Abstract
OBJECTIVES
To examine the association of sleep architecture, sleep disordered breathing, and cognition in older men.
DESIGN
A population-based cross-sectional study.
SETTING
6 sites in the United States.
PARTICIPANTS
2,909 community-dwelling men age 67 or older who were not selected on the basis of sleep problems or cognitive impairment.
MEASUREMENTS
Predictors were measured with in-home polysomnography: sleep architecture, nocturnal hypoxemia (any sleep time with SaO2<80%), apnea-hypopnea index (AHI), and arousal index. Cognitive outcomes were measured by the Modified Mini-Mental State Examination (3MS), Trails B test, and the Digit Vigilance Test (DVT).
RESULTS
Analyses adjusted by age, race, education, BMI, lifestyle, comorbidities and medication use show that those who spent less percent of time in rapid eye movement (REM) sleep had worse levels of cognition: compared to the highest quartile (≥23.7%), those in the lowest quartile (<14.8%) took an average of 5.9 seconds longer on the Trails B and 20.1 seconds longer on the DVT. Similarly, increased percent time spent in stage 1 sleep was related to poorer cognitive function. Those in the highest quartile of stage 1 sleep (≥8.6%) had worse cognitive scores on average compared to those in the lowest quartile (<4.0%). Those with nocturnal hypoxemia took longer to complete the DVT by an average of 22.3 seconds compared to those without, but no associations were found with 3MS or Trails B.
CONCLUSION
Spending less percent of time spent in REM sleep, more percent of time spent in stage 1 sleep, and having higher levels of nocturnal hypoxemia were associated with poorer cognition in older men. Further studies are needed to clarify the direction of these associations and to explore potential mechanisms.
Keywords: sleep architecture, sleep disordered breathing, cognitive function, hypoxemia
INTRODUCTION
At least 10% of people over 65 years old will develop cognitive impairment, with the rate increasing exponentially with advancing age.1,2 With life expectancies rising this number will continue to grow in future years, increasing the importance of identifying factors related to cognitive impairment among older adults which may lead to preventive strategies.
Data suggest that cognitive impairment may be exacerbated by poor sleep. Age-related changes in sleep architecture have been reported, including an increase in time spent in sleep stages 1 and 2 and a decrease in time spent in slow wave sleep (SWS) and rapid eye movement (REM) sleep with increasing age,3 but the potential association between sleep architecture and cognition has received little attention. Small experimental studies, however, have shown clear decrements in cognitive function associated with sleep deprivation or selective reduction of REM or SWS.4–9 These studies support the notion that SWS is needed for consolidation of declarative memory while REM sleep is more important to procedural memory.10,11 Although the associations between sleep architecture and cognition have been conducted in young, healthy volunteers, those studies have predominately tested learning or memory ability before and after sleep deprivation. No study to our knowledge has been conducted in community-dwelling older adults.
Previous studies among older adults have also reported a high prevalence of sleep disordered breathing (SDB) as measured by polysomnography.12,13 Studies have reported that those with diagnosed sleep apnea often have some level of cognitive impairment.13–17 This cognitive dysfunction may improve after treatment with continuous positive airway pressure (CPAP),18–20 and may be attributable to nocturnal hypoxemia (which may preferentially affect hippocampal function) or to sleep fragmentation leading to altered sleep architecture and/or daytime sleepiness.15,16,18 Past studies that have focused on objectively measured SDB or nocturnal hypoxemia have had conflicting results.17, 21–30 These discrepancies may be due to differences in populations and differing measures of cognition. Of note, most previous studies of the association of SDB and cognitive function have been performed in specific populations with clinical disorders, such as patients with insomnia,31,32 dementia,23 or SDB.14,15,17,24,25 Few studies have focused on large populations of community-dwelling older adults who had not been selected on the basis of disease status and examined the association of less severe levels of SDB and cognition.21,26,27,30
This analysis examined the cross-sectional relationship of sleep architecture, SDB, and nocturnal hypoxemia with cognitive function using data gathered on 2,909 community-dwelling older men in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study.
METHODS
Participants
During the Osteoporotic Fractures in Men Study (MrOS) baseline examination from 2000 to 2002, 5,994 community-dwelling men 65 years or older were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.33,34 In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, was conducted between December 2003 and March 2005 and recruited 3,135 of these participants (exceeding the goal of 3,000 participants) for a comprehensive sleep assessment. Men were screened for nightly use of mechanical devices during sleep including pressure mask for sleep apnea [CPAP or bilevel positive airway pressure (BiPAP)], mouthpiece for snoring or sleep apnea, or oxygen therapy and were generally excluded. Of the 2,859 men who did not participant in this ancillary study, 344 died before the sleep visit, 36 had already terminated the study, 332 were not asked because recruitment goals had already been met, 150 were not eligible, and 1,997 refused. Cognitive function data was available for 3,132 men. Of these, 2,909 had in-home overnight polysomnography (PSG) recordings and comprise our analytic cohort. All men with PSG data had information on SDB and nocturnal hypoxemia. Of the 2,909 PSG recordings, 39 had no data available on sleep architecture.
All men provided written informed consent, and the study was approved by the Institutional Review Board at each site.
Polysomnography Predictors
In-home sleep studies were completed using unattended, portable polysomnography (Safiro, Compumedics, Inc.®, Melbourne, Australia). The PSG recordings were to be gathered within 1 month of the clinic visit (mean 6.9 ± 15.8 days from visit), with 78% of recordings gathered within 1 week of the clinic visit. The recording montage was as follows: C3/A2 and C4/A1 electroencephalograms (EEG), bilateral electrooculograms and a bipolar submental electromyogram to determine sleep status; thoracic and abdominal respiratory inductance plethysmography to determine respiratory effort; airflow (by nasal-oral thermocouple and nasal pressure cannula); finger pulse oximetry; lead I EKG; body position (mercury switch sensor); and bilateral tibialis leg movements (piezoelectric sensors). Centrally-trained and certified staff performed home visits to set up the unit, verify the values of the impedances for each channel, confirm calibration of position sensors and note any problems encountered during set-up, similar to the protocol used in the Sleep Heart Health Study.35 Staff returned the next morning to collect the equipment and download the data to the Central Sleep Reading Center (Cleveland, OH) to be scored by certified research polysomnologists. PSG data quality was excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality.
