Abstract
The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia initiative, funded by an R13 conference grant from the National Institute of Mental Health, has sought to facilitate the translation of measures from the basic science of cognition into practical brain-based tools to measure treatment effects on cognition in schizophrenia. In this overview article, we summarize the process and products of the sixth meeting in this series, which focused on the identification of promising imaging paradigms, based on the measurement of cognitive evoked potentials (event-related potential) of cognition-related time-frequency analyses of the electroencephalography as well as functional magnetic resonance imaging. A total of 23 well-specified paradigms from cognitive neuroscience that measure cognitive functions previously identified as targets for treatment development were identified at the meeting as being recommended for the further developmental work needed in order to validate and optimize them as biomarker measures. Individual paradigms are discussed in detail in 6 domain-based articles in this volume. Ongoing issues related to the development of these and other measures as valid, sensitive and reliable measurement, and assessment tools, as well as the steps necessary for the development of specific measures for use as biomarkers for treatment development and personalized medicine, are discussed.
Keywords: CNTRICS, cognition, cognitive neuroscience, neuroimaging, biomarker, EEG/ERP, fMRI, schizophrenia
Despite an acute awareness of the disabling nature of cognitive impairments in schizophrenia, an increasing recognition of the treatment refractoriness of this aspect of the illness and a growing understanding of the cognitive and neural systems that underlie these deficits, very little progress has been made in developing effective treatments.1
Background of CNTRICS
In order to leverage the great advances that have been made over the last 3 decades in our understanding of the neurobiology of normal cognition, together with our growing understanding of potential pathophysiological processes in schizophrenia, it is necessary to approach the vertically tiered translational research processes with a common language and set of measurement tools that cross many different levels of analysis.2 Initial efforts to develop measurement tools for clinical trials of cognitive enhancing agents in schizophrenia led to the development of the Measurement Approaches to the Treatment of Impaired Cognition in Schizophrenia (MATRICS) battery. These clinical neuropsychological measures were selected because of their well-characterized psychometric properties and their history of use in clinical trials in schizophrenia. The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative, supported by an National Institute of Mental Health (NIMH) R13 conference grant, has sought advance our ability to measure treatment effects on cognition in schizophrenia by facilitating the development of a set of measures of cognitive functioning that are adapted from those used in basic cognitive neuroscience.3 Such measures have the advantage of being linked to the function of discrete cognitive and neural systems, with unique cellular and molecular characteristics that are the potential targets for treatments. However prior to CNTRICS, there was no consensus as to which cognitive and neural systems ought to be targeted for treatment in schizophrenia, no established “standards” for practical and reliable task administration in the treatment development setting, and minimal data on the psychometric properties of experimental cognitive tasks that could guide the selection of paradigms and their incorporation into treatment studies.4,5
The goal of CNTRICS has been to come up with specific measures and paradigms that could be used to measure specific aspects of cognition. These measures were required to have high construct validity for targeting specific cognitive processed generally agreed to be impaired in schizophrenia. During the first cycle of the initiative, 3 meetings were held that employed a consensus-based process that included premeeting questionnaires, the development of specific criteria to guide group decision making, presentations by basic cognitive neuroscientists, and group decision making by attendees who represented academia, the pharmaceutical industry, and the NIMH. During the course of these 3 meetings, a set of cognitive constructs (component processes underlying different aspects of several broad cognitive domains) were identified3 to be targeted for measurement, the psychometric and practical measurement issues related to using experimental cognitive tasks were explored, and guidelines were developed,6,7 and finally, a set of cognitive tasks were identified and recommended for further development as cognitive neuroscience based measurement tools.6 A detailed list of these tasks, organized according the cognitive systems or constructs that they measure, is shown in table 1. Further details including operational definitions of these cognitive constructs and detailed descriptions of the tasks may be found in the special issues of Biological Psychiatry (volume 64 (1) 2008) and Schizophrenia Bulletin (volume 35 (1) 2009) and on the CNTRICS web site at cntrics.ucdavis.edu.
Table 1.
