast gain control, which is a specific type of gain control, refers to the change in slope of the amplitude function as contrast increases and is present in M but not P neurons. The M response to contrast is an example of adapting and optimizing responses by showing high gain (slope) at low contrast and compression of responses at higher contrast (ie, divisive gain control). Divisive gain control refers to a situation in which the saturation of responses at high contrast utilizes inhibitory input from neighboring neurons to decrease response amplitude and keep it within a dynamic signaling range.8 The ssVEP task utilizes check stimuli that are kept within the low contrast region in order to emphasize contributions from the M pathway and modulates the checks around a high-static contrast to avoid the low contrast regions in order to emphasize the P pathway. M-biased ssVEP signal-to-noise ratios are correlated with behaviorally assessed contrast sensitivity10 as well as with behavioral ability to recognize facial emotions.11 N-Methyl-d-aspartate (NMDA) appears to be involved in the nonlinear contrast response function (for review, see10) and the divisive gain control at high contrast is thought to arise from Gamma Amino Butyric Acid (GABA)A-mediated shunting inhibition.12 M- and P-biased ssVEP measures correlated significantly with the Problem Solving Factor of the Independent Living Scales, a surrogate measure of community living in patients with schizophrenia and M-biased ssVEP performance also correlated significantly with scores on the Global Assessment of Functioning.10 Patients with schizophrenia show preferential deficits in M- vs P-biased ssVEP function, including lower initial steep slope and decreased plateau.10,11 Psychometric characteristics are good with the 95% CIs for 10 runs per person showing consistent data with good reliability within an individual.8 There are no ceiling effects. This paradigm shows good tolerability because it only involves one active electrode and each run takes only 7 seconds. The M- and P- contrast response functions using the ssVEP task described here are very similar to responses recorded from macaque retina and lateral geniculate nucleus (eg, see ref.3,9).
Efference Copy/Corollary Discharge
Neural Construct Validity.
Gain control over perception is implemented in a number of ways, from high-level top-down executive control of input (eg, focusing attention only to relevant features of input) to low-level bottom-up restriction of input at the end organ (eg, closing eyes). Automatic implementation of gain control leaves more resources for planning and evaluation of responses. A low-level mechanism has evolved to suppress sensations resulting from our own actions, and as such, is an example of gain control because it attenuates the sensory signal. Conceptually, it involves a “forward model”13 of the imminent sensations that will result from our actions and is called “efference copy,” “corollary discharge,”14 or “efference copy/corollary discharge.” Some use efference copy to refer to a copy of the motor command and corollary discharge to refer to the expected sensation resulting from action.15
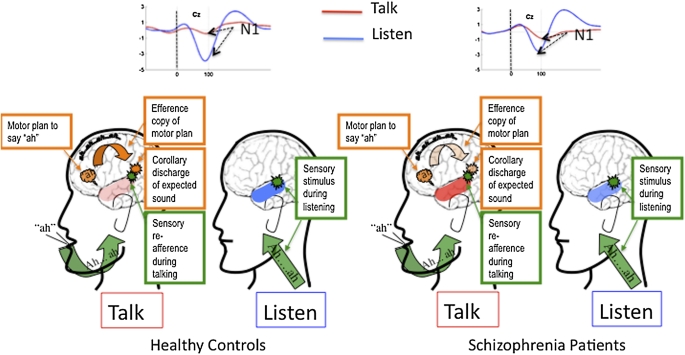
An example of this mechanism is illustrated in figure 1. In this example, a split second before a person speaks, motor cortex sends out 2 neural signals: a motor command to produce the sound and an efference copy of that plan, which in turn generates a corollary discharge in auditory cortex of the expected sound. As a result of the action of this mechanism, not only are sensations resulting from our actions suppressed but also they are tagged as coming from “self.”
Fig. 1.
