Abstract
As memory CD8 T cells form during acute viral infection, several changes in gene expression and function occur, but little is known about the control of this process. It was reported previously that the homodimer CD8αα was involved in generating IL-7Rαhigh memory CD8 T cell precursors, and consequently, protective memory CD8 T cells did not form in animals significantly impaired in CD8αα expression (E8I−/− mice). However, the precise contribution of CD8αα to sustained IL-7Rα expression and other memory CD8 T cell-associated changes has not been investigated. We found that IL-7Rα expression and generation of memory CD8 T cells that protect against secondary viral infection was considerably normal in E8I−/− animals. Interestingly, virus-specific CD4 T cell responses were elevated, and the relative surface levels of CD8αβ in activated T cells were reduced in E8I−/− mice compared with wild-type animals. Our results indicate that memory CD8 T cell development can occur independently of CD8αα.
Memory T cells are critical for mediating long-term protective immunity against infectious disease, but only a few of the key signals and pathways that govern their formation have been elucidated. Recent work has shown that IL-7 is essential for generation and maintenance of both memory CD8 and CD4 T cell populations (Ref. 1 and references within). The ability of an effector CD8 T cell to survive and become a memory CD8 T cell relies on both the availability of IL-7, as well as the effector cell’s ability to express the IL-7R α-chain (IL-7Rα)3 (2–4). During an acute viral or bacterial infection, the expression of IL-7Rα is down-regulated in the majority of activated effector CD8 T cells, but at the peak of the CD8 T cell response, a small subset of effector CD8 T cells express higher levels of IL-7Rα (and these are referred to as IL-7Rαhigh cells) (2–5). Additional experiments showed that the IL-7Rαhigh effector CD8 T cells preferentially survive and become the long-lived memory CD8 T cells that protect against reinfection, making IL-7Rα a marker that distinguishes the activated T cells that will survive (i.e., memory cell precursors) from those that will die following infection (2, 4).
A molecule recently implicated in directing IL-7Rα expression on memory CD8 T cell precursors is the homodimer CD8αα (5). CD8αα is a ligand for the nonclassical MHC molecule thymic leukemia (TL) Ag and can be detected with TL tetramers (6). One study found that CD8αα was coexpressed transiently on IL-7Rαhigh effector CD8 T cells during lymphocytic choriomeningitis virus (LCMV) infection (between days 7 and 14 postinfection (p.i.)). Moreover, IL-7Rαhigh effector CD8 T cells were not detected at the peak of clonal expansion (day 7 p.i.) in animals that are significantly defective in expressing CD8αα homodimers (5). These mutant animals (referred to as E8I−/− mice) contain a deletion of the E8I enhancer in the intergenic region of the CD8 αβ gene complex (7). Formation of protective memory CD8 T cells was also greatly impaired in E8I−/− mice, presumably due to defective IL-7Rαhigh memory CD8 T cell precursor development (5).
Several studies have highlighted that functionally competent memory CD8 T cells form over several weeks to months following acute infection, and during this maturation period, several changes are observed in gene expression and functional responses (Ref. 8 and references within). When integrated, these observations suggest that CD8αα acts early to induce formation of IL-7Rαhigh memory CD8 T cell precursors, but their subsequent maturation into memory CD8 T cells is conducted independently of CD8αα. Because of this temporal discordance between CD8αα expression and memory CD8 T cell maturation, we aimed to determine whether CD8αα controlled other aspects of memory CD8 T cell development, in addition to IL-7Rα expression. Through a comprehensive analysis of effector and memory CD8 T cell differentiation during LCMV infection, we found that the generation of memory CD8 T cells, based on number, form, and function, was relatively normal in E8I−/− mice compared with wild type (WT; C57BL/6). Our data indicate that CD8αα is not a primary signal controlling IL-7Rα expression on memory CD8 T cell precursors or their development into long-lived memory CD8 T cells that can protect against secondary infection.
Materials and Methods
Mice and viral infection
C57BL/6J (WT) mice were purchased from The Jackson laboratory, and E8I−/− knockout mice were kindly provided by D. Littman (Skirball Institute, New York, NY) and H. Cheroutre (La Jolla Institute for Allergy and Immunology, San Diego, CA). Mice were infected with 2 × 105 PFU of LCMV-Armstrong i.p. or 2 × 106 PFU of LCMV-clone 13 i.v. Viral titers were quantified from serum and tissues of infected mice by plaque assay on Vero cell cultures as described previously (9). The animals were housed and used under approved institutional animal care and use committee protocols.
