In the United States, an estimated 33, 550 cases of thyroid cancer were diagnosed in 2007 according to the American Cancer Society. Medullary thyroid cancer (MTC) accounts for approximately 5% of these carcinomas and arises from calcitonin-secreting parafollicular C cells.1 While up to 25% of MTCs are hereditary, the majority of cases are sporadic. Hereditary forms of MTC include both multiple endocrine neoplasia 2 types (MEN 2A and MEN 2B) and familial MTC. Mutations of the RET (Rearranged during Transfection) proto-oncogene on chromosome 10q11 are present in over 95% of hereditary MTCs and about 25% of sporadic MTCs.2 Dominant-activating or gain-of-function mutations in the RET proto-oncogene lead to the constitutive activation of receptor tyrosine kinases and downstream pathways involved in cell survival and proliferation.
Several signal transduction pathways have been identified as mediating the oncogenic properties of MTC, including both the phosphatidylinositol 3-kinase (PI3K)/Akt3 and Raf-1/MEK/extracelular signal-regulated kinase (ERK)4,5 pathways (also referred to as Ras/mitogen-activated protein kinase/MAPK). Other signaling molecules also have been implicated in the development of MTC, such as Notch1/Hairy Enhancer of Split-1 (HES-1)/acheate-scute complex like-1 (ASCL1)6 and glycogen synthase kinase-3β (GSK-3β).7 This review will focus on the role of PI3K/Akt signaling in MTC and the relationship of this pathway to mutations in the RET proto-oncogene.
THE PI3K/AKT PATHWAY
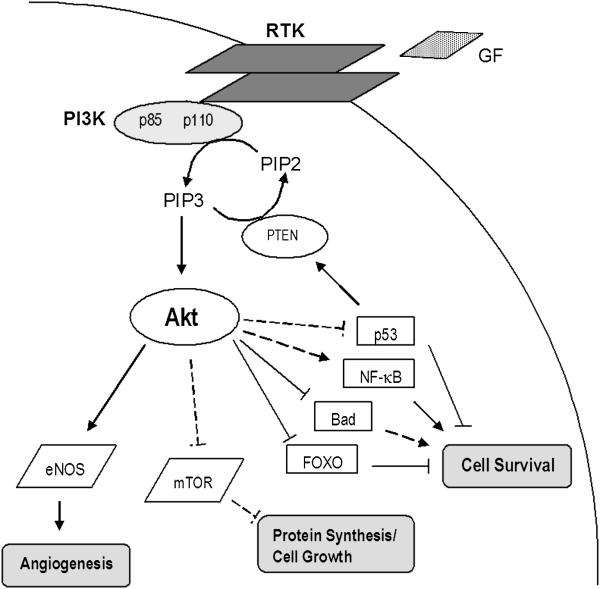
Over the past 20 years, hyperactivation of PI3K/Akt signaling has been recognized as playing a crucial role in the development of several different neoplasms in addition to MTC, including breast, prostate, colon, and ovarian cancers.8 PI3K is a growth factor receptor tyrosine kinase that contains two subunits, a p85 regulatory subunit and a p110 catalytic subunit (Fig. 1). This kinase acts by phosphorylating phosphatidylinositol-4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3) which, in turn, recruits both Akt (a serine threonine kinase, also known as protein kinase B) and inositol phosphate-dependent dehydrogenase kinase-1 (PDK1). PIP3 then phosphorylates Akt either directly or through PDK1. Full activation of Akt requires phosphorylation of two individual residues, threonine 308 and serine 473.
Fig 1.
The PI3K/Akt signaling pathway is involved in several human cancers. When bound, growth factor (GF) receptor tyrosine kinases (RTK) recruit PI3K, a heterodimer with both a regulatory p85 and a catalytic p110 subunit. PI3K phosphorylates PIP2 creating PIP3, which then activates Akt. Once phosphorylated by PIP3, Akt affects many cellular processes either directly (dark lines) or indirectly (dashed lines) through the regulation of downstream mediators. By inhibiting proteins that promote apoptosis, such as p53, Bad, and the FOXO family of forkhead transcription factors, Akt furthers cell survival. Akt also regulates survival by stimulating pro-survival proteins like NF-κB. Indirect activation of the mTOR pathway by Akt increases protein synthesis and cell growth, while the triggering of eNOS overexpression is thought to contribute to tumor angiogenesis. In addition, Akt is indirectly involved in the control of cell cycle progression through interactions with FOXO transcription factors, GSK3, Myc, and subsequent effects on cyclin D1 and p27KIP1 (not depicted).