(Left) Shows a cartoon profile of a healthy control subject talking (saying “ah”) and listening to a playback of ah. Above the heads, event-related potentials (ERPs) recorded from the vertex (Cz) elicited by the onset of the speech sound (dotted vertical line) during talking (red lines) and listening (blue lines) are shown. During talking, N1 to the speech sound is suppressed relative to N1 to the same sound during listening. Amplitude (microvolts) is on the y-axis and time (milliseconds) is on the x-axis. Vertex negativity is plotted down. The N1 of the ERP is generated in auditory cortex (colored orange during Talk and blue during Listen). Intensity of the color in the auditory cortex denotes the strength of the response to the speech sound. The intention to say ah is indicated as an orange “thought bubble” over Broca’s area. The orange curved arrow pointing from Broca’s area to auditory cortex indicates the transmission of the efference copy of the motor plan, which produces a corollary discharge (orange burst) of the expected sensation in auditory cortex. When the expected sensation (corollary discharge) matches the actual sensation in auditory cortex (green burst), perception is suppressed. (Right). The same is shown for schizophrenia patients, with a relative failure of the efference copy and corollary discharge being shown as faded orange. The slightly more intense orange color in auditory cortex during talking indicates relatively less suppression of the auditory cortical response to the spoken sound. The slightly less intense blue color during listening indicates an overall tendency of patients to generate a smaller N1 to sounds during passive listening.
In higher order animals, it also provides a mechanism for self-monitoring and action corrections in real time. For example, Eliades and Wang16 pointed out that this mechanism is useful for discrimination between self-generated and external sounds, as well as vocal convergence, allowing monkeys to match their vocalizations to their cage-mate’s. It flexibly allows us to both ignore expected sensations from our fingers as we type and heighten those sensations as we read Braille.
In their review article “Corollary Discharge Across the Animal Kingdom,” Crapse and Sommer14 concluded: “In addition to the usual flow of information from sensory systems to motor systems, there is extensive signaling in the opposite direction by motor systems reporting their activities to sensory structures. It is this coordination between the 2 systems that makes it possible to analyze the world while moving within it.”
In the primate auditory system, this mechanism has been studied using electrophysiological methods. Evidence of its successful action is seen as suppression of sensory responsiveness. Eliades and Wang16 recorded from primary auditory cortex single units in marmoset monkeys during vocalization and reported suppression of most units beginning before vocalization, with excitation of different units beginning shortly after onset of vocalization. In humans, this is best seen as a reduction of the amplitude of the N1 component of the ERP or its MEG counterpart, during talking compared with listening (reviewed in4), as illustrated in figure 1. However, because of the poor spatial resolution of the EEG and ERP signals, it is possible that activity recorded at the scalp is a combination of the motor command, the efference copy, the corollary discharge, and auditory cortical response to the spoken sound. While these signals are difficult to disentangle with scalp recordings, N1 suppression has been seen in recordings directly from auditory cortex in patients being evaluated for surgical removal of epileptogenic brain tissue.17
While reduction of the amplitude of N1 during talking signals the successful action of this mechanism, it has been suggested that synchrony of neural activity (in the gamma or beta range) preceding the action may be the instantiation of the efference copy being sent from motor to sensory areas.15 Whether it reflects the motor command or the efference copy itself, the neural activity preceding speech becomes synchronized about 100 ms before speech onset, and the amount of prespeech synchrony is directly related to the subsequent degree of auditory cortical suppression during speech.15 Similarly, premovement changes in neural activity have been shown to index efference copy/corollary discharge mechanisms in the visual system in nonhuman primates.14
It should be noted that the auditory N1 has also been studied in a simple form in response to trains of tones in patients with schizophrenia.18 In healthy controls, the amplitude of the N1 to a given tone increases as the interstimulus interval (ISI) between tones increases, reflecting increased gain. Patients with schizophrenia do not demonstrate the same gain function in N1 amplitude with ISI increases.18 In fact, N1 amplitude reductions in schizophrenia are greatest at long ISIs when gain is high.18
Relationship Between Individual Differences in Brain Activity and Performance.
The “Talking/Listening paradigm,” illustrated in figure 1, assesses suppression of N1 to the spoken sound during talking compared with when the subject is listening to a recording of previously spoken sounds.4 It is the human version of similar paradigms used with nonhuman primates, and suppression of cortical responses during talking is believed to reflect the successful action of the efference copy/corollary discharge mechanism.16 One advantage of this paradigm is that everyone who can talk can do it. Thus, there is not much variability in performance. However, when performance is perturbed by pitch-shifting the sound in real time, there is less suppression of auditory cortical response. That is, if what you said does not match what you hear, auditory cortex wants to know about it19!