Genotyping of E8I−/− mice
Genomic DNA was extracted from blood by DNeasy tissue kit (Qiagen) according to the manufacturer’s instructions and PCR amplified with gene-specific primers for WT and E8I alleles: a common forward primer, 5′-ATTCCCAACACCCACTACAAG-3′, reverse WT primer, 5′-AGCTATCTTCAGACGTGTCAG-3′, and reverse E8I primer, 5′-GGGGCTATAGCTCTGTAGGTCA-3′ that amplifies a 1.3- and a 1.6-kb product, respectively. Annealing temperatures for the WT and mutant primer sets was 54°C and 57°C, respectively.
Lymphocyte isolation and cell surface and intracellular cytokine staining
Lymphocytes were isolated from spleen, blood, liver, lung, and intestine as described previously (10). Intraepithelial lymphocytes (IELs) were isolated from the intestine as described in Ref. 11. Abs against IL-7Rα (CD127), CD3, CD8α, B220, IFN-γ, IL-2, and TNF-α were purchased from eBioscience, and CD4, Fas, and IgD were purchased from BD Biosciences. H-2Db tetramers bound to LCMV peptides NP396–404, GP276–86, and GP33–41 were generated as described previously (12). Surface and intracellular cytokine staining was performed as described previously (12). Cells were stained with TL tetramer (2.5–5 µg/ml), and Abs to CD3, CD4, and CD44 or for 30 min on ice (and CD8 T cells were examined by gating on CD3+CD4− cells), or TL tetramer was added first followed by the addition of Abs to CD8α, CD8β, and CD44.
LCMV-specific IgG ELISA
LCMV-specific IgG Ab titers in sera were determined by solid-phase ELISA, as described previously (9).
Results and Discussion
Normal IL-7Rα expression by LCMV-specific effector and memory CD8 T cells in E8I-deficient mice
Before initiating our studies, the genotype of the E8I−/− mice was verified by using functional and molecular assays (data not shown). By staining IELs for CD3, CD8α, CD8β, and TL tetramers, we confirmed that the development of CD8αα IELs was greatly reduced in the E8I−/− mice as shown previously (5, 7). Their genotype was also verified by PCR, which showed that all E8I−/− mice in our studies are homozygous for this deletion.
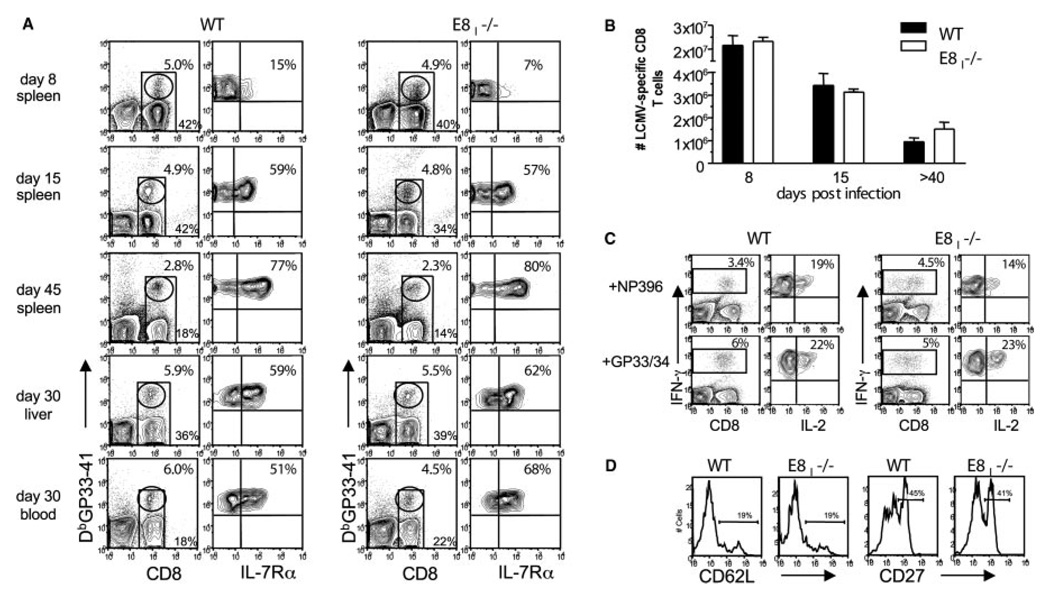
To examine the expression of IL-7Rα on effector and memory CD8 T cells, we infected WT and E8I−/− mice with LCMV and 8, 15, and 45 days later, cells were isolated from the blood and spleen and stained with Abs to CD8α and IL-7Rα and MHC class I tetramers DbGP33–41 and DbNP396–404, which bind LCMV-specific CD8 T cells (Fig. 1A and data not shown). At day 8 p.i., ~5–15% of LCMV-specific effector CD8 T cells expressed IL-7Rα in both WT and E8I−/− mice (Fig. 1A). Over the next several weeks, the proportion of IL-7Rαhigh LCMV-specific CD8 T cells in WT and E8I−/− mice continued to increase in an identical pattern. By day 45 p.i., between 70 and 80% of the memory CD8 T cells in both WT and E8I−/− mice expressed high amounts of IL-7Rα (Fig. 1A). The frequency of NP396–404- and GP276–86-specific CD8 T cells and their expression of IL-7Rα was also similar between WT and E8I−/− mice (data not shown). These data show that neither the initial formation of IL-7Rαhigh memory CD8 T cell precursors nor their ability to maintain IL-7Rα expression as they matured into memory CD8 T cells was abrogated by the lack of CD8αα during acute viral infection.
