Abstract
Attention is widely believed to be dysfunctional in schizophrenia. The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) group previously concluded that the processes involved in the top-down control of attention are particularly impaired in schizophrenia and should be the focus of future research. These processes determine which sources of input should be attended, linking goal representations in prefrontal cortex with more posterior regions that implement the actual selection of attended information. A more recent meeting of the CNTRICS group assessed several paradigms that might be useful for identifying biomarkers of attentional control and that could be used for treatment development and assessment. Two types of paradigms were identified as being particularly promising. In one approach, neural activity is measured (using electroencephalography or functional magnetic resonance imaging) during the period between an attention-directing cue and a target. In a second approach, neural activity is measured under low- and high-distraction conditions. These approaches make it possible to identify the goal representations that guide attention and the interactions between these goal representations and the implementation of selection. Although more basic science research with healthy volunteers and preclinical research with schizophrenia patients is needed before these paradigms will be ready to provide clinically useful biomarkers, they hold substantial promise for aiding in the development and assessment of new treatments.
Keywords: schizophrenia, biomarker, attention
The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) group convened a series of meetings to assess the specific cognitive constructs that are dysfunctional in schizophrenia, to create a consensus about the most promising behavioral tasks for assessing these constructs, and to discuss the present and future availability of biomarkers of these constructs. Here, we present the results of the discussion of biomarkers related to attention. We will begin by reviewing the construct of attention and the specific type of attentional dysfunction that appears to be present in schizophrenia. We will then discuss how the neural correlates of this construct are measured in the basic cognitive neuroscience literature. We will end by discussing 2 classes of experimental paradigms that hold promise as means of providing biomarkers of attentional control.
The Construct of Attention in Schizophrenia
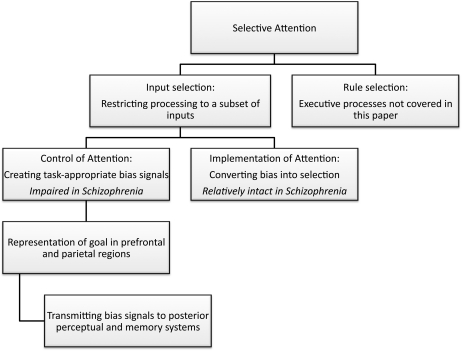
As discussed in the report on attention from the first CNTRICS meeting,1 the term “attention” has many meanings and is used so broadly that almost any impairment in task performance could be attributed to attentional dysfunction. Moreover, it is often difficult to distinguish between the constructs of attention, working memory, and executive function. To avoid these problems, the CNTRICS group agreed to focus on the variety of attention known as “selective attention.” The group further noted that selective attention can be subdivided further into “input selection” and “rule selection.” Figure 1 provides an overview of this conceptualization.
Fig. 1.
Scheme for subdividing the concept of selective attention.
Input selection is the process of restricting processing to a subset of inputs. For example, the Posner cuing paradigm uses spatial cues to direct attention toward one location and away from other locations, producing a competitive advantage for inputs that arise from the attended location. Input selection also includes tasks in which attention controls which inputs are stored in memory or sent to response systems. In contrast, rule selection is the process of determining which rules should be applied to a given source of information. In most laboratory tasks, the rules specify the stimulus-response mapping that should be used. In the Stroop task, for example, the observer sees a word drawn in a given ink color and applies either a read-the-word rule or a name-the-ink-color rule. Rule selection is usually considered to be an example of an executive process, and the CNTRICS group therefore decided that the attention construct should be limited to input selection (with rule selection being a part of the executive control construct). This is not intended to imply that rule selection is not a variety of attention; instead, this decision reflected a desire to minimize overlap between the constructs for the sake of efficiency.
Within the construct of input selection, it is important to distinguish between the “control of attention” and the “implementation of attention,”1,2 which is largely equivalent to the distinction between the sources of attentional control signals and the targets of these signals3 (see figure 1). Attentional control processes are mainly localized within prefrontal and parietal regions; they determine which inputs are relevant for the current task and send control signals to regions that are involved in actually processing these inputs (eg, perceiving them, storing them in memory, etc.). In the context of a spotlight metaphor, control processes determine where the spotlight is pointed and implementation processes determine the strength of the beam. In the context of the biased competition model,4 control processes provide the bias signal and implementation processes are responsible for converting this signal into an actual competitive advantage (eg, by means of lateral inhibition).