CNTRICS I: Constructs, Definitions, and Promising Tasks in Humans
| Perception | |
| Gain control: The processes whereby neurons adapt their response levels to take into account their immediate context, in order to make best use of a limited dynamic signaling range. | |
| Promising tasks in humans: | |
| Contrast-Contrast Effect (CCE) task | |
| Contrast sensitivity + steady-state visual evoked potentials to magnocellular vs parvocellular biased stimuli | |
| Mismatch negativity | |
| Prepulse inhibition of startle | |
| Integration: The processes linking the output of neurons—that individually code local (typically, small) attributes of a scene—into global (typically, larger) complex structure, more suitable for the guidance of behavior. | |
| Promising tasks in humans: | |
| Coherent motion detection task | |
| Contour integration task | |
| Working Memory | |
| Goal maintenance: The processes involved in activating task related goals or rules based on endogenous or exogenous cues, actively representing them in a highly accessible form, and maintaining this information over an interval during which that information is needed to bias and constrain attention and response selection. | |
| Promising tasks in humans: | |
| AX-CPT/dot pattern expectancy task(DPX) | |
| Interference control: The processes involved in protecting the contents of working memory from interference from either other competing internal representations or external stimuli. | |
| Promising tasks in humans: | |
| Recent probes task | |
| Operation span/symmetry span | |
| Attention | |
| Control of attention: The ability to guide and/or change the focus of attention in response to internal representations | |
| Promising tasks in humans: | |
| Guided search | |
| McGaughy and Sarter sustained attention task | |
| Executive control | |
| Rule generation and selection: The processes involved in activating task related goals or rules based on endogenous or exogenous cues, actively representing them in a highly accessible form, and maintaining this information over an interval during which that information is needed to bias and constrain attention and response selection. | |
| Promising tasks in humans: | |
| 1–2 AX-CPT | |
| ID/ED (Intradimensional/Extradimensional) Task | |
| Dynamic adjustments of control: The processes involved in detecting the occurrence of conflict or errors in ongoing processing, identifying the type of control adjustments needed, and recruiting additional control processes | |
| Promising tasks in humans: | |
| Stop signal task | |
| Stroop task | |
| Long-term memory | |
| Relational encoding and retrieval: The processes involved in memory for stimuli/elements and how they were associated with coincident context, stimuli, or events. | |
| Promising tasks in humans: | |
| Associative inference | |
| Relational (and Item Specific)Encoding and Retrieval Task (RISE) | |
| Item encoding and retrieval: The processes involved in memory for individual stimuli or elements irrespective of contemporaneously presented context or elements. | |
| Promising tasks in humans: | |
| Relational (and Item Specific)Encoding and Retrieval Task (RISE) | |
| Reinforcement learning/motivation | |
| Acquired behavior as a function of both positive and negative reinforcers, including the ability to (a) associate previously neutral stimuli with value, as in Pavlovian conditioning; (b) rapidly modify behavior as a function of changing reinforcement contingencies, and (c) slowly integrate over multiple reinforcement experiences to determine probabilistically optimal behaviors in the long run. | |
| Promising tasks in humans: | |
| PIzzagalli reward task | |
| Probabilistic reversal learning | |
| Probabilistic selection task | |
| Social/emotional processing | |
| Affective recognition and evaluation: The ability to detect, recognize, and judge the affective value of both linguistic (eg, seen or spoken words and their prosodic contour) and nonlinguistic (eg, images of people, facial expressions, eye gaze, scenes) stimuli. | |
| Promising tasks in humans: | |
| Facial affect recognition and the effects of situational context | |
| Penn emotion recognition task | |
Developmental Pathways for Biomarker Development
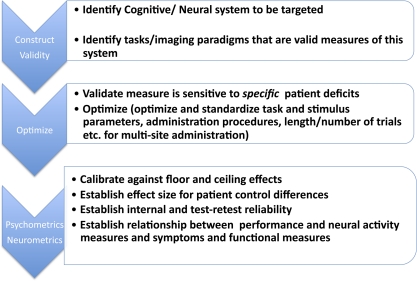
There is a lengthy developmental path that must be pursued in order to move from the identification of cognitive mechanisms to be targeted to the production of ready to use tools that provide valid and reliable measurement of the functioning of these mechanisms (figure 1). This process includes a number of steps, from construct identification and task selection, though psychometric development to optimize task administration (subject instructions and testing time) and measurement properties (absence of floor and ceiling effects, internal consistency, and test-retest reliability).
Fig. 1.
Developmental path required for the adaptation of measures from basic cognitive neuroscience for use as valid, efficient, and reliable biomarker tools to enhance treatment development.