Sensitivity to Manipulation by Pharmacological or Psychological Manipulations.
If pitch is perturbed during speaking in real time over several days, subjects begin to make subtle and unconscious adjustments in their speech to compensate for the perturbations.20 Converging lines of evidence suggest that deficits in the NMDA subtype of glutamate receptors may contribute to the negative symptoms and cognitive deficits seen in schizophrenia. Accordingly, data comparing responses during ketamine infusions have been collected and are being analyzed.
Linked to Functional Outcome in Schizophrenia.
Data comparing high- and low-functioning patients have been collected and are being analyzed.
Impairments in Neural Systems Associated With the Construct in Schizophrenia.
Neurophysiological evidence for dysfunction of the corollary discharge system in schizophrenia has been documented in auditory and somatosensory modalities (reviewed in4) and is consistent with the thinking of Feinberg.21 Using the N1 component of the ERP in the “talking/listening paradigm” described above, the normal dampening of the auditory cortical response during talking is less evident in patients with schizophrenia (eg, see ref.15). This is illustrated in figure 1.
Psychometric Characteristics.
Analyses of test-retest reliability are underway.
Practicability and Tolerability.
The paradigm, including how to set up the equipment to record data and how to analyze the data, has been described in detail.4 The task itself is well tolerated by subjects. Unlike paradigms involving passive listening or responding with a simple button press, talking is more activating and keeps subjects more alert.
Animal Models.
Corollary discharge has been studied in animals for 60 years, as described in the review by Crapse and Sommer.14 Only in the last decade has it been studied in humans.
Prepulse Inhibition
Neural Construct Validity.
The startle response is a set of reflexive responses to strong, sudden acoustic, or tactile stimuli that can be studied in all mammals. PPI is typically used as an operational measure of “sensorimotor gating” because it involves both sensory stimuli and motor responses.22,23 Although the CNTRICS group considered PPI to be an “already well-developed” psychophysiological measure of perceptual gain control since it reflects the adaptation of neural systems to immediately preceding events and context,3 more work is needed to develop PPI further for use in imaging environments. In PPI, the startle response elicited by a startling stimulus is measured in the presence or absence of a weak prepulse stimulus, which can be in the same or a different modality. The weak prepulse strongly inhibits the response to the subsequent startling stimulus. In humans, the eyeblink component of startle is typically assessed by electromyography (EMG).
Relationship Between Individual Differences in Brain Activity and Performance.
In both animals and humans, behavioral measures of PPI are correlated with specific aspects of cognitive performance, primarily related to speed of processing and distractibility. In the PPI imaging paradigm, nicotine alters PPI in healthy subjects and patients with schizophrenia, with performance being correlated with hippocampal activation.24 More such work is needed to assess the utility of PPI in imaging environments.
Sensitivity to Manipulation by Pharmacological or Psychological Manipulations.
Although rather insensitive to psychological manipulations, behavioral measures of PPI are sensitive to pharmacological manipulations, including both experimental drug challenges and acute and chronic pharmacotherapeutic interventions.22,23 Typical and atypical antipsychotic treatments have been demonstrated to differentially affect both the EMG and fMRI imaging measures in PPI paradigms in patients with schizophrenia.25
Linked to Functional Outcome in Schizophrenia.
Recent studies indicate some positive correlation of EMG measures of PPI with measures of functional outcome, but results with the fMRI version are not known at this time.
Impairments in Neural Systems Associated With the Construct in Schizophrenia.
PPI is reduced in patients with schizophrenia and other disorders characterized by deficits in gating, such as bipolar mania, panic disorder, and Huntington’s Disease.22 While most of this work has focused on the EMG measures of PPI, limited comparisons of psychiatric patient populations have also detected differences in fMRI measures of PPI.25,26 Because PPI is relatively homologous across species, neurobiological mechanisms are well understood in animals and are being confirmed in human imaging studies.26–28 PPI is modulated by the limbic cortex (medial prefrontal cortex, amygdala, and ventral hippocampus), the thalamus, the ventral striatum (nucleus accumbens), the ventral pallidum, and the pontine tegmentum. fMRI studies have shown that most of these regions are altered by startle and/or PPI in humans, some being affected differentially in schizophrenia patients relative to control subjects.25,26
Psychometric Characteristics.