In many attention paradigms, impaired performance could arise from dysfunction of either the control or the implementation of attention. However, it is possible to distinguish between control and implementation by using tasks that are challenging to one but not the other. For example, a typical Posner cuing paradigm is trivial for control processes because a salient cue indicates which location should be attended, and no other stimuli compete with this cue for attentional control. Consequently, the ability to exhibit faster or more accurate processing of stimuli at the cued location relative to the uncued location(s) largely reflects the implementation of attention—the ability to increase the processing gain at the cued location and decrease it at the uncued location(s). Many experiments have used this paradigm with schizophrenia patients and control subjects, and patients and controls are typically found to exhibit the same ability to exhibit faster and more accurate performance for targets at the cued location relative targets at uncued locations (reviewed in5). One exception to this occurs when a salient cue at one location indicates that attention should be directed elsewhere. Under these conditions, the attentional control system is challenged by the need to attend to one location even though a salient stimulus is present at a different location, and patients exhibit a reduced ability to attend to the appropriate location in this situation.6 Similar results are observed for eye movement performance in schizophrenia, which is impaired in antisaccade tasks (which require orienting away from the target) but not in prosaccade tasks (which require orienting toward the target).7 Thus, the existing evidence indicates that the implementation of attention is relatively intact in schizophrenia, whereas the top-down control of attention is impaired.1
Elements of Top-Down Attentional Control
The top-down control of attention requires 2 elements. First, a task or goal representation must be activated that specifies what kinds of information are relevant and should receive attention. Second, the goal representation must be linked to systems for implementation.
In a simple laboratory task such as the Posner cuing paradigm, goal representation might be as simple as representing the to-be-attended location that was indicated by the cue. In the natural environment, the task representation may be more complex, involving several features that define task-relevant objects. For example, if an individual is making a peanut butter sandwich, the goal at a given moment might be to locate the peanut butter jar, which in turn would involve activating the color, size, and shape of the jar so that objects with the appropriate features would attract attention.8 Once a goal representation is activated, the second element of attentional control is to create links between this representation and the processing systems in which the implementation of attention will occur. Without these links, the representation of the relevant features will not actually lead to the transmission of bias signals into the regions in which attentional selection is implemented.
Neural Measures of Goal Representation
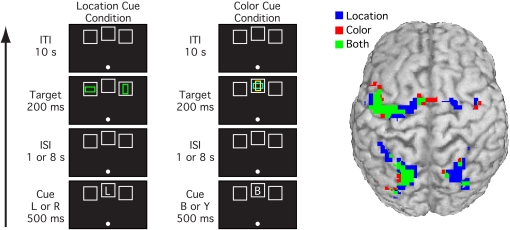
Goal representations are special cases of working memory representations. However, whereas working memory representations may involve posterior sensory and memory areas as well as prefrontal and parietal control areas,9–11 the goal representations that guide attention appear to be active primarily within prefrontal and parietal areas.12–14 Most of these areas are active both when attention is cued to a specific location or to nonspatial features such as color, but some regions are differentially active for spatial and nonspatial attention (see figure 2).13,15 In particular, regions that are closely linked with gaze control may be especially involved in the control of spatial attention.
Fig. 2.
Typical cuing paradigm used to assess neural activity related to top-down attentional control.13 In this version, the cue could either indicate a spatial location (L for left or R for right) or a color (Y for yellow or B for blue) that is likely to contain a subsequent target stimulus. Sustained activation during the period between the cue and target is observed primarily in prefrontal and parietal areas, presumably reflecting some kind of goal representation or attentional template, and this activity overlaps considerably for attention to spatial and nonspatial features. Data courtesy of Giesbrecht et al13.
As illustrated in figure 2, activation of goal representations is typically isolated in functional magnetic resonance imaging (fMRI) experiments by means of variants of the Posner paradigm in which a cue stimulus indicates which location or feature value should be attended on that trial. By using event-related designs with a relatively long interval between cue and target, it is possible to separate the goal representation (sustained activity following the cue but prior to the target) from the processes related to the implementation of attention (activity following target presentation).