CNTRICS only addressed the initial steps in this process, construct identification and task selection, along with guidance on task refinement and optimization. A subset of measures have been further developed into valid and reliable tools with the support of RO1 funding, and 4 such tasks (2 variants of the AX-CPT (modified AX version of the continuous Performance Task) Task for measurement of goal maintenance in support of working memory and executive control,8–10 the Relational and Item-Specific Encoding and Retrieval (RISE) task for measurement of relational and item encoding and retrieval in episodic memory,11 the Jittered Orientation Visual Integration Paradigm (JOVI)12,13 paradigm for measurement of context effects during visual perception, and the Contrast-Contrast Effect (CCE) task for the measurement of surround suppression effects during visual perception14,15) are available (presently as beta versions) for downloading from the CNTRACS link (Cognitive Neuroscience Tests Reliability and Clinical applications in Schizophrenia) from the CNTRICS web site. This effort represents just a small proportion (5 of 20 selected tasks) of the CNTRICS recommended tasks and substantial work remains to be done in order to refine and optimize the remaining behavioral paradigms.
The Value of the Cognitive Neuroscience Approach for Imaging Biomarker Development Related to Cognition
An important strength of using behavioral paradigms from cognitive neuroscience is that they are amenable for use in functional imaging studies; indeed an important element in establishing the construct validity of these measures is their ability to recruit neural systems associated with specific cognitive mechanisms. The logical extension of developing such tasks as behavioral measures is to incorporate them into functional imaging paradigms that might serve as biomarkers of reflecting the state of illness-related functional neural systems. Such measures should enhance treatment development through their sensitivity to targeted treatment effects making them a useful tool for experimental medicine. The use of these measures may provide objective evidence of pharmacodynamic activity at the level of neural circuitry, proof of mechanism for first into human and phase I clinical studies, as well as individual predictors of treatment response in the service of personalized medicine approaches to treatment development.2
CNTRICS 2: Imaging Biomarker Development
The second phase of CNTRICS has had, as one of its aims, the development of imaging biomarkers for enhancing treatment development of impaired cognition. The results of work in pursuit of an important second Aim, the development of more informative animal models for treatment development in schizophrenia will be described in future articles. Like the initial phase of CNTRICS, pursuing imaging biomarkers has focused upon elucidating the development pathway involved in validating and optimizing specific measures and identifying strong candidates for further development. Additional efforts will be required to refine and optimize these specific measures for ready use.
In this Special Issue of Schizophrenia Bulletin, we are describing the results of the sixth CNTRICS Meeting in 7 articles. At this meeting, we focused on developing a set of functional magnetic resonance imaging (fMRI) and event-related potential (ERP)/electroencephalography (EEG) measures that have strong validity for measuring activity in functional neural systems associated with cognitive systems that were identified to be targeted during the first CNTRICS meeting. Many of the paradigms involved implementation, in the EEG or fMRI setting, of tasks that were selected during the third CNTRICS meeting. Additional tasks were also considered, and some measures identified in this third meeting were not recommended for further development at the present meeting because they are not readily incorporated into an imaging context.