Extensive studies have demonstrated that behavioral measures of PPI exhibit excellent parametric sensitivity, multisite comparability, and test-retest reliability in both humans and animals.29 Further work is needed to extend these findings to the fMRI paradigm.
Practicability and Tolerability.
Startle and PPI tests are robust, readily quantitated, automated, and well tolerated. In fMRI or positron emission tomography (PET) settings, both acoustic and tactile (eg, airpuffs to neck or arms) stimuli have been used for prepulse and startle stimuli. Early fMRI and PET studies of PPI utilized blocked-trial designs,26 with only the PET paradigms using concurrent EMG measurement of the eyeblink. Movement artifacts have not proven problematic. Given concerns about the noisy MRI environment, tactile stimuli are preferred, although acoustic stimuli have been used successfully without simultaneous EMG recordings.27 Recent fMRI work, however, has shown that single-trial event-related PPI-fMRI paradigms with concomitant EMG recordings are also feasible.28
Animal Models.
An enormous literature details the genetic, pharmacological, developmental, and neuroanatomical influences on PPI in rodent models and to some extent in infrahuman primates.23
Mismatch Negativity
As mentioned above, MMN is a well-established measure for schizophrenia research and was described in the previous CNTRICS perception article.3 MMN is an auditory ERP response reflecting the automatic detection of auditory deviance and likely reflecting sensory auditory echoic memory5; (see30,31 for reviews). It occurs whether or not stimuli are attended or task relevant and is elicited when a series of standard auditory stimuli are interrupted by randomly interspersed infrequent deviant stimuli. The response to the deviant is larger than the response to the standard, so that MMN can be observed as the difference between responses to the standards and deviants.
Infrequent stimuli that elicit MMN can differ from standards in duration, pitch, or intensity. MMN is thought to be a measure of gain control because the auditory cortical response to a given auditory stimulus depends on whether the stimulus is deviant with respect to the temporal context created by surrounding stimuli presented in the auditory stream. In addition, the amplitude of MMN will increase to a given stimulus as the context of surrounding “standard” auditory stimuli render it more deviant (eg, greater deviations in pitch, intensity, or duration between standard and deviant stimuli are associated with larger MMN amplitudes to the deviant stimulus). Thus, MMN reflects neural adaptation to the immediate context.
Primary neural generators for MMN have been localized to primary and secondary auditory cortices using ERP, MEG, and fMRI techniques (for review see30). A relationship between individual differences in brain activity and performance is seen in significant correlations between MMN and behavioral performance on a tone-matching task.32 MMN deficits have been extensively replicated in schizophrenia and have an effect size of approximately 1 SD (see ref.30,31 for reviews). Deficits are seen in both medicated and unmedicated patients with schizophrenia.30 NMDA appears to be important both for the generation of MMN, as seen in studies using NMDA antagonists in monkeys and humans.30 MMN is linked to functional outcome in patients with schizophrenia as seen in relationships with the Global Assessment of Functioning scores and a measure of independent living.33 Psychometric characteristics of MMN are very good and include high test-retest reliability and stability over time.3 Practicability and tolerability are excellent for an ERP paradigm. Indeed, MMN requires no active attention and can be obtained while the participant is doing a concomitant visual task. Finally, MMN has been extensively investigated in animal models and has been recorded from rats, cats, chimpanzees, and monkeys.3
Integration
Contour Integration Task
Neural Construct Validity.
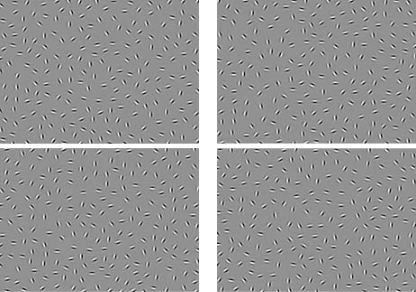
Visual integration has been successfully studied using variants of a contour integration task (see figure 2). Here, participants are typically asked to either identify the location of a straight or circular contour within a background of randomly oriented Gabors or to determine in which direction an egg-shaped contour is pointing. Gabor elements are Gaussian-modulated sinusoidal luminance distributions that closely model the known spatial frequency processing properties of cells in area V1. They are therefore ideal for examining properties of interactions among these cells, such as those observed during visual integration. The embedded contours in stimuli employing Gabor elements cannot be detected by feature or spatial frequency detectors in V1 or by the known types of orientation-tuned neurons with large receptive fields.