Analogous effects can be observed in event-related potential (ERP) experiments, which take advantage of the contralateral organization of the brain. Specifically, when a cue directs attention to one hemifield, a sustained voltage can be measured over the contralateral hemisphere between the time of the cue and the time of the target.16 However, it is not yet clear whether these effects reflect the goal representation itself or the resulting implementation of attention within perceptual processing areas, which leads to sustained changes in the BOLD signal12 and neural firing rates17 within visual cortex. In addition, prefrontal electroencephalography (EEG) oscillations in the theta band have been linked with the across-region coordination of mental representations,18 which presumably underlies top-down attentional control.
It is much more challenging to identify the top-down control signals than it is to identify the goal representation because attentional control is defined as the interaction between the goal representation and the areas involved in the implementation of attention. As an analogy, motor cortex can be activated on the basis of response expectations and even motor imagery without leading to actual hand movements; some additional process is needed before these signals will actually take control of lower-level motor neurons. Similarly, activation of attentional control areas cannot alone produce increased gain for specific inputs; this activation must be linked with the more posterior areas in which attentional selection is actually implemented to produce a change in gain. This linkage between systems is not as easily measured as the activation of the individual systems.
Neural Measures of Anterior-Posterior Linkage in the Control of Attention
The best evidence of anterior-to-posterior attentional control linkage has been observed in macaque monkeys by Moore and his colleagues, who have studied the links between goal-related activity in the frontal eye fields (FEF) and the implementation of selection in area V4 of visual cortex.19–21 Maintaining spatial representations causes increased firing rates in FEF neurons that code the region of space being maintained in memory, even in tasks that do not involve saccades.19 Moreover, microstimulation of the FEF causes a change in the sensory responsiveness of V4 neurons that code the same location as the stimulated FEF neurons,21 leading in turn to changes in behavioral performance.20 This paradigm directly reveals the link between the goal representation in FEF and the implementation of selection in V4.
This level of precision is difficult to achieve in noninvasive measures of human brain activity, but recent studies have developed methods to assess links between prefrontal or parietal activity and either perception-related neural activity or behavioral measures. Some of these studies have also varied the level of competition between top-down control and bottom-up salience, providing a tight link with the behavioral measures of impaired attentional control in schizophrenia that were described earlier.
One approach to assessing attentional control has been to use functional connectivity, coherency, and Granger causality methods with fMRI data, asking whether activity within a control area at one moment in time predicts activity within an implementation area at that moment or shortly afterward. Studies using these approaches have shown that prefrontal and parietal activity at one moment significantly predicts concurrent and/or future activity in visual cortex.22–24 Moreover, one study found that the anterior-to-posterior connectivity was stronger when measured during an antisaccade task than during a prosaccade task,23 presumably because top-down control systems play a stronger role when individuals are asked to orient away from rather than toward a salient stimulus.
EEG measures have also been used to assess anterior-posterior linkage in the context of attentional control. Specifically, posterior alpha oscillations vary according to the location being attended and whether attention is directed to the auditory or visual modality,25,26 which appears to reflect the implementation of attention within posterior brain regions. A recent study found that the magnitude of the alpha activity following an attentional cue was correlated with the magnitude of prefrontal theta activity in typically developing children, but this correlation with prefrontal theta was absent in children with attention deficit hyperactivity disorder (ADHD).26 This correlation between anterior theta and posterior alpha likely reflects a linkage between prefrontal goal representations and the implementation of selection within posterior cortex, and the reduction of this correlation in children with ADHD provides further evidence for the validity of this measure. Interestingly, there was no difference in behaviorally measured attention effects between the typically developing children and the children with ADHD. This presumably reflects the fact that this task does not provide much of a challenge to attentional control: As long as the mechanisms of implementation are intact, even a weak control signal will be sufficient to drive effective selection in tasks that do not stress control. Control signals may be measured effectively in paradigms such as this that do not put stress on control as long as a behavioral measure of control is not also required.