Consensus-Based Process
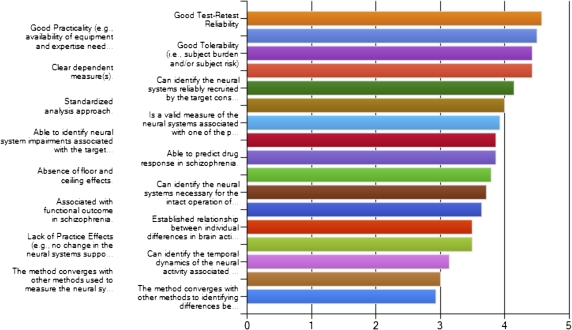
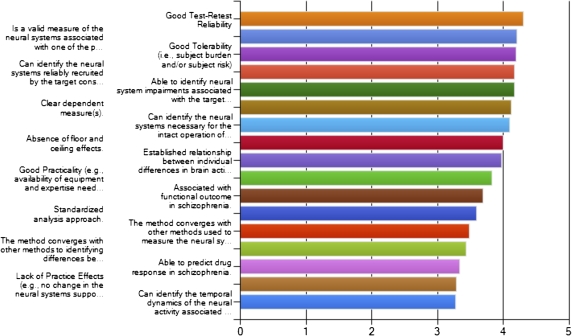
Prior to the meeting, 2 surveys were undertaken. The first sought to identify a set of criteria that experts in the field felt were most important in selecting candidate biomarkers. These surveys were an essential element in the consensus building process, ensuring broad participation in paradigm nominations and providing key criteria to help constrain the decision-making process at the meeting. Survey completion was solicited through the web and a very broad range of experts were invited to participate. This list included previous participants in CNTRICS meeting or surveys, lists of editorial board members for major journals in cognitive neuroscience, clinical neuroscience, and psychopharmacology, major participants in the pro-cognitive treatment development process in the Pharmaceutical Industry. The results of this survey can be seen in figures 2 and 3, broken down according to whether the survey responders were from academia or the pharmaceutical industry. One hundred and 3 experts rated a total of 17 items. There was a high level of convergence between the rankings of the proposed criteria for evaluating potential biomarkers between industry and academia. Both groups rated highly (either somewhat essential or very essential) the ability of a paradigm to reliably recruit the neural systems associated with a specific cognitive construct, test-retest reliability, tolerability of the procedure, and the presence of a clear dependant measure. In addition, industry representatives emphasized practicality of administration (the availability of equipment and expertise), while academic raters further emphasized construct validity. These ratings served to weight the use of these criteria in the breakout groups evaluating candidate measures during the meeting.
Fig. 2.
Ratings provided by industry participants of key criteria for selection of measures for development as biomarkers of cognitive and neural processing deficits in the human brain.
Fig. 3.
Ratings provided by academic participants of key criteria for selection of measures for development as biomarkers of cognitive and neural processing deficits in the human brain.
Paradigms designed to measure brain activity across the entire group of constructs were nominated through the web-based survey. This was followed by a detailed review by the Executive Committee that focused on the construct validity of the measures. A small number of measures were excluded because they had very poor construct validity and the rest were moved forward for consideration at the meeting. A total of 61 paradigms distributed across 12 constructs or component processes, spanning the “domains” of attention, executive control, working memory, episodic memory, perception, and emotional processing were nominated as candidate biomarker measures via the second web-based survey. Thirty-five of the nominated tasks were fMRI paradigms, and 25 were ERP or EEG time frequency domain measures.
At the meeting, a series of framing presentations by basic cognitive and affective neuroscientists revisited the individual constructs and known data about the neural systems supporting them. Then breakout groups focusing on measures from individualized domains evaluated and ranked each measure using the criteria described above, and selected, where possible, the top 2 or 3 measures per method (fMRI and EEG/ERP) to be recommended for further development. Final recommendations were made after the leaders of each of the 6 breakout groups reported back to the entire group and their recommendations were discussed.
The candidate imaging biomarkers recommended for further development are listed in table 2. A total of 23 distinct paradigms (16 fMRI, 3 EEG/ERP, and 4 for both methodologies) were recommended. There was some redundancy across domains since from the perspective of cognitive neuroscience domains such as working and long-term memory and executive functions are not considered to be orthogonal and, indeed, are seen to overlap to a significant degree at the component process level. Some paradigms were also identified that have been developed to address multiple-component processes within a domain (eg, the RISE task for both relational- and item-based long-term memory). The ability to measure multiple discrete component processes within a cognitive domain was considered to be a particularly advantageous.
Table 2.