Fig. 2.
Samples of images from the contour integration task. Top left: 0 jitter, top right: 7–8 jitter, bottom left: 11–12 jitter, and bottom right: 15–16 jitter.
In a recently developed behavioral version of the task, stimuli are presented in blocks that differ in difficulty level; difficulty is manipulated by jittering the orientation of the contour elements from their original position34 (see figure 2). Because contour element orientation deviates more, the ability to perceive the circular shape is reduced. Recent work has focused on optimizing the task to increase its sensitivity to patient-control differences and to create a version with the minimum number of trials necessary for group discrimination. For example, in a recent multisite behavioral study by the Cognitive Neuroscience Task Reliability and Applications Consortium (CNTRACS), the task was modified to include more conditions at an intermediate level of difficulty and to eliminate conditions associated with floor or ceiling effects. This version of the task, which is now called the Jittered-Orientation Visual Integration Test, version 2 (JOVI-2), uses 5 conditions of jitter: 7–8°, 9–10°, 11–12°, 13–14°, and 15–16°. The JOVI-2 uses 48 trials at each of the 5 conditions for a total of 240. Each trial involves a 2-second stimulus presentation followed by a 1-second presentation of a blank gray screen. Including practice trials and instructions, the JOVI-2 typically takes 12–15 minute. Note however that data from the first 120 trials (ie, 24 from each condition) produces results that are equal to those of the full version.
The behavioral findings using contour integration tasks are supported by behavioral studies in healthy people,35 and microelectrode studies in visual cortex in animals36 that indicate excitatory (facilitating) effects of flanker elements with orientations similar to, or strongly correlated with, a target element, and inhibitory effects of random orientation surrounds. These effects are markedly reduced in patients with schizophrenia.35 fMRI data in humans and monkeys indicate a visual cortex basis for contour integration.34 In particular, areas V1, V2, V3, V4, and the lateral occipital complex (LOC) are significantly activated when processing Gabor-defined contours compared with a field of randomly oriented Gabor elements.
A recent ERP study in humans, in which participants are asked to identify the direction of the contour, showed a much greater negative peak at approximately 285 ms for the low-jitter, easy to identify, contours than for the high-jitter, difficult to identify, contours. A subtraction of the high-jitter from the low-jitter negativity provides a measure of contour integration.37 This ERP component was previously termed the Ncl for closure negativity and was found to occur when fragmented pictures can be identified.38 Thus, the Ncl appears to be important for both perceptual closure and contour integration. Source localization has identified the contour integration Ncl in the lateral occipital object recognition area, which is located in ventral fusiform gyrus (for review, see38).
Relationship Between Individual Differences in Brain Activity and Performance.
In the ERP version of contour integration, a greater Ncl was significantly related to ability to identify the direction of the Gabor contour.37
Sensitivity to Manipulation by Pharmacological or Psychological Manipulations.
Preliminary evidence that the behavioral task may be sensitive to medication effects and/or clinical changes comes from 2 studies (see,39 for review). In one, patients were tested on admission to an acute care inpatient unit and then again at discharge (∼3 wk later). For schizophrenia patients with disorganized symptoms, the only group that demonstrated impairment on admission, performance improved significantly during treatment. Moreover, degree of normalization of contour integration was significantly correlated with degree of reduction of disorganized (but not positive or negative) symptoms. In a second study, ketamine (a noncompetitive NMDA receptor antagonist) users were tested on a contour integration test the night of ketamine use and then again 3 days later. Task performance was abnormal only on the night of ketamine use. These data are consistent with the hypothesis that contour integration impairment is related to altered cognitive coordination (ie, context-based modulation of feedforward input), secondary to NMDA receptor hypofunction (see,39 for review).