Linking Neural Measures With the Degree of Attentional Control
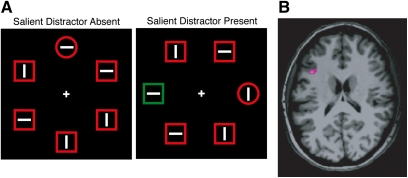
Other studies have examined the neural correlates of control signals by assessing how neural activity varies with the need for, or the success of, attentional control. For example, one study used the “additional singleton paradigm” along with fMRI to examine how brain activity differed between trials on which top-down control was successful or unsuccessful.27 As illustrated in figure 3A, this paradigm requires participants to search for a form-defined target and report the orientation of a line embedded within the target. A salient but irrelevant color singleton is sometimes present in the display to provide competition for control.28 This salient singleton typically attracts attention, increasing the time needed for the participant to find the target. However, by maintaining a strong representation of the shape of the target, participants may avoid being distracted by the irrelevant singleton.29 Trial-by-trial variations in reaction time (RT) can be used to determine how well an individual is able to avoid being distracted by the salient but irrelevant singleton and the pretrial BOLD signal in the left middle frontal gyrus (MFG) significantly predicts the degree of distraction (figure 3B).27 The left MFG is one of the areas that is commonly found to be active following an attention-directing cue (see, eg, figure 2), and in this study, greater activity in the left MFG was correlated with less distraction, consistent with the idea that it is possible to overcome distraction in this paradigm by means of a strong top-down attentional control signal. This may be exactly the kind of top-down control of attention that is impaired in schizophrenia.
Fig. 3.
Example of the Theeuwes additional singleton paradigm (A).28 Subjects search for the item with the unique shape and press one of 2 buttons to indicate whether the line inside this shape is horizontal or vertical. A task-irrelevant color singleton is present on some trials; this singleton captures attention, especially when top-down control is poor, slowing responses to the target. Activity in the left middle frontal gyrus is correlated with the degree of attention capture (B).27 Data courtesy of Leber.27
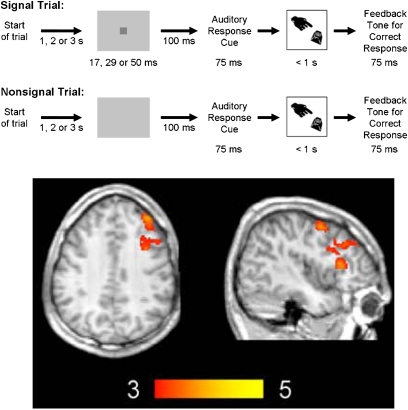
Another approach is to compare brain activity in blocks of trials in which strong top-down control is needed to perform the task with blocks of trials in which performance is less dependent on control. For example, one study30 used arterial spin labeling to measure regional cerebral blood flow in 2 versions of the McGaughy and Sarter31 sustained attention task (SAT),32 which involves detection of a visual target that occurs at an unpredictable point in time (see figure 4). In the basic SAT, no distractors are present; in the distraction version of the SAT (dSAT), the background continuously flashes to disrupt attention. The right MFG was more active in the dSAT than in the SAT. The relatively specific involvement of right MFG in the distractor version is particularly remarkable in light of a recent meta-analysis identifying this region as being disrupted in schizophrenia.33
Fig. 4.
Top: Sustained Attention Task (SAT). Subjects attempt to detect a short signal that appears (signal trials) or does not appear (nonsignal trials) with equal probability after a variable interval of 1, 2, or 3 seconds. Upon hearing a response cue, participants make a button-press response to indicate whether a signal had been perceived. Correct responses elicit a feedback tone signaling a monetary reward. In the distractor version of the SAT (dSAT), the screen flashes between silver and black at 10 Hz. Bottom: Arterial spin labeling results30 (activation in the dSAT condition after controlling for SAT activation and a passive perceptual control condition), which included activation in right dorsolateral prefrontal cortex as well as bilateral motor, cingulate, and insular regions. The color scale indicates z scores. Reprinted from NeuroImage with permission from Elsevier30.
Importantly, right MFG activation during dSAT performance was greater than the sum of activation during SAT performance and the flashing background presented separately. That is, this region was specifically sensitive to performance under challenge and was not merely driven by the increased visual stimulation provided by the flashing background. Furthermore, the degree of right MFG activation was significantly correlated with performance in the dSAT version and with the difference in performance between the dSAT and SAT versions.