Once activated, Akt regulates several normal cellular processes that can promote tumorigenesis when unregulated (Table 1). In many models, Akt facilitates growth-factor mediated cell survival by phosphorylating and deactivating pro-apoptotic proteins, such as the Bcl-2 family (Bcl-2 and Bad), p53, and the FOXO family of Forkhead transcription factors (AFX, FKHRL1, and FKHR). Activation of Akt is also linked to other downstream mediators that facilitate the evasion from apoptosis, enhance protein synthesis, or support tumor angiogenesis, such as NF-κB, mTOR, and eNOS, respectively. Phosphatase and tensin homolg (PTEN), a well-known tumor suppressor, converts PIP3 back to PIP2 and prevents further PI3K signaling. Loss-of-function mutations of PTEN have been shown to activate Akt.8 Components of the PI3K/Akt pathway represent potential targets for novel cancer therapies which have pushed researchers to elucidate further the role of this signal transduction pathway in MTC and other cancers.
Table 1.
Cellular processes regulated by PI3K/Akt Signaling
| Survival |
| Growth/Protein Synthesis |
| Proliferation |
| Motility |
| Angiogenesis |
RET MUTATIONS
MTC is linked strongly to dominant-activating mutations of the RET proto-oncogene that have been identified in about 25% of all cases of MTC and 95% of hereditary MTC.2 The RET gene serves a critical role in the development of the renal and enteric nervous systems and codes for a transmembrane receptor tyrosine kinase. RET mutations lead to continual autophosphorylation of specific tyrosine residues, thereby inducing constitutive activation of intracellular signal transduction. MEN 2A and familial MTC are initiated frequently by a single point mutation at one of six cysteine codons triggering constitutive dimerization and activation of the RET kinase.9 Mutations within the tyrosine kinase domain itself are identified in familial MTC. In addition, germline mutations occur commonly within one of two specific sites in the kinase catalytic domain in MEN 2B. Receptor tyrosine kinase inhibitors similar to Gleevac (imitanib mesylate) have been developed against RET and show promising results both in vitro and in vivo as emerging therapies for the treatment of MTC. Substantial work has also focused on identifying downstream effectors of RET for the development of more directed treatments.
THE ASSOCIATION BETWEEN RET AND PI3K/AKT SIGNALING
In 2000, Segouffin-Cariou et al from France established that the transforming ability of RET in MEN2A requires activation of the PI3K/Akt signaling pathway.9 In rat fibroblasts, clones expressing a MEN2A mutant form of RET (cysteine mutation at codon 634, C634W) were found to have a marked increase in PI3K and Akt activity compared to wild type. In addition, overexpression of Akt in these clones potentiated the oncogenic activity of the MEN2A mutant RET, while a dominant-interfering form of PI3K suppressed the clones’ transforming ability. Furthermore, the forkhead transcription factor, FKHRL1, was phosphorylated and activated by this RET mutation. FKHRL1 is thought to promote cell survival by inhibiting apoptosis. These findings represent some of the earliest data linking RET to PI3K/Akt signaling in MTC. Importantly, this work suggested that Akt is a limiting factor in the expression of the MEN2A associated RET neoplastic phenotype and works by evading apoptosis.
Drosten and associates also implicated the PI3K/Akt pathway in the pathogenesis of MTC in MEN2A.1 Human medullary thyroid carcinoma TT cells, which also harbor the C634W RET mutation, were transfected with adenoviral vectors expressing a dominant-negative truncated RET protein (RETΔTK) that inhibits RET autophosphorylation. Active, phosphorylated Akt was either decreased or not detectable in tumor cells expressing the RETΔTK vector demonstrating that PI3K/Akt signaling contributes to RET-mediated transformation in MTC. Moreover, TT cells transfected with the RETΔTK vector had decreased levels of Bcl-2, an anti-apoptotic protein. Bcl-2 is increased typically in MTC and is known to be mediated by Akt. These results suggested that oncogenic RET activity in MTC relies, in part, on overactivation of the PI3K/Akt pathway. Furthermore, up-regulated Akt suppresses apoptosis and stimulates pro-survival signaling by increasing Bcl-2 expression.