FIGURE 1.
Normal IL-7Rα expression pattern and numbers of LCMV-specific CD8 T cells in E8I-deficient animals. A,WT and E8I−/− mice were infected with LCMV-Armstrong, and on days 8, 15, and 30–55 p.i., the lymphocytes were isolated from the spleen, liver, and blood and stained for CD8α, IL-7Rα, and DbGP33–41 MHC class I tetramers. Plots on left show percentage of CD8 T cells (bottom numbers) and of tetramer+ CD8 T cells (top numbers), and plots on the right show percentage of IL-7Rαhigh GP33–41-specific CD8 T cells. The frequency of NP396–404- and GP276–86-specific CD8 T cells and their expression of IL-7Rα was also similar between WT and E8I−/− mice (data not shown). B, Bar graph shows the combined numbers of LCMV-specific CD8 T cell populations (NP396–404, GP33–41/34–41, GP276–86, and NP205–212) in the spleen that were calculated based on intracellular IFN-γ staining after 5 h of stimulation with peptides. C, Spleno-cytes from WT and E8I−/− mice (~30–55 days p.i.) were stimulated with NP396–404 and GP33–41/34–41 peptides for 5 h and stained for CD8α, IFN-γ, and IL-2. Left panel shows the percentage of IFN-γ+CD8 T cells, and the right panel (gated on IFN-γ+ cells) shows the percentage of cells that coproduce IL-2.D, Splenocytes from WT and E8I−/− mice infected ~50 days prior were stained for CD8α, CD62L, CD27, and DbGP33–41 MHC class I tetramers. Histograms show CD62L and CD27 expression levels on tetramer+ CD8 T cells.
The expression of CD8αα on effector CD8 T cells at day 8 p.i. was also examined to see if there was a correlation between IL-7Rα and CD8αα expression as reported previously (5). Using TL tetramers, we failed to reproducibly identify a clear population of CD8αα+ effector CD8 T cells in the spleens of WT animals, whereas the same staining protocols routinely detected CD8αα+ T cells within the IEL population, indicating that the TL tetramer was functional (data not shown). Perhaps more optimized staining protocols than those used here are required to detect CD8αα+ effector CD8 T cells in the spleen because their overall amounts of CD8αα expression is significantly lower than that of IELs.
Memory CD8 T cell maturation appears normal in E8I-deficient animals
Because the LCMV-specific CD8 T cells that survived the contraction phase in E8I−/− mice showed a typical pattern of IL-7Rα expression, we next examined the numbers of memory CD8 T cells that formed. The frequency and number of LCMV-specific CD8 T cells in the spleens of E8I−/− and WT mice were calculated at days 8, 15, and 30–55 p.i. (Fig. 1). Using both MHC class I tetramer staining (Fig. 1A) and intracellular cytokine staining for IFN-γ (Fig. 1C), we found similar numbers of CD8 T cells specific for the dominant (DbNP396–404, DbGP33–41, and KbGP34–41) and subdominant (DbGP276–86 and DbNP205–12) LCMV epitopes in WT and E8I −/− mice at all time points (Fig. 1B). At days 8 and 15 p.i., both groups of mice contained ~20–25 × 106 and 3–5 × 106 LCMV-specific CD8 T cells, respectively. The similarity in numbers at day 15 p.i. indicates that effector cell apoptosis was not occurring at higher than normal rates in E8I−/− mice. At days 30–55 p.i., the WT and E8I−/− mice contained equivalent numbers of LCMV-specific memory CD8 T cells ranging from ~1 to 3 × 106 cells/spleen (Fig. 1, B and C). Similar numbers of memory CD8 T cells were also found in the liver, lung, and blood of E8I−/− mice, indicating that memory CD8 T cells were also generated in nonlymphoid organs (Fig. 1A and data not shown).