Sleep stages (REM, stages 1–4 NREM) were scored using standard criteria.36,37 Data from EEG leads C3 and C4 were used to score sleep stages 3 and 4, slow wave sleep. Sleep architecture was presented as the percent of total sleep time spent in each stage. Thirty-nine PSG recordings had no information available on sleep architecture. There was additional missing information for specific sleep stages when the PSG scorer thought the data was unreliable (246 for sleep stage 1, 160 for stage 2, 127 for SWS, and 97 for REM). The arousal index was defined as the number of EEG arousals per hour of sleep. The reliability of these measures, determined by rescoring studies over time, indicates that the inter-scorer reliability (ICC) for the percent of sleep time spent in sleep stages 1, 2, slow wave sleep, and REM and the arousal index were .60, .91, .96, .94, and .82, respectively. Similar, although higher levels of intra-scorer reliability were also documented.
Parameters of sleep disordered breathing and nocturnal hypoxemia examined in this analysis included the apnea-hypopnea index (AHI), the percent of time during overnight sleep in which arterial oxygen saturation was below 90% (% of sleep time with SaO2<90%), and having any time during overnight sleep with SaO2<80%. Apnea was defined as complete or near complete cessation of airflow for >10 seconds, and hypopneas were scored if clear reductions in breathing amplitude (at least 30% below baseline breathing) occurred, and lasted >10 seconds.38 In these analyses, only apneas and hypopneas that were each associated with a 3% or greater desaturation were included. AHI was calculated as the total number of apneas and hypopneas per hour of sleep. The inter-scorer reliability for AHI was high (ICC=.99).
Ascertainment of Cognitive Function
Three cognitive function tests were administered at the clinic visit by trained staff: the Trail Making Test – Part B (Trails B), the Modified Mini-Mental State examination (3MS), and the Digit Vigilance Test (DVT).
The Trails B is a timed test that measures attention, sequencing, visual scanning and executive function. Executive function is a measure of the ability for planning or decision making, error correction or trouble shooting, and abstract thinking. The Trails B test requires the participant to continuously scan a page to identify numbers and letters in a specified sequence while shifting from number to letter sets.39 A lower time for completion (in seconds) represents better cognitive functioning.
The 3MS is a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. The 3MS test is a broad sampling of cognitive domains. Scores range from 0 to 100, with higher scores representing better cognitive functioning.40
The DVT is a paper-and-pencil test designed to measure vigilance during rapid visual tracking and accurate selection of target stimuli.41 The DVT measures selectivity and the ability to maintain attention and alertness during repetitive tasks. The standard test requires participants to cross out 6s which appear randomly within 59 rows of 35 single digits. This test was modified to increase difficulty by requiring participants to cross out only those 6s that are followed by a number higher than 6 (7, 8, or 9). A faster time for completion (in seconds) represents better cognitive functioning.
Other Measurements
All participants completed questionnaires at the time of the clinic visit, which included items about demographics, medical history, self-reported health status, physical activity, smoking, caffeine intake and alcohol use. The number of prior medical conditions was calculated as the summed total of self-reported prior diagnoses of common chronic illnesses [stroke or transient ischemic attack (TIA), diabetes, Parkinson’s disease, chronic obstructive pulmonary disease (COPD), hypertension, coronary heart disease (CHD)]. The chronic illness of depression was assessed using the number of depressive symptoms from the Geriatric Depression Scale (GDS), with higher scores corresponding to higher levels of depression.42 Participants were asked to bring in all current medications used within the preceding 30 days. All prescription and nonprescription medications were entered into an electronic database and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).43 The use of antidepressants, benzodiazepines, and sleep medications (non-benzodiazepine, non-barbiturate sedative hypnotics) were categorized. Level of physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).44 Functional status was assessed by collecting information on 5 instrumental activities of daily living (IADL), which included walking 2 to 3 blocks on level ground, climbing up to 10 steps, preparing meals, doing heavy housework, and shopping for groceries or clothing.45,46 Self-reported caffeine intake was calculated based on answers to questions regarding intake of caffeinated coffee, tea and soda.47
A comprehensive examination included measurements of body weight and height. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
The predictor variables were expressed as categorical variables, many of which were defined similarly to previous publications for comparability (sleep architecture predictors and arousal index as quartiles; AHI: <5, 5 to <15, 15 to <30, 30+; percent of sleep time with SaO2 <90%: <1%, 1 to <3.5%, 3.5 to <10%, 10%+; any sleep time with SaO2<80% vs. not).
Characteristics of participants were compared across quartiles of the percent of time spent in REM sleep using chi-square tests for categorical variables, ANOVA for normally distributed continuous variables, and Kruskal-Wallis tests for continuous variables with skewed distributions. Similar comparisons were performed across categories of the other predictors (data not shown).
The association between a given predictor and cognitive function expressed continuously was examined with linear regression models. Results are presented as adjusted means and 95% confidence intervals. The cognitive scores were transformed to meet model requirements (log transformation for both Trails B and DVT, cube transformation for 3MS) and back-transformed for display of results. All models were minimally adjusted for age, ethnicity and clinic. Additional covariates were included in a multivariable model if they were related to one or more of the cognitive outcomes and one or more of the predictors at p<.10.