In contrast to the additional singleton paradigm, where greater left MFG activation was correlated with less distractibility, for the dSAT, greater right MFG activity was correlated with more vulnerability to the distractor. There are several differences between the irrelevant-singleton task and the dSAT that might contribute to the different patterns of correlations. One key difference is that the additional singleton task was optimized to measure the trial-by-trial deployment of attentional control, whereas the dSAT study was optimized to measure the tonic engagement of attentional control. This raises the possibility that right prefrontal cortex may be particularly involved in tonic task or goal representations, whereas left prefrontal cortex may be particularly involved in linking that representation to the current stimulus event and to posterior representations of the stimulus and appropriate response rules. The development of “mixed” or “state-item” fMRI designs that allow the separation of tonic and phasic activity34 represents an opportunity to test this hypothesis in future research.
It should also be noted that the relatively intense flickering used in the dSAT makes it inappropriate for use with individuals who are susceptible to photic seizures.
Paradigm Recommendations
The participants at the CNTRICS meeting reached consensus about recommending some approaches for further development. This was based mainly on evidence of strong construct validity for these approaches. In most cases, the approaches with excellent construct validity have not yet been widely used in studies of schizophrenia, so substantial work will be needed before specific biomarkers can be validated. Moreover, some of the recommended approaches are fairly general, and substantial work will be needed to develop specific versions that have other desirable properties (eg, high reliability, ease of application to patient populations). Other approaches may be identified in the future that would also be valuable, and the goal was not to preclude the development of all other approaches. However, some specific alternative approaches were considered to be unlikely to bear fruit.
Paradigms Not Recommended for Further Development
We will begin by briefly discussing paradigms that were nominated but were not viewed as appropriate at this time. First, the N2pc component is a well-validated ERP measure of the focusing of visual attention35 that has been successfully used in schizophrenia patients.36 However, this component reflects the implementation of attention rather than the control of attention, so it does not match the specific construct that was identified in previous CNTRICS meetings. Similarly, the MONSTER (Manipulation of Orthogonal Neural Systems Together in Electrophysiological Recordings) approach provides a general means of isolating multiple ERP components,37 including N2pc, but it does not provide a specific measure of attentional control.
The general class of dual-task paradigms was nominated, with the psychological refractory period (PRP) paradigm described as a prototypical example. Although the PRP paradigm has many virtues,38 it is mainly valuable in measuring the speed of individual processing stages and does not provide a specific measure of attentional control. Many other dual-task paradigms are available, but they also lack specificity for this construct.
The spatial delayed response paradigm was also nominated. This paradigm isolates spatial working memory activity, which may be related to the goal maintenance element of attentional control. However, it is not known whether the working memory activity isolated by this task actually contributes to attentional control.
Attentional Cuing Tasks
Attentional cuing paradigms using fMRI and EEG/ERPs, such as those shown in figures 2 and 4, have been used in many basic science studies to isolate the goal representation activity used in the control of attention. As noted above, neural measures make it possible to observe impairments in control signals in these tasks, even though these tasks do not typically lead to substantial behavioral deficits in schizophrenia (because the tasks do not require strong control signals). Similar paradigms have been used in nonhuman primates39 and in rodents.40 Given the good construct validity of this approach and the availability of animal models, the consensus of the CNTRICS participants was to recommend this approach for further development.
However, attentional cuing paradigms are not ready for adoption in treatment studies because neuroimaging versions of these paradigms have not yet been used to demonstrate dysfunction in schizophrenia. It seems likely that evidence of dysfunction would be seen in these tasks because working memory tasks that isolate similar brain regions have shown abnormal activity in these regions in patients. The pattern of abnormal working memory activity in patients is complex, however, with reports of both increased and decreased frontal lobe activation. This seeming inconsistency is likely due to inefficient frontal lobe function in schizophrenia, which is best described by an inverted-U shaped function relating frontal lobe activation to working memory load. As the load increases, neural activity rises to a peak and then falls off once working memory capacity has been exceeded.41,42 Thus, with smaller loads, patients may appear to have more activation than controls, but at higher loads, less. Future work aimed at studying impaired goal representations in the context of attentional cuing tasks will need to keep this complex pattern in mind.