Importantly, the maturation of memory CD8 T cells also appeared to occur normally in E8I−/− mice based on expression of IL-7Rα, L-selectin (CD62L), CD27, and production of IL-2 (Fig. 1, C and D). At days 30–55 p.i., the memory CD8 T cells in both WT and E8I−/− mice were ~70–80% IL-7Rαhigh, ~20–30% CD62Lhigh, ~40–50% CD27high, and ~15–25% could produce IL-2 (13). Taken together, it appeared that memory CD8 T cell differentiation and survival was not defective in animals lacking CD8αα expression.
Increased virus-specific CD4 T cell responses in E8I-deficient animals
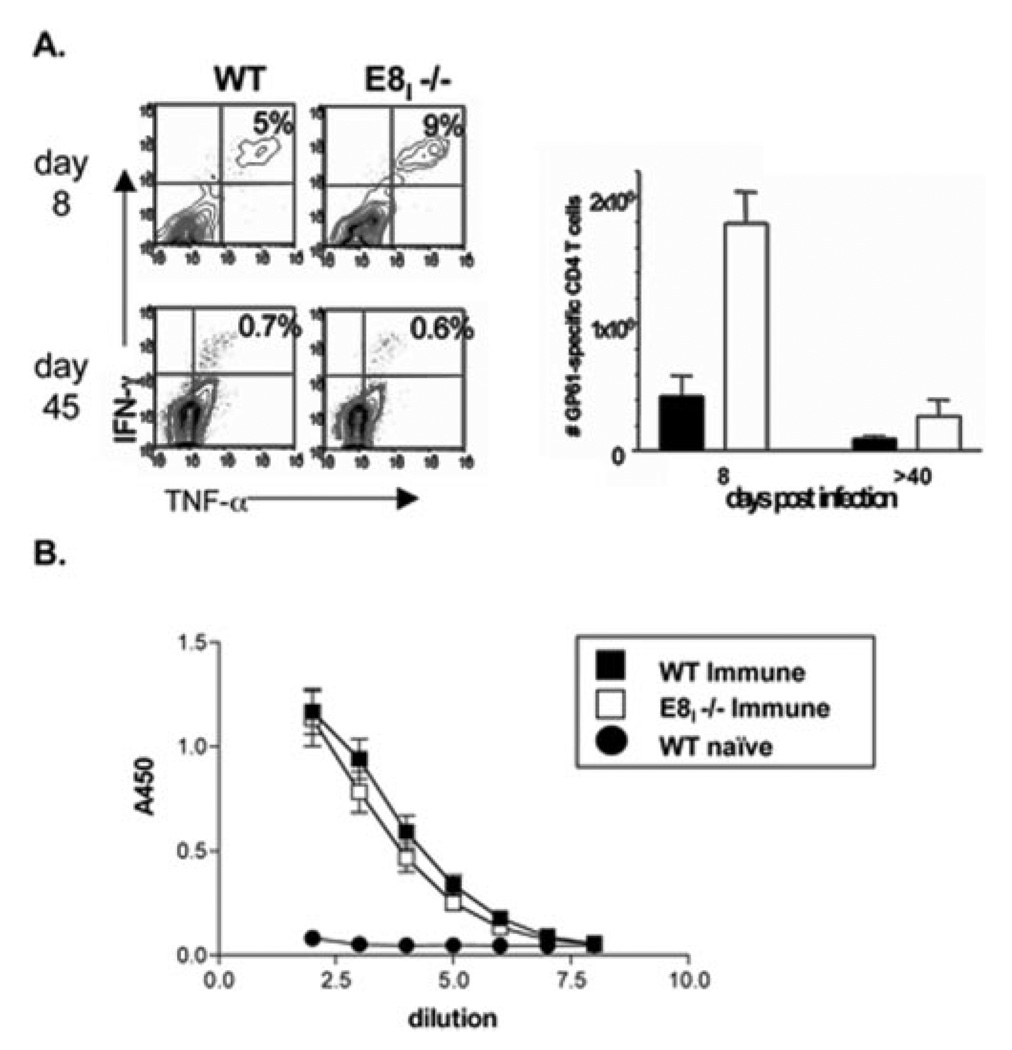
In addition to the CD8 T cell responses, the virus-specific CD4 T and B cell responses were examined. The number of LCMV-specific CD4 T cells specific for the GP61–80 epitope was measured at days 8 and 45 p.i. by intracellular cytokine staining for IFN-γ and TNF-α (Fig. 2A). Interestingly, there was a 2- to 4-fold increase in the number effector and memory GP61–80-specific CD4 T cells in E8I−/− mice. This correlated with a slight increase in the number of germinal center B cells in E8I−/− mice at day 15 p.i. as assessed by flow cytometry of B220+, IgD−, PNA+, Fas+ B cells, but there was no apparent difference in the titers of LCMV-specific IgG