To enable comparability to other studies with much older populations, the interactions of the predictors with age (<80 vs ≥80 years old) were explored in secondary analyses. Stratifications by these parameters were performed where appropriate (when interaction p<.10). Multivariable models for the sleep architecture predictors were further adjusted by total sleep time (TST) to examine if any association observed was driven by low TST. Associations between COPD and cognition were examined, and further adjustment by COPD for models with nocturnal hypoxemia or SDB predictors were performed to determine if any associations seen between the predictors and cognition were driven by COPD. Finally, to examine whether any associations between sleep stages and cognition were independent of greater nocturnal hypoxemia, and vice-versa, models were performed including both predictors as independent variables.
All significance levels reported were two-sided and all analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the Study Population
The analysis cohort was comprised of 2,909 men with an average age of 76 ± 6 years and 91% were Caucasian. The average percent of time spent in stages of sleep were as follows: stage 1, 7% ± 4%; stage 2, 63% ± 10%; SWS, 11% ± 9%, REM, 19% ± 6%. About half of the men (43%) had moderate or more severe sleep apnea as defined by an AHI≥15, 12% had SaO2<90% for over 10% of sleep time during the night, and 7% of the men spent at least some time during the night with SaO2<80%. The AHI was modestly correlated to both nocturnal hypoxemia predictors (Spearman correlation rho=.60 for percent of sleep time spent with SaO2< 90%; rho =.30 for any SaO2<80% during sleep, p<.001).
The means of the cognitive function outcomes for the cohort were as follows: 121 ± 54 seconds for Trails B completion time, 92 ± 6 points for the 3MS, and 555 ± 188 seconds for the DVT. There were 148 (5.1%) men with probable dementia defined by a 3MS score<80 (n= 97), taking medications for dementia (n = 41), or both (n=10).
Many covariates differed significantly across quartiles of the percent of sleep time spent in REM (Table 1). Those in the lowest quartile(<14.8%) on average were older, had more IADL impairments, had more self-reported medical conditions including hypertension, stroke or TIA, COPD, and CHD, and had more depressive symptoms. Those with lower percent time spent in REM sleep also had lower levels of education, had higher rates of use of antidepressants, were less physically active, and had worse self-reported health (p<.05).
Table1.
Characteristics by Quartile of Percent of Sleep Time Spent in REM
| Quartile | |||||
|---|---|---|---|---|---|
| Q1: <14.8 | Q2: 14.8 to <19.6 | Q3: 19.6 to <23.7 | Q4: >=23.7 | ||
| Variable | (N= 690) | (N= 684) | (N= 698) | (N= 701) | P-value |
| Age, years, mean ± SD | 77.5 ± 5.7 | 76.5 ± 5.6 | 75.8 ± 5.5 | 75.6 ± 5.2 | <.001 |
| Body mass index, kg/m2, mean ± SD | 27.3 ± 4.1 | 27.1 ± 3.9 | 27.0 ± 3.6 | 27.2 ± 3.6 | .68 |
| Race/Ethnicity, % | |||||
| Caucasian | 91.9 | 91.8 | 90.8 | 88.5 | .24 |
| African American | 3.0 | 2.3 | 3.2 | 4.4 | |
| Other | 5.1 | 5.9 | 6.0 | 7.1 | |
| One or more IADL impairments, % | 25.7 | 21.5 | 17.5 | 17.6 | <.001 |
| History of medical conditions, % | |||||
| Hypertension | 55.9 | 48.5 | 48.4 | 47.5 | <.01 |
| Stroke or transient ischemic attack | 13.9 | 12.4 | 8.9 | 9.0 | <.01 |
| Diabetes | 14.8 | 12.4 | 12.9 | 12.4 | .51 |
| Parkinson’s disease | 1.7 | 0.9 | 0.6 | 0.7 | .12 |
| COPD | 7.0 | 5.9 | 3.3 | 4.9 | .02 |
| Coronary heart disease* | 38.0 | 32.7 | 29.1 | 30.0 | <.01 |
| Number of medical conditions, %† | |||||
| 0 | 23.8 | 29.9 | 33.8 | 35.1 | <.001 |
| 1–2 | 63.6 | 59.7 | 59.5 | 56.4 | |
| 3+ | 12.6 | 0.4 | 6.8 | 8.4 | |
| Current antidepressant use, % | 11.6 | 6.3 | 6.7 | 5.1 | <.001 |
| Current benzodiazepine use, % | 5.4 | 4.8 | 2.7 | 4.4 | .09 |
| Current sleep medication use, % | 2.0 | 1.3 | 3.0 | 1.7 | .14 |
| Geriatric Depression Scale score (range 0 to 15), mean ± SD | 2.1 ± 2.4 | 1.7 ± 2.1 | 1.5 ± 1.9 | 1.6 ± 2.0 | <.001 |
| Education, % | |||||
| < high school | 7.4 | 5.1 | 3.6 | 4.0 | <.01 |
| high school | 17.5 | 17.1 | 14.8 | 15.6 | |
| >high school | 75.1 | 77.8 | 81.7 | 80.5 | |
| Alcohol intake, drinks/week, % | |||||
| 0–2 | 64.2 | 57.8 | 57.2 | 59.3 | .09 |
| 3–13 | 32.0 | 36.1 | 36.8 | 35.6 | |
| 14+ | 3.8 | 6.2 | 6.0 | 5.2 | |
| Smoking, % | |||||
| Never | 40.4 | 37.6 | 42.1 | 38.9 | .44 |
| Past | 57.1 | 61.0 | 55.9 | 59.3 | |
| Current | 2.5 | 1.5 | 2.0 | 1.7 | |
| Caffeine use, mg/day, mean ± SD | 225.3 ± 239.4 | 235.7 ± 232.2 | 232.7 ± 247.4 | 250.2 ± 268.2 | .51 |
| Physical Activity Scale for the Elderly (PASE) score, mean ± SD | 136.6 ± 73.4 | 148.4 ± 71.3 | 148.0 ± 69.8 | 151.8 ± 69.4 | <.001 |
| Self-rated health good/excellent, % | 83.0 | 87.3 | 90.7 | 88.0 | <.001 |
P-values for continuous data are from an ANOVA for normally distributed data, Kruskal-Wallis test for skewed data.