Linked to Functional Outcome in Schizophrenia.
No studies have been carried out examining links to functional outcome using fMRI or ERP recording with the contour integration task.
Impairments in Neural Systems Associated With the Construct in Schizophrenia.
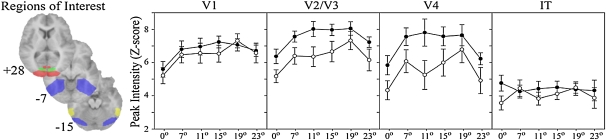
The contour integration task has shown evidence of impairment in schizophrenia in all behavioral studies in which it has been used.39 A recent fMRI study34 found that among controls and patients, regions known to be involved in contour processing (V1, V2/V3, and V4) were most sensitive to the jitter manipulation, with activity increasing over baseline as the demand on contour integration increased; once the contours could not be perceived, however, activity returned to baseline levels. Moreover, in all comparisons except one, controls and patients were equivalent in their signal change in area V1. In contrast, signal strength was consistently relatively reduced in patients in higher visual cortex areas, with the group differences increasing from V2 to V4 (see figure 3). This suggests that it was processing of larger areas of (fragmented) curvature that was impaired in patients, as opposed to linking of small numbers of closely spaced elements.40 In a recent ERP study, patients with schizophrenia showed a significantly reduced Ncl compared with controls to contour stimuli.37 This indicates that patients with schizophrenia are not able to utilize the same circuitry, including the LOC, as controls to integrate the Gabor patches into a contour. In summary, the contour integration test is sensitive to schizophrenia and seems to reliably distinguish patient from control groups on behavioral, ERP, and fMRI indices.
Fig. 3.
Regions of interest for extraction of the peak signal change (Y-axis) within V1 (green), V2/V3 (red), V4 (blue), and inferotemporal cortex (IT) (yellow). Peak signal intensity for controls (black circles) and patients (white circles) for areas involved in visual processing. X-axis depicts degree of orientation jitter of contour elements. Figure reprinted with permission from Imperial College Press, ©2009.34
Psychometric Characteristics.
The test-retest reliability of the JOVI, in terms of both test scores and fMRI data, are currently being investigated as part of the CNTRACS study, and these data have been presented at meetings and should be available on the CNTRACS website in 2011. In these CNTRACS studies, the JOVI was found to have strong internal consistency and discriminating power in terms of differentiating patients from controls. Moreover, group differences, providing further evidence for validity, were demonstrated at 5 sites, and there were no site differences in behavioral data. Past behavioral data indicate good reliability and minimal practice effects.39 No data are available on psychometric characteristics of the ERP version.
Practicability and Tolerability.
The behavioral contour integration test has good practicability and tolerability. As noted above, the JOVI-2 is relatively brief, and valid data can be obtained using only half of the trials. The ERP version takes approximately 30 minute to administer and consists of approximately 300 trials each of low- and high-jitter stimuli, and thus has reasonable tolerability and practicability for an ERP procedure.
Animal Models.
The role of contextual interactions in contour linking has been convincingly demonstrated in nonhuman primates and in cat,41 and these data fit with computational models of contour integration.42 Also, as noted above, fMRI studies have demonstrated similar visual cortex regions as being active during contour integration in healthy humans and macaque monkeys, and these same regions show reduced activity during task performance in schizophrenia patients.34
Visual Motion Processing in Schizophrenia
Neural Construct Validity.
fMRI paradigms for visual motion processing were developed for understanding brain mechanisms underlying this perceptual domain.43 One representative fMRI paradigm measures cortical activations for 2 different aspects of motion perception—detection of coherent motion and speed discrimination.44 Detection of coherent motion requires perceptual judgments of the direction of motion from a random dot pattern. Speed discrimination requires discerning which of 2 low-spatial frequency gratings moves faster (the gratings are identical except for speed). Performance of the motion perception tasks is supported by the visual areas including the striate and extrastriate cortices.
Application of the fMRI paradigms, including the one recommended by CNTRICS,44 identifies middle temporal area (MT) in the extrastriate cortex as the center for visual motion processing in humans. While other cortical regions such as the striate cortex (V1) are also involved, only MT responds selectively to motion signals. The fMRI result agrees with the findings from previous neurophysiological and brain lesion studies,45 thus validating the fMRI paradigm for studying the neural construct of normal and abnormal visual motion processing.