Abs compared with WT mice (Fig. 2B and data not shown). Although the underlying cause for the elevated CD4 T cell response is not clear, it is possible that CD8αα functions in another cell type, such as CD8α+ dendritic cells, to negatively regulate CD4 T cell expansion. Further investigation of this finding is needed.
FIGURE 2.
CD4 T cell and B cell responses in E8I−/− mice during LCMV infection. A, Splenocytes from WT and E8I−/− mice infected 8 and 45 days previously were stimulated with GP61–80 peptide for 5 h and stained for CD4, IFN-γ, and TNF-α. Plots are gated on CD4 T cells and show the percentage of IFN-γ+ and TNF-α+ cells. Bar graph on right shows numbers of GP61–80-specific CD4 T cells in the spleen in WT (■) and E8I−/− (□) animals. B, LCMV-specific IgG were measured in the serum of uninfected animals (naive) or in animals infected ~40–55 days previously using ELISA.
The E8I enhancer is needed for optimal CD8α expression in activated CD8 T cells
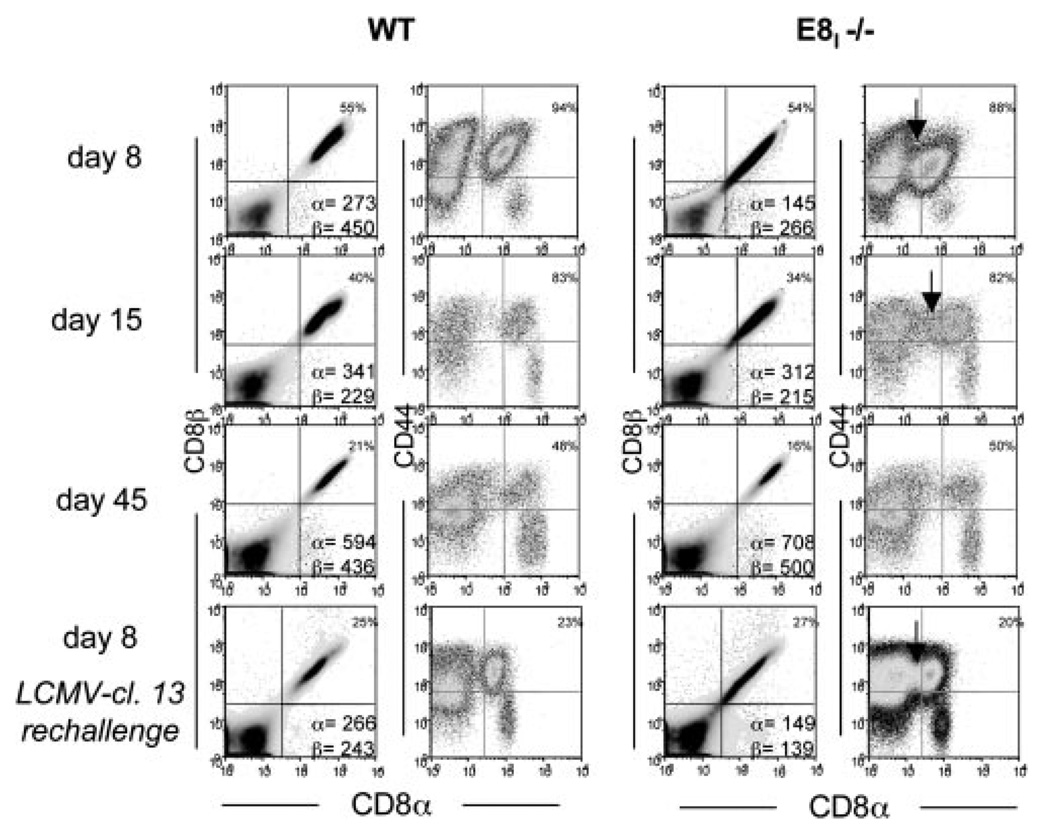
During the above experiments, we noted that amounts of surface CD8α was lower in a portion of the activated LCMV-specific CD8 T cells in E8I−/− mice when analyzed directly ex vivo or after 5 h of stimulation in vitro (Figs. 1C, 3, and 4C). At day 8 p.i. the