P-values for categorical data are from a chi-square test for homogeneity.
Coronary heart disease includes myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty, or heart valve replacement.
Medical conditions include stoke/transient ischemic attack, coronary heart disease, diabetes mellitus, chronic obstructive pulmonary disease, Parkinson's disease, and hypertension.
SD=standard deviation; REM=rapid eye movement; IADL=instrumental activities of daily living; COPD=chronic obstructive pulmonary disease.
Associations between Sleep Architecture and Cognition
There was an association of both REM sleep and stage 1 sleep with cognition after adjustment for multiple confounders (age, race, clinic, BMI, presence of IADLs, depression level, comorbidities, antidepressant use, benzodiazepine use, education, alcohol use, smoking, physical activity level, self-reported health status). After multivariate adjustment, cognitive function increased linearly as levels of the percent of time spent in REM sleep increased (p-trend ≤.008, Table 2). Compared to those with more percent time spent in REM sleep (highest quartile, ≥23.7%), those with less percent time spent is REM sleep (lowest quartile, <14.8%) took an average of 5.9 seconds longer to complete the Trails B and 20.1 seconds longer to complete the DVT. No significant associations were seen with the percent of time spent in REM sleep and the 3MS score. A similar but inverse relationship was seen with stage 1 sleep and cognition after multivariate adjustment (p-trend ≤.01). Compared to those with less percent time spent in stage 1 sleep (lowest quartile, <4.0%), those with more percent time spent is stage 1 sleep (highest quartile, ≥8.6%) took an average of 7.4 seconds longer to complete the Trails B, had about 1 point lower 3MS score, and took an average of 20.6 seconds longer to complete the DVT after multivariate adjustment. There were no significant associations with the percent of time spent in stage 2 sleep and cognition, or the percent of time spent in SWS and cognition. There were no significant associations seen between the arousal index and cognitive function.
Table 2.
Associations of Sleep Architecture and Cognition. Adjusted Means (95% Confidence Interval).
| Trails B Completion Time (seconds) | 3MS Score | DVT Completion Time (seconds) | ||||
|---|---|---|---|---|---|---|
| Minimally | Multivariate | Minimally | Multivariate | Minimally | Multivariate | |
| Predictor | Adjusted* | Adjusted† | Adjusted* | Adjusted† | Adjusted* | Adjusted† |
| % of Sleep Time Spent in Stage 1 | ||||||
| Q1:<4.0% | 106.0 (103.0, 109.2) | 106.2 (103.2, 109.3) | 93.7 (93.3, 94.1) | 93.7 (93.3, 94.0) | 519.7 (509.0, 530.6) | 519.2 (508.7, 529.9) |
| Q2: 4.0 to <5.9% | 108.0 (104.9, 111.2) | 108.6 (105.5, 111.7) | 93.4 (93.1, 93.8) | 93.5 (93.1, 93.9) | 518.3 (507.6, 529.4) | 519.5 (508.9, 530.3) |
| Q3: 5.9 to <8.6% | 110.7 (107.6, 114.0) | 110.4 (107.3, 113.6) | 93.1 (92.7, 93.5) | 93.1 (92.8, 93.5) | 521.2 (510.5, 532.2) | 520.0 (509.4, 530.8) |
| Q4: 8.6%+ | 114.4 (111.1, 117.8) | 113.6 (110.4, 116.9) | 92.7 (92.3, 93.1) | 92.8 (92.4, 93.2) | 540.7 (529.4, 552.2) | 539.8 (528.8, 551.1) |
| P-trend | <.001 | <.001 | <.001 | <.001 | .010 | .013 |
| % of Sleep Time Spent in Stage 2 | ||||||
| Q1:<56.3% | 110.7 (107.5, 113.9) | 111.0 (107.9, 114.2) | 93.4 (93.0, 93.7) | 93.4 (93.0, 93.7) | 527.0 (516.0, 538.2) | 527.8 (517.1, 538.8) |
| Q2: 56.3 to <62.9% | 109.1 (106.0, 112.3) | 109.7 (106.7, 112.8) | 93.0 (92.7, 93.4) | 93.0 (92.6, 93.3) | 521.7 (510.9, 532.7) | 525.0 (514.3, 535.9) |
| Q3: 62.9 to <69.4% | 110.1 (107.0, 113.3) | 110.4 (107.3, 113.5) | 93.2 (92.8, 93.6) | 93.2 (92.9, 93.6) | 525.5 (514.6, 536.5) | 526.8 (516.1, 537.6) |
| Q4: 69.4%+ | 111.6 (108.4, 114.9) | 110.0 (106.9, 113.2) | 93.2 (92.8, 93.5) | 93.3 (92.9, 93.6) | 534.2 (523.1, 545.6) | 526.7 (515.9, 537.8) |
| P-trend | .611 | .750 | .602 | .981 | .324 | .947 |
| % of Sleep Time Spent in Stage SWS | ||||||
| Q1:<3.8% | 109.7 (106.6, 112.9) | 109.2 (106.1, 112.3) | 93.0 (92.6, 93.3) | 93.0 (92.6, 93.3) | 531.1 (520.1, 542.5) | 529.2 (518.3, 540.2) |
| Q2: 3.8 to <9.9% | 111.4 (108.2, 114.6) | 111.4 (108.3, 114.5) | 93.0 (92.7, 93.4) | 93.1 (92.7, 93.4) | 528.6 (517.7, 539.7) | 529.3 (518.6, 540.2) |
| Q3: 9.9 to <16.8% | 109.9 (106.8, 113.0) | 109.7 (106.7, 112.8) | 93.2 (92.9, 93.6) | 93.3 (92.9, 93.6) | 524.2 (513.3, 535.2) | 524.0 (513.4, 534.8) |
| Q4: 16.8%+ | 111.5 (108.4, 114.8) | 111.9 (108.8, 115.1) | 93.4 (93.0, 93.8) | 93.4 (93.0, 93.7) | 528.3 (517.3, 539.4) | 527.9 (517.2, 538.8) |
| P-trend | .591 | .369 | .074 | .085 | .606 | .715 |
| % of Sleep Time Spent in REM | ||||||
| Q1:<14.8% | 115.9 (112.6, 119.2) | 114.2 (111.0, 117.5) | 93.0 (92.6, 93.3) | 93.1 (92.8, 93.5) | 546.5 (535.4, 558.0) | 538.7 (527.8, 549.8) |
| Q2: 14.8 to <19.6% | 111.1 (107.9, 114.3) | 110.9 (107.9, 114.1) | 93.2 (92.8, 93.5) | 93.2 (92.9, 93.6) | 530.7 (520.0, 541.7) | 529.7 (519.3, 540.5) |
| Q3: 19.6 to <23.7% | 108.1 (105.0, 111.2) | 108.9 (106.0, 112.0) | 93.4 (93.0, 93.8) | 93.3 (92.9, 93.6) | 520.6 (510.1, 531.2) | 524.5 (514.2, 535.1) |
| Q4: 23.7%+ | 107.9 (104.9, 111.0) | 108.3 (105.4, 111.4) | 93.2 (92.8, 93.5) | 93.1 (92.8, 93.5) | 516.1 (505.7, 526.6) | 518.6 (508.4, 529.0) |
| P-trend | <.001 | .007 | .283 | .904 | <.001 | .008 |
Adjusted for age, race, and clinic.