CNTRICS II: Constructs, Definitions, and Promising Imaging Biomarker Paradigms in Humans
| Perception | |
| Gain control: The processes whereby neurons adapt their response levels to take into account their immediate context, in order to make best use of a limited dynamic signaling range. | |
| Promising tasks in humans: | |
| Corollary discharge—ERP | |
| Steady-state visual evoked potentials—ERP | |
| Auditory mismatch negativity—ERP | |
| Integration: The processes linking the output of neurons—that individually code local (typically, small) attributes of a scene—into global (typically, larger) complex structure, more suitable for the guidance of behavior. | |
| Promising tasks in humans: | |
| Motion processing—fMRI | |
| Contour integration task—fMRI or ERP | |
| Working memory | |
| Goal maintenance: The processes involved in activating task related goals or rules based on endogenous or exogenous cues, actively representing them in a highly accessible form, and maintaining this information over an interval during which that information is needed to bias and constrain attention and response selection. | |
| Promising tasks in humans for GOAL maintenance: | |
| AX-CPT/DPX—fMRI | |
| Switching stroop—fMRI or ERP | |
| Promising tasks in humans for ITEM maintenance: | |
| Visual delayed response task—fMRI | |
| Sternberg item recognition—fMRI | |
| Promising tasks in humans for CAPACITY: | |
| Change Detection—fMRI or ERP | |
| Interference control: The processes involved in protecting the contents of working memory from interference from either other competing internal representations or external stimuli. | |
| Promising tasks in humans: | |
| Suppress task—fMRI | |
| Sternberg item recognition task—fMRI | |
| Attention | |
| Control of attention: The ability to guide and/or change the focus of attention in response to internal representations. | |
| Promising tasks in humans: | |
| Theeuwes attention capture Task—fMRI or ERP | |
| McGaughy and Sarter sustained attention task—fMRI | |
| Executive control | |
| Rule generation and selection: The processes involved in activating task related goals or rules based on endogenous or exogenous cues, actively representing them in a highly accessible form, and maintaining this information over an interval during which that information is needed to bias and constrain attention and response selection. | |
| Promising tasks in humans | |
| AX-CPT/DPX—fMRI | |
| Switching stroop—fMRI or ERP/EEG | |
| Dynamic adjustments of control: The processes involved in detecting the occurrence of conflict or errors in ongoing processing, identifying the type of control adjustments needed, and recruiting additional control processes. | |
| Promising tasks in humans: | |
| Switching stroop—fMRI or ERP | |
| Long-term memory | |
| Relational encoding and retrieval: The processes involved in memory for stimuli/elements and how they were associated with coincident context, stimuli, or events. | |
| Promising tasks in humans: | |
| Hannula face scene task—fMRI | |
| Relational and item-specific encoding and retrieval task (RISE)—fMRI | |
| Item encoding and retrieval: The processes involved in memory for individual stimuli or elements irrespective of contemporaneously presented context or elements. | |
| Promising tasks in humans: | |
| Relational and item-specific encoding and retrieval task (RISE)—fMRI | |
| Reinforcement learning/motivation | |
| Acquired behavior as a function of both positive and negative reinforcers, including the ability to (a) associate previously neutral stimuli with value, as in Pavlovian conditioning; (b) rapidly modify behavior as a function of changing reinforcement contingencies, and (c) slowly integrate over multiple reinforcement experiences to determine probabilistically optimal behaviors in the long run. | |
| Promising tasks in humans: | |
| Probabilistic reversal learning—fMRI | |
| Probabilistic selection task—fMRI | |
| Social/emotional processing | |
| Affective recognition and evaluation: The ability to detect, recognize, and judge the affective value of both linguistic (eg, seen or spoken words and their prosodic contour) and nonlinguistic (eg, images of people, facial expressions, eye gaze, scenes) stimuli. | |
| Least well-developed area, but promising tasks in humans: | |
| Penn emotion recognition task—fMRI | |
| Faces in context—fMRI | |
| Bubbles task—fMRI | |
| Emotional point light walkers—fMRI | |
Note: ERP, event-related potential; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; DPX, dot pattern expectancy.
Each of these paradigms, along with the rationale for including them (and for excluding others), are described in the 6 accompanying articles in this volume. This is an important step in the development of cognitive neuroscience-based imaging biomarkers but substantial additional development work remains. Only a small subset of these tasks have entered the later phases of the process described in figure 1. As noted above individual measures of goal maintenance (supporting executive control attentional control and working memory), the AX-CPT and Dot Pattern version of the AX-CPT (DPX), relational and item encoding into long-term memory, the RISE paradigm, together with measures of visual integration (JOVI paradigm), and visual gain control (the CCE task) have been undergoing validation, refinement optimization, and psychometric evaluation supported by NIMH RO1 funding, and the behavioral paradigms may be downloaded from cntrics.ucdavis.edu. Three of these paradigms are also being validated and characterized in a multisite effort as fMRI biomarkers and data, and protocols and paradigms will be available at the same site in the coming months. This leaves a very large amount of developmental work to complete, with many fMRI and EEG/ERP candidate measures remaining to be moved through the developmental pipeline.