Attentional cuing paradigms using EEG were also recommended for further development. As described earlier, posterior alpha-band EEG oscillations vary according to whether a cue directs attention to the auditory or visual modality, and this alpha modulation is correlated with prefrontal theta oscillations (which likely reflect goal representations). This theta-alpha correlation goes beyond merely indexing the goal representation and is a promising measure of anterior-posterior linkage in attentional control. Its utility has already been proven in the context of ADHD.26 Moreover, EEG-based measures have many practical advantages as biomarkers in the context of psychiatric treatment research,43 including being relatively easy and inexpensive to obtain in large-scale genetic studies or clinical trials. Consequently, this alpha-theta measure is worth pursuing to determine whether it reveals cognitive impairment in schizophrenia and whether it has good measurement properties.
Distraction Paradigms
Two distraction paradigms, the additional singleton task (figure 3) and the dSAT (figure 4), were also recommended for further development. These tasks are very promising because they examine attentional control in the face of competition from potent distractors, which appears to be the situation that most clearly leads to impaired attentional performance in schizophrenia patients. Thus, they have excellent construct validity. However, these tasks have only recently been extended to include noninvasive measures of neural activity in healthy individuals, and it is also not yet known whether patient behavioral performance or neural activity is disrupted in these specific tasks (although impairment seems likely given the disruption observed in similar tasks). Thus, both basic science work with healthy individuals and preclinical research with schizophrenia patients will be necessary before these tasks can provide biomarkers for clinical research. Moreover, the specific neural measure of attention control in the additional singleton paradigm, shown in figure 3, is likely impractical in large-scale clinical studies because it requires large numbers of trials and is based on trial-by-trial correlations between RT and the BOLD signal. Consequently, substantial basic science research is needed before this general approach can be used to provide a useful measures of attentional control in patients.
In addition to good construct validity, it should be noted that the dSAT has the advantage of extensive prior research in rodents. This research has characterized the role of the basal forebrain cholinergic system in mediating frontal-parietal responses to attentional performance demands and the increase in those demands imposed by distractors.44,45 Most of this research has been conducted using microdialysis techniques that measure performance-related tonic increases in levels of cholinergic neurotransmission (on the scale of minutes). More recently, methods have been developed that allow measurement of the phasic cholinergic increases that occur during successful detection of a signal (on the scale of seconds).46,47 Dissociations between the tonic and phasic actions of the cholinergic system have at least a surface similarity to the idea of tonic representations that maintain goals throughout the task and phasic responses that interpret specific stimulus events in light of those task goals and connect them to posterior representations of the appropriate response rules.48 However, further work is needed to verify this conceptual link.
Finally, a version of the task has been used to test an amphetamine-pretreatment rodent model of schizophrenia.44,49 These animals showed spared performance but increased tonic cholinergic release during the standard SAT, reflecting abnormally high levels of cognitive control required to mediate performance even in the absence of distractors. Additional demands on top-down control in the dSAT severely disrupted these animals’ performance. This effect was mediated via a return of levels of cholinergic neurotransmission to pretask baselines.
Additional experiments are needed to demonstrate that dysregulation in cholinergic systems cause the disruption in attentional performance in this model. Likewise, additional behavioral and neuroimaging studies with patients are needed to determine similarities and any differences with the results produced by the animal model. If there is good correspondence, the dSAT has strong potential for guiding pharmaceutical treatment research targeting the specific aspects of cholinergic neurotransmission that may contribute to attention deficits in schizophrenia.50
Conclusions
Impairments of attention in schizophrenia have been postulated since the earliest clinical descriptions of the disease. However, attention is a broad term with multiple meanings, and the field has only recently begun to isolate the specific nature of attentional dysfunction in schizophrenia and to distinguish between the related constructs of attention, working memory, and executive control. By focusing on the specific construct of top-down control of attention, it may be possible to develop biomarkers that can be very useful in developing and testing new treatments for cognitive dysfunction in schizophrenia. However, this specificity means that basic and preclinical research has not yet progressed to the point where biomarkers of attentional control are fully ready for use in treatment research. Nonetheless, the CNTRICS participants have identified 4 paradigms that are highly promising. One of these paradigms (cuing using fMRI) has been used extensively in the basic cognitive neuroscience literature and is ripe for preclinical research to determine whether patients show reliable reductions in cue-elicited goal representations. Another of these paradigms (cuing using EEG) has already proven useful in ADHD research. Two additional paradigms (additional singleton and dSAT) have extremely good construct validity and are closely related to paradigms that have revealed behavioral manifestations of impaired attentional control in schizophrenia patients, but need more basic science development of neural measures. Thus, the field is well on the road to developing biomarkers for attentional control, and additional basic science research on attentional control may yield even better biomarkers in the future.