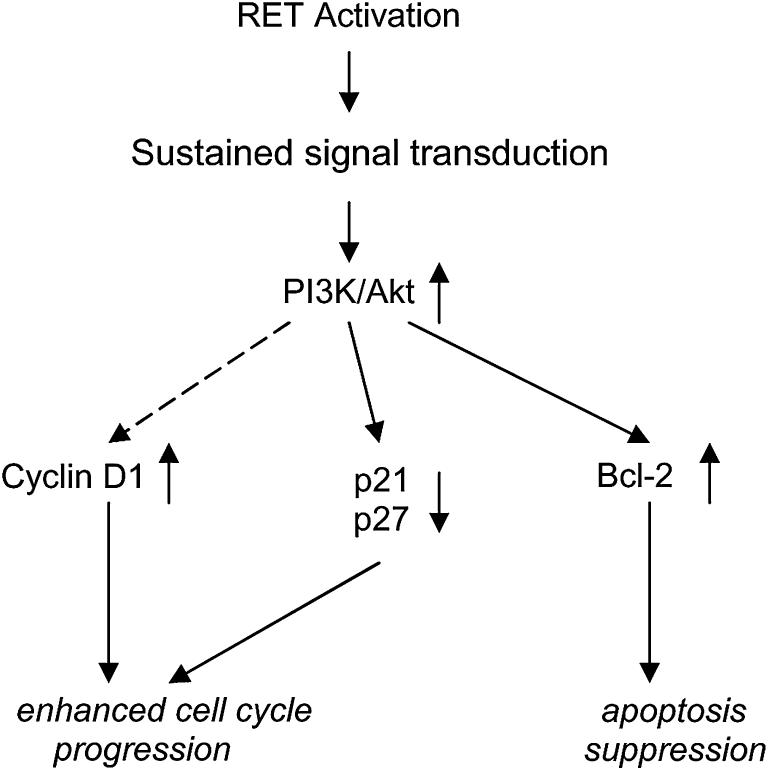
In the study by Drosten et al, RETΔTK transfected cells also were observed to exhibit decreased cyclin D1 levels and increased expression of p21CIP1/WAF1 and p27KIP1 mRNAs, all of which regulate the cell cycle (Fig.2).1 The downregulation of p21CIP1/WAF1 and p27KIP1 by Akt occurs through phosphorylation of the transcription factor AFX/FKHR. Therefore, the oncogenic potential of RET also manifests itself through the PI3K/Akt pathway by enhancing cell cycle progression and decreasing p21CIP1/WAF1 and p27KIP1 production.
Fig 2.
Schematic illustration of the mechanisms that lead to cell survival in MEN2A associated RET mutations that activate PI3K/Akt signaling: apoptosis suppression and enhanced cell cycle progression. RET mutations cause sustained signal transduction and up-regulation of the PI3K/Akt pathway, which then stimulates the expression of antiapoptotic proteins, such as Bcl-2. In addition, production of p21CIP1/WAF1 and p27KIP1, two cyclin-dependent kinase inhibitors, is decreased, while cyclin D1 generation increases. These changes in p21CIP1/WAF1, p27KIP1, and cyclin D1 enhance cell cycle progression, likely at the S-phase. Adapted from Drosten M, Hilken G, Böckmann M, Rödicker F, Mise N, Cranston AN, et al. Role of MEN2A-derived RET in maintenance and proliferation of medullary thyroid cancer. J NCI 2004;96:1231-9.
The activation of the PI3K/Akt cascade appears to play an essential role in the pathogenesis of MEN2B as well. RET mutations found in MEN2B leading to autophosphorylation specifically at tyrosine 1062 have been shown to cause activation of the p85 regulatory subunit of PI3K and subsequent phosphorylation of Akt.7 Salvatore and colleagues demonstrated increased tyrosine 1062 and Akt phosphorylation in cells expressing RET mutations associated with MEN2B compared to those expressing MEN2A.7 In addition, their work established that MEN2B associated RET expression binds the Shc docking protein, which couples receptors to downstream pathways, more actively than MEN2A RET mutations thereby differentially potentiating PI3K/Akt signaling. These authors postulated that taken together, the findings of increased tyrosine 1062 phosphorylation, Shc coupling, and Akt activity in cells expressing a MEN2B associated RET mutation may account for the more aggressive phenotype often displayed in MEN2B patients.