Relationship Between Individual Differences in Brain Activity and Performance.
A linear relationship has been demonstrated between the cortical activations in MT, measured using blood oxygenation level-dependent, and the strength of motion signal (ie, the coherence level of random dot patterns).43 This relationship is highly consistent with the relationship between the magnitudes of neuronal response in MT, measured using electrophysiological paradigms,46 and the strength of motion signal. However, further work with larger samples of patients and with the specific paradigm nominated by CNTRICS is necessary.
Sensitivity to Pharmacological or Psychological Manipulations.
Our knowledge about the effects of pharmacological and psychological factors on fMRI responses to visual motion signals is limited at this point, though behavioral sensitivity to motion signals can be modified by various types of neurotransmission such as serotonin.47 In addition, behavioral training such as perceptual learning can significantly enhance the capacity of motion perception,48 yet its roles on the fMRI response to motion signals has not been examined.
Linked to Functional Outcome in Schizophrenia.
While motion processing provides sensory information for a variety of real world activities including social interaction, little work has been done on this in schizophrenia. There is currently no fMRI study examining relationships between motion processing and functional outcome in schizophrenia. One recent study showed that perceptual detection of coherent motion, among a series of visual perception measures, is only indirectly related to real-world functioning measures through social perception in schizophrenia.49
Impairments in Neural Systems Associated With the Construct in Schizophrenia.
Neuroimaging has been used to examine motion processing in schizophrenia. Applying fMRI in conjunction with psychophysically defined motion perception tasks, the paradigm recommended by CNTRICS has demonstrated that cortical activations in patients were significantly decreased in MT and significantly increased in the inferior convexity of prefrontal cortex (ICPFC).44 The altered pattern of cortical activation occurred during 2 motion discrimination tasks (direction and speed) but not during a nonmotion visual discrimination task (contrast). This result has 2 implications. First, it highlights a specific functional cortical abnormality in the visual motion domain in schizophrenia. Second, it points to not only the sensory (ie, MT in the posterior cortex) but also putative cognitive (ie, ICPFC in the anterior cortex) systems as neural substrates for motion processing dysfunction in schizophrenia. In behavioral studies,50 deficient performances on motion discrimination have been found in schizophrenia patients.
Further neuroimaging studies should consider combining MRI (high spatial resolution) and EEG/MEG (high temporal resolution) so that in both spatial and temporal domains, cortical responses to motion information can be more precisely evaluated in schizophrenia.
Psychometric Characteristics and Practicality.
Implementation of the fMRI motion paradigms is straightforward, from both the experimenters’ and subjects’ perspectives, because it deals with simple and concrete stimuli and tasks. Yet, reliability and tolerability of the fMRI motion paradigm have not been formally evaluated in schizophrenia. Psychometric characteristics of the behavioral motion perception paradigm are also only sparsely evaluated.50 Additional work is needed to adapt this laboratory-based fMRI research paradigm for clinical settings.
Animal Models.
Motion processing in mammals including primates has been widely studied using electrophysiological methods. In monkeys, application of the fMRI motion paradigms has yielded results similar to that in humans.43 Across species, motion processing is mediated by very similar neural mechanisms. These animal studies focus primarily on physiology of normal motion processing and remain to be adapted to schizophrenia research.
Funding
National Institutes of Health (MH84848 to P.D.B., MH61824 to Y.C., MH58262 and MH067967 to J.M.F., MH052885 to M.A.G., MH084828 to S.M.S., MH043292 to M.F.G.); Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center to M.F.G. and M.A.G.; VA Merit to J.M.F.
Acknowledgments
We thank all the people who nominated tasks and participated in the breakout discussion. We also thank Dr Daniel C. Javitt, Dr Daniel Mathalon, and Dr Vance Zemon for their helpful comments about the manuscript. The authors report the following conflicts of interest: Dr Geyer has received consulting compensation from Omeros, San Diego Instruments, Takeda, and Teva. Drs Butler, Chen, Ford, Silverstein, and Green have no conflicts of interest in relation to the subject of this study.