Funding
National Institute Mental of Health (R01MH076226, R01MH065034 to S.J.L., RO1MH080332, RO1MH086530 to M.S.); National Science Foundation (0726285 to C.L.).
Acknowledgments
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute Mental of Health or the National Science Foundation. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luck SJ, Vecera SP. Attention. In: Yantis S, editor. Stevens' Handbook of Experimental Psychology: Vol. 1: Sensation and Perception. 3rd ed. New York, NY: Wiley; 2002. pp. 235–286. [Google Scholar]
- 3.Posner MI, DiGirolamo GJ. Attention in cognitive neuroscience: an overview. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. Vol 2. Cambridge, MA: MIT Press; 2000. pp. 623–632. [Google Scholar]
- 4.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 5.Gold JM, Fuller RL, Robinson B, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 6.Hahn B, Robinson BM, Kaiser ST, et al. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103:277–287. [PubMed] [Google Scholar]
- 8.Hayhoe MM, Shrivastava A, Mruczek R, Pelz JB. Visual memory and motor planning in a natural task. J Vis. 2003;3:49–63. doi: 10.1167/3.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 10.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 11.Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 13.Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 14.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harter MR, Miller SL, Price NJ, LaLonde ME, Keyes AL. Neural processes involved in directing attention. J Cogn Neurosci. 1989;1:223–237. doi: 10.1162/jocn.1989.1.3.223. [DOI] [PubMed] [Google Scholar]
- 17.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 18.Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong KM, Chang MH, Moore T. Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci. 2009;29:15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc Natl Acad Sci U S A. 2007;104:9499–9504. doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Lauritzen TZ, D'Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis. 2009;9:18.11–18.14. doi: 10.1167/9.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang K, Velanova K, Luna B. Strengthinging of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magentic resonance imaging effective connectivity study. J Neurosci. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. J Neurosci. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS, Corbett BA. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Leber AB. Neural predictors of within-subject fluctuations in attentional control. J Neurosci. 2010;30:11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theeuwes J. Perceptual selectivity for color and shape. Percept Psychophys. 1992;51:599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- 29.Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- 30.Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54:1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- 32.Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35:182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- 35.Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York, NY: Oxford University Press; In press. [Google Scholar]
- 36.Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Kappenman EK, Luck SJ. doi: 10.1093/schbul/sbr147. Manipulation of orthogonal neural systems together in electrophysiological recordings: the MONSTER approach to efficient neurocognitive assessment. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuechterlein KH, Pashler H, Subotnik KL. Translating basic attentional paradigms to schizophrenia research: reconsidering the nature of the deficits. Dev Psychopathol. 2006;18:831–851. doi: 10.1017/s095457940606041x. [DOI] [PubMed] [Google Scholar]
- 39.Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- 40.Bushnell PJ. Overt orienting in the rat: parametric studies of cued detection of visual targets. Behav Neurosci. 1995;109:1095–1105. doi: 10.1037//0735-7044.109.6.1095. [DOI] [PubMed] [Google Scholar]
- 41.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 42.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 43.Luck SJ, Mathalon DH, O'Donnell BF, et al. A roadmap for the development and validation of ERP biomarkers in schizophrenia research. Biol Psychiatry. doi: 10.1016/j.biopsych.2010.09.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarter M, Martinez V, Kozak R. A neurocognitive animal model dissociating between acute illness and remission periods of schizophrenia. Psychopharmacology (Berl) 2009;202:237–258. doi: 10.1007/s00213-008-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- 47.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32:2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- 50.Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. doi: 10.1016/j.neuropharm.2010.12.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]