Adjusted for age, race, clinic, body mass index, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
DVT=Digit Vigilance Test; SWS=slow wave sleep; REM=rapid eye movement.
Associations between AHI and Nocturnal Hypoxemia with Cognition
There were no significant associations seen between AHI categories and cognitive function. (Table 3) There was an association between the percent of sleep time spent with SaO2<90% and the DVT after adjustment for age, race and clinic but the association was not significant after multivariate adjustment. This loss of significance was primarily driven by the adjustment of BMI, presence of IADLS, and comorbidities. Those with any sleep time with SaO2<80% took longer to complete the DVT (multivariate adjusted average 22.3 seconds longer completion time). Neither of the nocturnal hypoxemia predictors was related to the Trails B test or the 3MS test.
Table 3.
Associations of Sleep Disordered Breathing, Nocturnal Hypoxemia and Cognition. Adjusted Means (95% Confidence Interval).
| Trails B Completion Time (seconds) | 3MS Score | DVT Completion Time (seconds) | ||||
|---|---|---|---|---|---|---|
| Minimally | Multivariate | Minimally | Multivariate | Minimally | Multivariate | |
| Predictor | Adjusted* | Adjusted† | Adjusted* | Adjusted† | Adjusted* | Adjusted† |
| Apnea-Hypopnea Index (per hour sleep) | ||||||
| <5 (n = 618) | 109.5 (106.3, 112.9) | 109.4 (106.1, 112.8) | 93.0 (92.6, 93.4) | 93.0 (92.6, 93.4) | 527.9 (516.3, 539.7) | 527.6 (516.1, 539.3) |
| 5 to <15 (n = 1,029) | 110.1 (107.5, 112.7) | 110.3 (107.8, 112.8) | 93.2 (92.9, 93.5) | 93.2 (92.9, 93.5) | 530.4 (521.5, 539.6) | 531.5 (522.7, 540.4) |
| 15 to <30 (n = 759) | 113.1 (110.0, 116.2) | 113.3 (110.4, 116.4) | 93.0 (92.7, 93.4) | 93.1 (92.8, 93.5) | 530.4 (520.0, 541.1) | 532.2 (522.0, 542.7) |
| 30+ (n = 503) | 112.8 (109.1, 116.8) | 111.7 (108.0, 115.5) | 92.9 (92.5, 93.4) | 93.0 (92.6, 93.5) | 534.7 (521.7, 548.1) | 527.4 (514.6, 540.4) |
| P-trend | 0.087 | 0.166 | 0.595 | 0.926 | 0.492 | 0.963 |
| % Sleep Time with SaO2<90% | ||||||
| <1% (n = 1,410) | 110.9 (108.7, 113.2) | 111.4 (109.1, 113.7) | 93.1 (92.8, 93.3) | 93.1 (92.8, 93.3) | 526.7 (519.0, 534.5) | 528.7 (520.9, 536.6) |
| 1 to <3.5% (n =767) | 111.2 (108.3, 114.3) | 111.3 (108.4, 114.3) | 93.1 (92.7, 93.4) | 93.1 (92.7, 93.4) | 529.4 (519.1, 539.9) | 529.9 (519.8, 540.1) |
| 3.5 to <10%(n=374) | 111.0 (106.8, 115.5) | 110.6 (106.4, 114.9) | 93.2 (92.6, 93.7) | 93.2 (92.7, 93.7) | 532.7 (517.8, 548.1) | 530.2 (515.6, 545.3) |
| 10%+ (n = 358) | 112.4 (108.0, 117.0) | 110.2 (105.8, 114.8) | 93.0 (92.5, 93.5) | 93.2 (92.6, 93.7) | 546.9 (531.2, 563.1) | 536.2 (520.6, 552.4) |
| P-trend | 0.640 | 0.643 | 0.901 | 0.635 | 0.037 | 0.475 |
| Any Sleep Time with SaO2<80% | ||||||
| No (n = 2,715) | 111.1 (109.5, 112.7) | 111.1 (109.6, 112.7) | 93.1 (92.9, 93.3) | 93.1 (92.9, 93.3) | 528.5 (523.0, 534.1) | 528.7 (523.3, 534.1) |
| Yes (n = 194) | 113.2 (107.1, 119.6) | 111.3 (105.4, 117.7) | 92.6 (91.9, 93.4) | 92.8 (92.1, 93.5) | 561.2 (539.5, 583.6) | 551.0 (529.8, 573.2) |
| P-value | 0.524 | 0.941 | 0.215 | 0.382 | 0.004 | 0.046 |
Adjusted for age, race, and clinic.