References
- 1.Carter CS, Barch DM, and the CNTRICS Executive Committee Imaging biomarkers for treatment development for impaired cognition: report of the sixth CNTRICS meeting: biomarkers recommended for further development. Schizophr Bull. 2012;38:26–33. doi: 10.1093/schbul/sbr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MF, Butler PD, Chen Y, et al. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JM, Roach BJ, Mathalon DH. How to assess the corollary discharge in humans using non-invasive neurophysiological methods. Nat Protoc. 2010;5:1160–1168. doi: 10.1038/nprot.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naatanen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biol Psychol. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- 6.Lu BY, Edgar JC, Jones AP, et al. Improved test-retest reliability of 50-ms paired-click auditory gating using magnetoencephalography source modeling. Psychophysiology. 2007;44:86–90. doi: 10.1111/j.1469-8986.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 7.McMains SA, Kastner S. Defining the units of competition: influences of perceptual organization on competitive interactions in human visual cortex. J Cogn Neurosci. 2010;22:2417–2426. doi: 10.1162/jocn.2009.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zemon V, Gordon J. Luminance-contrast mechanisms in humans: visual evoked potentials and a nonlinear model. Vision Res. 2006;46:4163–4180. doi: 10.1016/j.visres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Shapley RM, Victor JD. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. J Physiol. 1981;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler PD, Abeles IY, Weiskopf NG, et al. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull. 2009;35:1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 13.Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br J Psychiatry. 1988;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- 14.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 16.Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen C-M, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM. The efference copy in humans is reflected in synchronous neural oscillations. J Cogn Neurosci. 2010; published online ahead of print 14 October 2010. doi: 20946054. [Google Scholar]
- 18.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 19.Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- 20.Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 22.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 23.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 24.Postma P, Gray JA, Sharma T, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl) 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- 25.Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- 26.Kumari V, Gray JA, Geyer MA, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 27.Campbell LE, Hughes M, Budd TW, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- 28.Neuner I, Stocker T, Kellermann T, et al. Electrophysiology meets fMRI: neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Hum Brain Mapp. 2010;31:1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow NR, Sprock J, Light GA, et al. Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2007;92:237–251. doi: 10.1016/j.schres.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 33.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009;8:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- 35.Keri S, Kelemen O, Benedek G. Attentional modulation of perceptual organisation in schizophrenia. Cogn Neuropsychiatry. 2009;14:77–86. doi: 10.1080/13546800902757936. [DOI] [PubMed] [Google Scholar]
- 36.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 37.Abeles I, Sehatpour P, Dias EC, Ross M, Javitt DC, Butler PD. An Event Related Potential Investigation of Contour Integration Deficits in Schizophrenia. San Diego, CA: Society for Neuroscience Abstracts; 2010. [Google Scholar]
- 38.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 39.Silverstein SM, Keane BP. Perceptual organization in schizophrenia: plasticity and state related change. Learn Percept. 2009;1:229–261. [Google Scholar]
- 40.Connor CE, Brincat SL, Pasupathy A. Transformation of shape information in the ventral pathway. Curr Opin Neurobiol. 2007;17:140–147. doi: 10.1016/j.conb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Piech V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50:951–962. doi: 10.1016/j.neuron.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Kay JW, Phillips WA. Coherent infomax as a computational goal for neural systems. Bull Math Biol. 2010;73:344–372. doi: 10.1007/s11538-010-9564-x. [DOI] [PubMed] [Google Scholar]
- 43.Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Grossman ED, Bidwell LC, et al. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cogn Affect Behav Neurosci. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maunsell JHR, Newsome WT. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- 46.Britten K, Shadlen M, Newsome W, Movshon J. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX. Psilocybin impairs high-level but not low-level motion perception. Neuroreport. 2004;15:1947–1951. doi: 10.1097/00001756-200408260-00023. [DOI] [PubMed] [Google Scholar]
- 48.Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- 49.Brittain P, Ffytche DH, McKendrick A, Surguladze S. Visual processing, social cognition and functional outcome in schizophrenia. Psychiatry Res. 2010;178:270–275. doi: 10.1016/j.psychres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]