Discussion
The CNTRICS process sought to realize the potential, and address the limitations, of bringing the tools and constructs of cognitive neuroscience to bear on the challenge of developing effective treatments for impaired cognition for schizophrenia.5 Through a consensus-based approach, we have identified a set of cognitive systems to be targeted for impaired cognition in schizophrenia, and asset of tasks adapted from basic cognitive neuroscience that may be used to engage these cognitive processes along with the neural systems that support them. Many of these cognitive systems are also relevant for measuring the function of cognitive and neural systems in other disorders, including ADHD (Attention Deficit Hyperactivity Disorder),16 autism,17 MCI (Mild Cognitive Impairment), and Alzheimer’s disease.18 With support from the NIMH, a subset of these measures has been moved along the developmental pathway toward the goal of having downloadable behavioral measures that are valid and reliable and ready to use in treatment development research. The second phase of CNTRICS has focused on the development of improved animal models and imaging biomarkers. The products of the 2 imaging biomarker meetings includes the conceptual and methodological articles published in a special issue of Biological Psychiatry2,19–21 and the 6 articles describing the specific recommended biomarker paradigms in the present volume. A small subset of these measures are already being moved along the development pathway and should be broadly available in the near future. Substantial additional opportunities exist for expanding on these measures through the development of the remaining paradigms identified in this meeting.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix
Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) Executive Committee:
Robert W. Buchanan, Maryland Psychiatric Research Center
Edward Bullmore, M.D., University of Cambridge and GlaxoSmithKline
Pamela Butler, New York State University
Jonathan D. Cohen, Princeton University
Mark Geyer, University of California at San Diego
Randy Gollub, Massachusetts General Hospital
Michael F. Green, Semel Institute at UCLA and VA Greater Los Angeles Healthcare System
Judy Jaeger, AstraZeneca
John H. Krystal, Yale University
Holly Moore, Columbia University
Keith Nuechterlein, University of California at Los Angeles
Trevor Robbins, University of Cambridge
Steven Silverstein, University of Medicine and Dentistry of New Jersey
Edward E. Smith, Columbia University
Milton Strauss, University of New Mexico
Til Wykes, Institute of Psychiatry, King’s College London
References
- 1.Barch DM. Pharmacological strategies for enhancing cognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:43–96. doi: 10.1007/7854_2010_39. [DOI] [PubMed] [Google Scholar]
- 2.Carter CS, Barch DM, Bullmore E, et al. Cognitive neuroscience treatment research to improve cognition in schizophrenia II: developing imaging biomarkers to enhance treatment development for schizophrenia and related disorders. Biol Psychiatry. 2011;70:7–12. doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter CS, Barch DM, Buchanan RW, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter CS. Applying new approaches from cognitive neuroscience to enhance drug development for the treatment of impaired cognition in schizophrenia. Schizophr Bull. 2005;31:810–815. doi: 10.1093/schbul/sbi046. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barch DM, Carter CS, Arnsten A, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35:109–114. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barch DM, Carter CS. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: a report of the second consensus building conference of the CNTRICS initiative. Schizophr Bull. 2008;34:613–618. doi: 10.1093/schbul/sbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 9.Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 10.Jones JA, Sponheim SR, MacDonald AW., III The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22:131–141. doi: 10.1037/a0017828. [DOI] [PubMed] [Google Scholar]
- 11.Ragland JD, Cools R, Frank M, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MF, Butler PD, Chen Y, et al. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozma-Wiebe P, Silverstein SM, Feher A, Kovacs I, Ulhaas P, Wilkniss SM. Development of a world-wide web based contour integration test. Comput Hum Behav. 2006;22:971–980. [Google Scholar]
- 14.Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:R822–R824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JH, Rokem AS, Silver MA, et al. Diminished orientation-specific surround suppression of visual processing in schizophrenia. Schizophr Bull. 2009;35:1078–1084. doi: 10.1093/schbul/sbp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Dev Disabil Res Rev. 2008;14:261–267. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. Int J Dev Neurosci. 2008;26:239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnaiz E, Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol Scand Suppl. 2003;179:34–41. [PubMed] [Google Scholar]
- 19.Barch DM, Mathalon DH. Using brain imaging measures in studies of procognitive pharmacologic agents in schizophrenia: psychometric and quality assurance considerations. Biol Psychiatry. 2011;70:13–18. doi: 10.1016/j.biopsych.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luck SJ, Mathalon DH, O'Donnell BF, et al. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry. 2011;70:28–34. doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]