Adjusted for age, race, clinic, body mass index, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
DVT=Digit Vigilance Test.
Additional Analyses
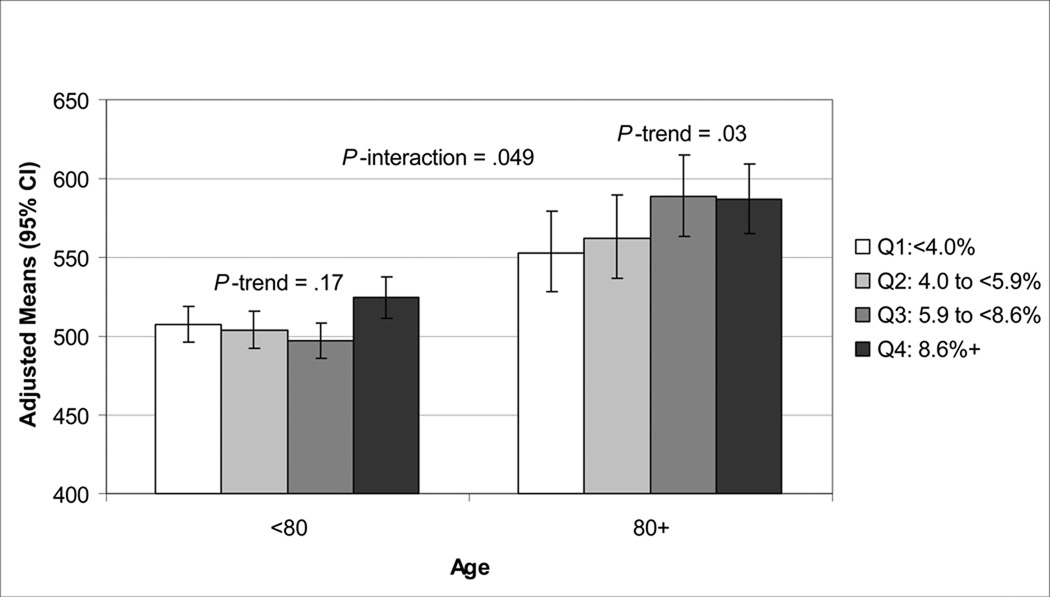
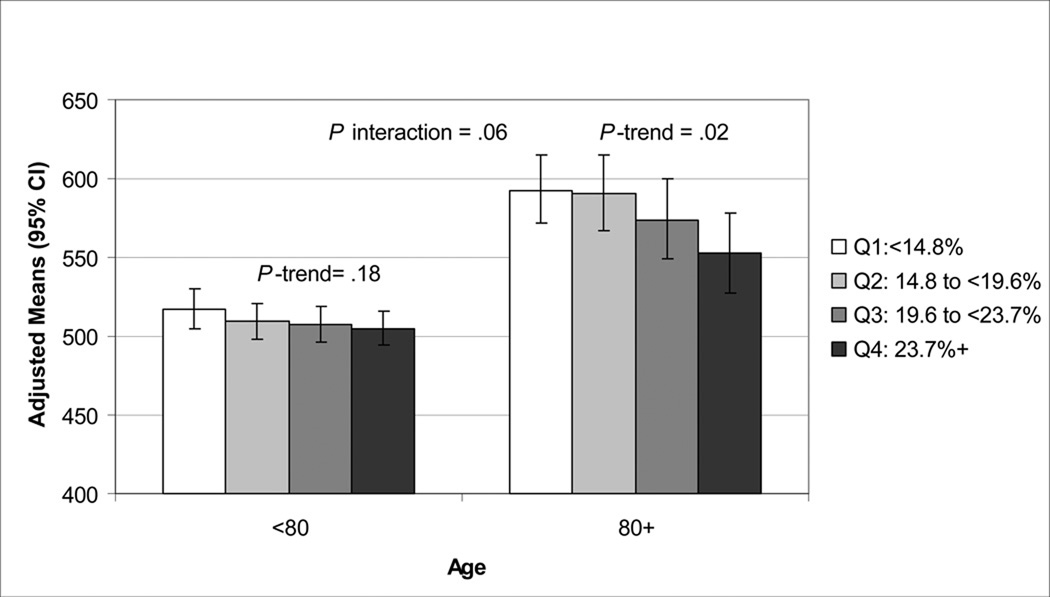
There were significant interactions between age, REM sleep and stage 1 for the DVT outcome (p interaction ≤.06). There was a significant multivariate-adjusted association between DVT completion time with percent of sleep time spent in REM sleep and stage 1 in men 80 years old or older but not in younger men. (Figures 1, 2). Further adjustment of the multivariable models by TST for the sleep architecture analyses did not alter results. Self-report of a history of COPD was not related to AHI or any sleep time spent with SaO2<80%, but was related to any sleep time spent with SaO2<90% (p<.001). History of COPD was associated with Trails B and DVT in minimally adjusted models (p<.02), but lost significance after adjustment for multiple confounders. Further adjustment of the multivariable model by COPD for the outcome of DVT and predictor of any sleep time with SaO2<80% did not significantly alter the association of severe nocturnal hypoxemia with DVT. In multivariable models including both percent of time spent in REM sleep and severe nocturnal hypoxemia (any sleep time with SaO2<80%) as predictor variables, the association of REM sleep and cognition (Trials B, DVT) remained significant. In similar models, the association of stage 1 sleep and cognition (Trials B, 3MS, DVT) also remained significant. The association of severe nocturnal hypoxemia with DVT remained significant after further adjustment for stage 1 sleep, but was attenuated after adjustment for REM sleep (p = .09).