CD8α median fluorescent intensity was ~20–45% lower in E8I−/− animals compared with WT (Fig. 3). Because murine CD8β cannot traffic to the plasma membrane independent of CD8α (14), CD8β was decreased correspondingly. As time postinfection proceeded, the reduced CD8αβ expression became less apparent (days 15 and 45; Fig. 3). However, the CD8α down-regulation was observed again 5 and 8 days after secondary LCMV-cl.13 infection (Figs. 3 and 4C and data not shown). The extent of CD8α down-regulation was variable between E8I−/− mice, but it was consistently observed in over 30 animals analyzed. Thus, it appears that the E8I enhancer may operate to maintain normal expression of CD8αβ on recently activated CD8 T cells.
FIGURE 3.
Decreased expression of CD8αβ is observed in activated CD8 T cells in E8I-deficient mice. Splenocytes from WT and E8I−/− mice infected 8, 15, and 45 days previously with LCMV-Armstrong were stained for CD8α, CD8β, and CD44. Bottom row shows data from LCMV immune animals 8 days after LCMV-cl.13 reinfection. The median fluorescent intensity of CD8α and CD8β is indicated in lower right quadrant of left panels. Arrows highlight CD44high CD8 T cells with reduced CD8α expression.
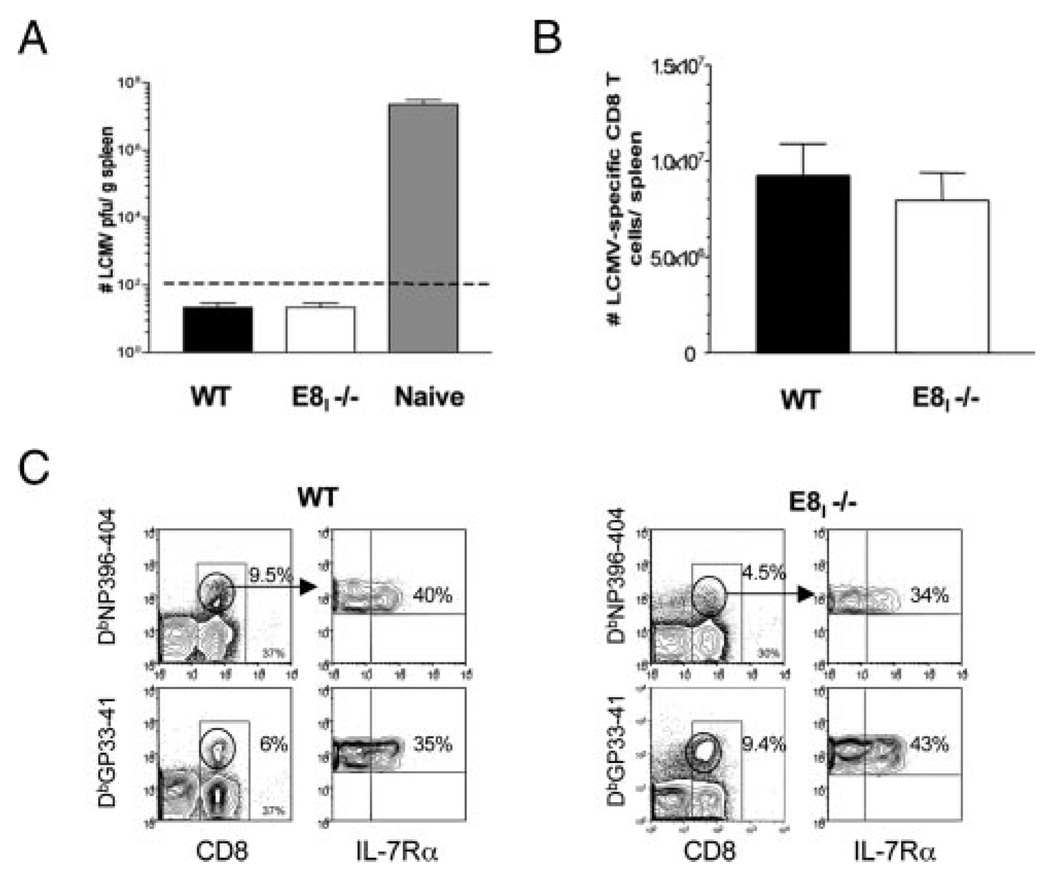
FIGURE 4.