The relationship between RET and the PI3K/Akt pathway in MTC also has been studied by interfering with intermediate tyrosine kinases involved in the signaling cascade. Activation of Src, a family of kinases known to mediate growth factor signaling and mitogenesis, has been linked to expression of the oncogenic RET phenotype and resultant cellular proliferation.2 In the human MTC TT cell line, inhibition of c-Src decreased TT cell growth and DNA synthesis, while increasing apoptosis.2 Moreover, phosphorylation of Akt decreased with c-Src inhibition. These observations suggest that proliferation and survival of TT cells is mitigated through signals involving the PI3K/Akt pathway that inhibit apoptosis.
While c-Src inhibition mediates PI3K/Akt signaling upstream, our group examined the role of PI3K inhibition in MTC. We treated TT cells with LY294002, a well-known PI3K inhibitor that works by binding the p110 catalytic site of the kinase, in order to suppress PI3K/Akt signaling. Inhibition of PI3K led to decreased cell proliferation, Akt phosphorylation, and production of the neuroendocrine hormones human achaete-scute homolog1 and chromogranin A.3 The mechanism of this growth inhibition appeared to occur through increased apoptosis as evidenced by an increase in cleaved poly(ADP-ribose) polymerase (PARP) and caspase-3. Our work further supports the role of the PI3K/Akt pathway in MTC and the importance of Akt in promoting MTC cell survival by evading apoptosis.
CONCLUSION
Overactivation of the PI3K/Akt signaling pathway appears to be an important event in the pathogenesis of medullary thyroid cancer (MTC) and the expression of oncogenic RET in both MEN2A and MEN2B. Despite intensive work elucidating the role of the PI3K/Akt cascade in MTC, little is known about the exact network of downstream mediators. Most researchers believe that this pathway works primarily by suppressing apoptosis; however, more recent evidence suggests that regulation of cell cycle progression may be a parallel mechanism resulting in prolonged cellular survival. The PI3K/Akt signal transduction pathway also has been implicated in the control of the production of the neuroendocrine hormones calcitonin and chromogranin A. The RET mutation clearly plays a role in MTC tumorigenesis and upregulation of PI3K/Akt signaling; however, the RET gene likely signals through a variety of mechanisms. In particular, the Raf/MEK/ERK pathway has also been shown to playa central role in MTC development.4,5 The interaction between these and various other pathways has yet to be fully described. Evidence exists supporting crosstalk between the PI3K/Akt and Raf/MEK/ERK cascades (Fig. 3).1,2 We expect that components of these pathways will emerge as targets for novel therapies in patients with MTC. Over the next 20 years, the development of specific inhibitors of the PI3K/Akt pathway will likely change the treatment of MTC.8
Acknowledgment
Funded in part by the American College of Surgeons Resident Research Scholarship and NIH grant T32 CA009614 Physician Scientist Training in Cancer Medicine.
Abbreviations
- MTC
medullary thyroid cancer
- PI3K
phosphatidylinositol-3 kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drosten M, Hilken G, Böckmann M, Rödicker F, Mise N, Cranston AN, et al. Role of MEN2A-derived RET in maintenance and proliferation of medullary thyroid cancer. J NCI. 2004;96:1231–9. doi: 10.1093/jnci/djh226. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Falola J, Zhu X, Gu Y, Kim LT, Sarosi GA, et al. Antiproliferative effects of Src inhibition on medullary thyroid cancer. J Clin Endocrinology. 2004;89:3503–9. doi: 10.1210/jc.2003-031917. [DOI] [PubMed] [Google Scholar]
- 3.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–15. doi: 10.1016/j.surg.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–71. doi: 10.1016/s0039-6060(03)00418-5. [DOI] [PubMed] [Google Scholar]
- 5.Vaccaro A, Chen H, Kunimalaiyaan M. In-vivo activation of Raf-1 inhibits tumor growth and development in a xenograft model of human medullary thyroid cancer. Anticancer Drugs. 2006;17:849–53. doi: 10.1097/01.cad.0000217424.36961.47. [DOI] [PubMed] [Google Scholar]
- 6.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–30. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 7.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase-3β, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–8. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 8.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 9.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2000;275:3568–76. doi: 10.1074/jbc.275.5.3568. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore D, Melillo RM, Monaco C, Visconti R, Gianfranco F, Vecchio G, et al. Increased in vivo phosphorylation of Ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res. 2001;16:1426–31. [PubMed] [Google Scholar]