Figure 1.
Association of Percent of Sleep Time Spent in Stage 1 and DVT Completion Time (Seconds).
Adjusted for age, race, clinic, body mass index, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
DVT=Digit Vigilance Test; CI=confidence interval.
Figure 2.
Association of Percent of Sleep Time Spent in REM and DVT Completion Time (Seconds).
Adjusted for age, race, clinic, body mass index, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
DVT=Digit Vigilance Test; CI=confidence interval; REM=rapid eye movement.
DISCUSSION
This cross-sectional analysis of 2,909 older community-dwelling men suggests associations of sleep architecture with poorer cognition. As expected, severe nocturnal hypoxemia (any sleep time with SaO2<80%) was also associated with poorer cognitive function. There was, however, no associations between less severe hypoxemia (sleep time spent with SaO2<90%), AHI or arousal index and cognition.
Spending less percent of sleep time in REM and more in stage 1 was related to poorer cognitive function in this cohort of older men. Specifically, less percent of time spent in REM sleep was related to measures of executive function and attention, while more percent of time spent in stage 1 sleep was related to these two domains and to the global measure of cognition. REM sleep is thought to be important for procedural memory.10,11 While no studies on older adults have been preformed, small studies on young, healthy volunteers have found that subjects with higher amounts of REM sleep performed better on learning motor tasks and memory.4–6,8,9 It has been suggested that SWS is needed for consolidation of declarative memory, but our study found no association with SWS and cognition.10,11 One study examining SWS and cognitive performance among insomniacs found an association with lower levels of SWS and worse performance, but no such relationship among normal controls.32 Changes in both sleep staging and cognition may be partially attributed to changes in health status. While a number of health status confounders have been adjusted for here, there may be unmeasured confounders that have not been accounted for that affect the relationship of sleep staging and cognition. Studies examining the longitudinal relationship of change in sleep architecture with change in cognition are needed to further clarify the association. Future studies that quantify the delta power may have improved ability to detect potential associations between SWS and cognition.
In addition to the relationship of sleep stages and cognition, we also found an association between severe overnight hypoxemia (any sleep time with SaO2<80%) and our measure of attention, the DVT. Numerous studies focusing on those diagnosed with obstructive sleep apnea syndrome (OSAS) have found apneics had worse levels of cognitive function than those without OSAS.14,16,17,20 A review of past studies of the association of OSAS and cognition found the most consistent relationship to be in the domain of attention/vigilance, as we have seen with the association of the DVT and overnight hypoxemia.48 The mechanisms for reduced cognition have been speculated to include effects due to hypoxemia, sleep fragmentation with daytime sleepiness, and altered sleep architecture.14–16,18, 21,22,24,29 Animal studies have shown that experimental hypoxemia can cause a wide range of effects that impact brain function, including cholinergic nerve damage, oxidative stress and inflammation, which in turn, have been shown to be associated with spatial memory and attention deficits.49 An association between nocturnal hypoxemia and executive function tasks has been observed in other studies.15,16,24 It has been recently reported that intermittent hypoxemia is associated to altered cerebral blood flow and reduced verbal memory.50 Our findings support a role for overnight hypoxemia in the pathophysiology of cognitive deficits associated with SDB. Indeed, we see this in the extremes, with worse average completion scores on the DVT for those men with any sleep time spent with SaO2<80%. However, we did not observe associations between AHI or arousal index and cognition, suggesting that in older adults cognitive impairment is more strongly associated with nocturnal hypoxemia than the frequency of respiratory disturbances or arousals. In contrast to these findings, an association between SDB and cognition has been described among elderly community-dwelling women in the Study of Osteoporotic Fractures (SOF).30 In both the SOF and MrOS studies there was no association seen between SDB and the Trails B test, but among the SOF women there was an association seen between SDB and a global measure of cognition, the 30-point Mini-Mental State Exam (MMSE). These differing results found may be related to gender, or to the advanced age of the women in the SOF study. We did stratify by age in the current study and found no association between SDB and cognition among men 80 years or older. The 3MS test which was used in the MrOS study was developed to improve upon the MMSE test by extending the ceiling and floor of the test and to sample a wider range of cognitive abilities, with the MMSE test being restricted in its range of difficulty.40 Perhaps the differing results are due in part to the contrast in instruments used to test global cognition.
This study has several strengths. The study had a large population of community-dwelling older men who were not selected for inclusion based on sleep problems or cognitive impairment. The predictors were gathered objectively with in-home PSG. There were 3 measures of cognition which tested different domains, including a global assessment, executive function and vigilance. The data collected allowed for adjustment for multiple potential confounding factors, with results suggesting these associations were not explained by other covariates including depression, comorbidities, medication use, education, or lifestyle.
This study also had several limitations. The findings may not be generalizable to populations other than community-dwelling older men. Causality cannot be established due to the cross-sectional study design. The association of cognition and sleep staging may be bi-directional, so further research investigating direction of association on incident cognitive decline is needed. Adjustment for numerous covariates was performed, but there may be unmeasured confounders that could affect the results. None of the cognitive tests used specifically measure declarative memory, which is thought to be related to sleep staging.
These cross-sectional findings suggest an association with percent of sleep time spent in both stage 1 and REM with cognition, with less time spent in REM sleep and more in stage 1 sleep being related to poorer cognitive function. Severe nocturnal hypoxemia was related to vigilance, but no association was observed with the apnea-hypopnea index or arousals and cognition in these older community-dwelling men. Due to the lack of studies examining the relationship of cognitive function and sleep staging, these findings need to be replicated in other cohorts. Further study needs to be done to examine if these measures predict longitudinal cognitive decline.
ACKNOWLEDGMENT
Conflict of Interest
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Cancer Institute (NCI), the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, UL1 RR024140, and AG08415.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study "Outcomes of Sleep Disorders in Older Men" under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Dr Yaffe received funding from K 24 grant AG031155.