Protective memory CD8 T cell responses in E8I−/− mice. A–C, WT and E8I−/− mice were infected with LCMV-Armstrong, and ~50–55 days later, the mice were reinfected with LCMV-cl.13 and analyzed 8 days later. A, Bar graph shows cl.13 virus titers in the spleen at day 8 p.i. Naive mice were infected for a positive control of infection. Dashed line denotes level of detection. B, Bar graph shows the combined numbers of DbNP396–404, DbGP33–41, and DbGP276–86-specific CD8 T cells in the spleen 8 days p.i. that were calculated based on MHC class I tetramer staining. C, IL-7Rα expression on NP396–404- and GP33–41-specific CD8 T cells 8 days p.i. Left panels show percentage of CD8 T cells (bottom numbers) and tetramer+ CD8 T cells (top numbers). Right panels show percentage of IL-7Rαhightetramer+ CD8 T cells. Note the reduction in CD8α expression on tetramer+ CD8 T cells in E8I−/− mice; these CD8αlow cells were not included in the calculations shown in B.
Protective memory CD8 T cell recall responses in E8I-deficient mice
The above data showed that the formation of memory CD8 T cells was not defective in E8I−/− mice. Therefore, we tested the ability of the memory CD8 T cells to protect against a secondary LCMV infection by reinfecting LCMV-immune WT and E8I−/− mice with a more virulent strain of LCMV (cl.13).WT immune animals mount rapid recall responses and clear the virus within 5 days (9). No virus was detected in the serum (day 5 p.i., data not shown) or in the spleen (day 8 p.i.) in either WT or E8I−/− mice, indicating that the E8I−/− mice can efficiently clear a secondary LCMV-cl.13 infection (Fig. 4A). Overall, the secondary clonal expansion of the memory CD8 T cells was very similar between WT and E8I−/− mice, and both groups contained ~8–10 × 106 LCMV-specific CD8 T cells at day 8 p.i. (Fig. 4B). When the different epitope-specific CD8 T cell populations were analyzed individually, small differences between WT and E8I−/− mice were observed. WT mice contained ~25–30% more DbNP396–404- and DbGP276–86-specific CD8 T cells in their spleens at day 8 p.i. compared with E8I−/− mice (Fig. 4C and data not shown). However, there was a compensatory ~30% increase in the number of DbGP33–41-specific CD8 T cells in the E8I−/− compared with WT mice. Thus, the total number of LCMV-specific secondary effector CD8 T cells was very similar between WT and E8I−/− mice. The pattern of IL-7Rα expression on these cells did not differ between the two groups, again indicating that virus-specific CD8 T cells can express IL-7Rα independent of CD8αα signals (Fig. 4C). Altogether, these data show that the LCMV-specific memory CD8 T cells in E8I−/− mice were capable of profound expansion and viral clearance in response to a secondary infection, indicating that their protective responses were intact.
In summary, our analysis of the E8I−/− mice indicates that CD8α signals are not necessary for IL-7Rα expression or for memory CD8 T cell formation during LCMV infection. However, it should be noted that the expression of CD8αα in E8I−/− mice is significantly reduced but not entirely ablated (7). Therefore, it is plausible that greatly reduced to undetectable amounts of CD8αα are sufficient to promote memory CD8 T cells to develop. A better test of this model may be to develop transgenic animals that contain specific mutations in CD8α that inhibit CD8αα homodimer but not CD8αβ heterodimer formation. The results of such a study should be revealing.