Terri Blackwell, Dr. Ancoli-Israel, Dr. Redline, Dr. Ensrud, Dr. Stefanick and Dr. Stone have received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page.
Dr. Yaffe is a consultant for Novartis Inc, serves on DSMBs for Pfizer, Medivation, and NIMH, and is a board member for the Beeson Scientific Advisory Committee.
Dr. Ancoli-Israel has consulted for or is on the advisory board of Ferring Pharmaceuticals, GaxoSmithKline, Merck, NeuroVigil, Pfizer, Philips Respironics, Sanofi-Aventis, Sepracor, Scherling-Plough, and Perdue and has received research support from Sepracor and Litebook.
Footnotes
Author Contributions:
Terri L. Blackwell – analysis and interpretation of data, preparation of manuscript
Kristine Yaffe, MD – interpretation of data, critical review of manuscript
Sonia Ancoli-Israel, PhD- study concept and design, interpretation of data, critical review of manuscript
Susan Redline, MD, MPH- study concept and design, acquisition of data, interpretation of data, critical review of manuscript
Kristine E. Ensrud, MD, MPH - study concept and design, acquisition of data, interpretation of data, critical review of manuscript
Marcia Stefanick, PhD- study concept and design, acquisition of data, interpretation of data, critical review of manuscript
Alison Laffan, PhD– interpretation of data, critical review of manuscript
Katie L Stone, PhD- study concept and design, acquisition of data, interpretation of data, critical review of manuscript
Sponsor’s Role:
The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript.
REFERENCES
- 1.Ott A, Breteler MM, van Harskamp F, et al. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 4.Empson JA, Clarke PR. Rapid eye movements and remembering. Nature. 1970;227:287–288. doi: 10.1038/227287a0. [DOI] [PubMed] [Google Scholar]
- 5.Fischer S, Hallschmid M, Elsner AL, et al. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karni A, Tanne D, Rubenstein BS, et al. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 7.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 8.Stickgold R, Whidbee D, Schirmer B, et al. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 9.Tilley AJ, Empson JA. REM sleep and memory consolidation. Biol Psychol. 1978;6:293–300. doi: 10.1016/0301-0511(78)90031-5. [DOI] [PubMed] [Google Scholar]
- 10.Rauchs G, Bertran F, Guillery-Girard B, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- 11.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 12.Mehra R, Stone KL, Blackwell T, Ancoli Israel S, et al. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–1364. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedard MA, Montplaisir J, Richer F, et al. Obstructive sleep apnea syndrome: Pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–964. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 15.Findley LJ, Barth JT, Powers DC, et al. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–262. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 17.Mazza S, Pépin JL, Naëgelé B, et al. Most obstructive sleep apnoea patients exhibit vigilance and attention deficits on an extended battery of tests. Eur Respir J. 2005;25:75–80. doi: 10.1183/09031936.04.00011204. [DOI] [PubMed] [Google Scholar]
- 18.Borak J, Cieślicki JK, Koziej M, et al. Effects of CPAP treatment on psychological status in patients with severe obstructive sleep apnoea. J Sleep Res. 1996;5:123–127. doi: 10.1046/j.1365-2869.1996.d01-60.x. [DOI] [PubMed] [Google Scholar]
- 19.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: A preliminary study. J Clin Sleep Med. 2009;5:305–309. [PMC free article] [PubMed] [Google Scholar]
- 20.Naegele B, Pepin JL, Levy P, et al. Cognitive executive dysfunction in patients with obstructive sleep apnea syndrome (OSAS) after CPAP treatment. Sleep. 1998;21:392–397. doi: 10.1093/sleep/21.4.392. [DOI] [PubMed] [Google Scholar]
- 21.Boland LL, Shahar E, Iber C, et al. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: The Sleep Heart Health Study. J Sleep Res. 2002;11:265–272. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Zion M, Stepnowsky C, Marler M, et al. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–1627. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: Relation to sleep apnea. J Am Geriatr Soc. 1991;39:258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 24.Adams N, Strauss M, Schluchter M, et al. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163:1626–1631. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli Incalzi R, Marra C, Salvigni BL, et al. Does cognitive dysfunction conform to a distinctive pattern in obstructive sleep apnea syndrome? J Sleep Res. 2004;13:79–86. doi: 10.1111/j.1365-2869.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 26.Foley DJ, Masaki K, White L, et al. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 27.Kim HC, Young T, Matthews CG, et al. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 28.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 29.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: The SYNAPSE study. Sleep. 2010;33:515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 31.Bastien CH, Fortier-Brochu E, Rioux I, et al. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 32.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–492. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 33.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 36.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington DC: National Institutes of Health; 1968. NIH publication 204. [Google Scholar]
- 37.American Sleep Disorders Association. EEG arousals: scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 38.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 39.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 40.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 41.Lewis RF, Rennick PM. Manual for the Repeatable Cognitive-Perceptual-Motor Battery. Gross Point Park, MI: Axon Publishing; 1979. [Google Scholar]
- 42.Sheikh J, Yesavage J. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. Geriatric Depression Scale: recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 43.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 44.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and Evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 45.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital & Health Statistics-series 1: Programs & collection procedures. 1987;21:1–115. [PubMed] [Google Scholar]
- 46.Pincus T, Summey JA, Soraci SA, Jr, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 47.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 48.Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc. 2004;10:772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 49.McCoy JG, McKenna JT, Connolly NP, et al. One week of exposure to intermittent hypoxia impairs attentional set-shifting in rats. Behav Brain Res. 2010;210:123–126. doi: 10.1016/j.bbr.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiratli PO, Demir AU, Volkan-Salanci B, et al. Cerebral blood flow and cognitive function in obstructive sleep apnea syndrome. Hell J Nucl Med. 2010;13:138–143. [PubMed] [Google Scholar]