The memory CD8 T cells in E8I-deficient animals could mount protective recall responses and control secondary viral infection. The overall secondary expansion of memory CD8 T cells in E8I−/− mice following LCMV reinfection was similar to that of WT mice; however, minor differences were noted when different epitope-specific CD8 T cell populations were examined. The reduction in CD8αβ expression was acutely evident on the secondary effector CD8 T cells during LCMV-cl.13 reinfection in E8I−/− animals, and perhaps the decreased expression of CD8αβ contributed to the reduced expansion or survival of NP396–404- and GP276–86- specific effector CD8 T cells. Recent work shows that lowered amounts of surface CD8α can exacerbate proliferative and functional CD8 T cell responses (15). Therefore, it is a formal possibility that variations in CD8 T cell responses in the E8I−/− mice can be attributed to reduced levels of CD8αβ rather than, or in addition, to CD8αα.
An underlying aspect of many models of memory CD8 T cell development is that the “strength of signal” experienced by a T cell impacts its ability to become a memory CD8Tcell (16, 17). Indeed, the proposed role of CD8αα in memory CD8 T cell development is to down-modulate TCR signals and promote effector cell survival by sequestering lck away from the TCR via its exclusion from lipid rafts (5). This model holds much credibility, and our studies here do not directly examine this point. Our results indicate that if TCR signaling is heightened in E8I−/− animals (as put forth in Ref. 5), then this level is not sufficient to inhibit formation of IL-7Rαhigh memory CD8 T cell precursors or their ability to develop into protective memory CD8 T cells during acute LCMV infection. The reason(s) for the discrepancies between our study and Madakamutil et al. (5) is not clear because, for the primary infection, similar doses of LCMV-Armstrong and routes of infection were used. Therefore, the generation of IL-7Rα-expressing memory CD8 T cells in E8I−/− mice cannot be attributed to differences in viral infection. Moreover, a recent study by Zhong and Reinherz (18) have found that the formation of functional memory CD8 T cells also occurs normally in E8I−/− mice after influenza infection. Another recent study by Williams and Bevan have demonstrated that a single MHC class Ia molecule is sufficient for formation of memory CD8 T cells, suggesting that TL signals are not required for memory CD8 T cell development (19). Altogether, these studies question the role of CD8αα in memory CD8 T cell differentiation, and thus, more direct and comprehensive studies are needed.
Acknowledgments
We thank T. Pasqualini for technical assistance, Drs. G. Shadel, E. J. Wherry, M. Krishna, R. Ahmed, F. Lakkis, G. Chalasani, and J. Obhrai and N. Joshi for critical reading of the manuscript, and W. Ellmeier for PCR protocols. H. Cheroutre kindly provided the TL-tetramer and E8I−/− mice (with permission from D. Littman).
Footnotes
This work was supported by Burroughs-Wellcome Fund 1004313 (to S.M.K.), National Institutes of Health Grant R01 AI 066232-01 (to S.M.K.), Edward Mallinckrodt, Jr., Foundation (to S.M.K.), and Cancer Research Institute (to S.M.K.).
Abbreviations used in this paper: IL-7Rα, IL-7R α-chain; TL, thymic leukemia; LCMV, lymphocytic choriomeningitis virus; p.i., postinfection; WT, wild type; IEL, intraepithelial lymphocyte; CD62L, L-selectin.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 3.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 4.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, Ellmeier W, von Herrath MG, Jensen P, Littman DR, Cheroutre H. CD8αα-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 6.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8αα and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 7.Ellmeier W, Sunshine MJ, Losos K, Littman DR. Multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity. 1998;9:485–496. doi: 10.1016/s1074-7613(00)80632-9. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ, Ahmed R. Memory CD8 T cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MD, Parrott DM. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22:481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 14.Hennecke S, Cosson P. Role of transmembrane domains in assembly and intracellular transport of the CD8 molecule. J. Biol. Chem. 1993;268:26607–26612. [PubMed] [Google Scholar]
- 15.Maile R, Siler CA, Kerry SE, Midkiff KE, Collins EJ, Frelinger JA. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J. Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 18.Zhong W, Reinherz EL. Complexity of CD8αα homodimer expression and function during antiviral CD8 T cell memory generation. Eur. J. Immunol. 2005 doi: 10.1002/eji.200535162. In press. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, Bevan MJ. Cutting edge: a single MHC class Ia is sufficient for CD8 memory T cell differentiation. J. Immunol. 2005;175:2066–2069. doi: 10.4049/jimmunol.175.4.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]