Abstract
Introduction
The soleus H-reflex is dynamically modulated during walking. However, modulation of the gastrocnemii H-reflexes has not been studied systematically.
Methods
The medial and lateral gastrocnemii (MG and LG) and soleus H-reflexes were measured during standing and walking in humans.
Results
Maximum H-reflex amplitude was significantly smaller in MG (mean 1.1 mV) or LG (1.1 mV) than in soleus (3.3 mV). Despite these size differences, the reflex amplitudes of the three muscles were positively correlated. The MG and LG H-reflexes were phase- and task-dependently modulated in ways similar to the soleus H-reflex.
Discussion
Although there are anatomical and physiological differences between the soleus and gastrocnemii muscles, the reflexes of the three muscles are similarly modulated during walking and between standing and walking. The findings support the hypothesis that these reflexes are synergistically modulated during walking to facilitate ongoing movement.
Keywords: synergist, task-dependent modulation, phase-dependent modulation, locomotion, spinal reflex
Introduction
The soleus H-reflex is dynamically modulated during motor tasks in humans. The soleus H-reflex amplitude at a given background electromyography (EMG) level decreases from standing to walking, and from walking to running (i.e., task-dependent modulation) 1–4. Furthermore, the soleus H-reflex is modulated during walking depending on the phase of the gait cycle (i.e., phase-dependent modulation) 3–5. In contrast to the large number of investigations of modulation of the soleus H-reflex, the number of studies of H-reflexes in its synergists, medial and lateral gastrocnemius (MG and LG, respectively) have been limited to specific tasks such as hopping 6, 7, landing 7, 8, and lengthening and shortening contraction 9. In general, the H-reflexes of the soleus and the gastrocnemii were modulated during a motor task in similar ways, although Pinniger et al. 9 found that the ratio of maximum H-reflex to maximum M-wave was larger in the soleus than in the MG. On the other hand, Moritani et al. 6 reported that the amplitude of the MG H-reflex during hopping elicited shortly after foot contact increased as force and speed of the motor task increased, whereas the soleus H-reflex showed an opposite pattern. However, this study had only one subject, and the H-reflex size was not evaluated in relation to the background EMG level; thus, it is not clear how differently the H-reflex is modulated during hopping between the soleus and the MG. The MG and LG, together with the soleus, are traditionally considered as one functional unit of ankle plantarflexor muscles (i.e., the triceps surae)10. However, to date, whether and to what extent the MG and LG H-reflexes are modulated between and during standing and walking have not been studied.
There are several clear differences between the gastrocnemii and the soleus. Anatomically, the gastrocnemius muscles are two-joint muscles which operate at both the ankle and knee joints, whereas the soleus is a single-joint plantarflexor muscle. Thus, ankle and/or knee joint motion influences their lengths and consequent motor outputs differently 11. Histochemical properties (i.e., muscle fiber type) are also different between the soleus and the gastrocnemii 12–14. Their fiber type differences affect their resistance to fatigue 15, contractile properties 16, and EMG activity patterns during standing and walking 17, 18. The gastrocnemii are often categorized as phasic muscles, because they are mainly activated during phasic activity (e.g., walking) to provide impetus to a motion 18, whereas the soleus is categorized as a tonic muscle, because it has a main role in tonic actions such as postural control 17, 19. Duysens et al. 20 reported that the soleus was constantly activated during standing, while the MG was almost silent. This causes the EMG activity of the soleus to always be larger than that of the MG. During walking, the MG was nearly as active as the soleus, but its activation started later in the stance phase.
As for the differences in spinal reflexes, Levy 21 measured stretch reflexes and H-reflexes of the human soleus and MG in a prone position and found that the amplitudes of both reflexes were always greater in the soleus than in the MG. He linked the amplitude differences to an observation in previous animal studies, which demonstrated that muscle spindle density is greater in the soleus than in the MG in cats 22, 23. Furthermore, in cats, the soleus motoneurons were reported to have larger total excitatory post-synaptic potentials (EPSPs) produced by Ia afferent volleys from a variety of muscles than its synergists MG and LG 24, 25. The larger total EPSP in the soleus than in the MG and LG was also reported in a non-human primate study 26. Moreover, in decerebrate cats, Nichols 27 showed that organizations of the heterogenic reflexes (either excitatory or inhibitory) among the three muscles were different between the quiescent condition (i.e., resting) and activated conditions and thus suggested that the soleus and gastrocnemii have different roles in coordinating posture and movement.
In sum, based on the available anatomical, histological, and electrophysiological evidence, it is conceivable that gastrocnemii and soleus spinal reflexes are differentially modulated during motor tasks. However, to our knowledge, there has been no systematic investigation of task- and/or phase-dependent modulation of the MG or LG H-reflexes. In this study, we examined the MG and LG H-reflexes during standing and walking to delineate the characteristics of MG and LG H-reflex modulation and to compare it to the well-known soleus H-reflex modulation.
Materials and Methods
General Procedure
Twenty-four subjects with no known neurological disorders (13 men and 11 women aged 21–54 yrs) participated in the study. The subjects understood the purposes and procedures of the experiments and signed the consent form before participation. All protocols were approved by the Institutional Review Boards for Human Research of the University of North Carolina at Chapel Hill (Chapel Hill, NC) and Helen Hayes Hospital (West Haverstraw, NY).
In all experiments, EMG activity was recorded from the soleus, MG, LG, and tibialis anterior (TA) muscles with self-adhesive surface Ag-AgCl electrodes (Vermed, Bellows Falls, VT). After placing the EMG electrodes on the skin over the muscles, maximum voluntary contraction (MVC) was measured for soleus, MG, and LG during standing. Then, the H-reflexes and the M-waves of the three muscles were elicited by stimulating the tibial nerve while the subject was standing upright or walking on a treadmill.
This study consisted of three subsets of experiments. All 24 subjects participated in the H-reflex and M-wave recruitment curve measurement. Nineteen subjects participated in the measurement of H-reflex during standing with a fixed level of background EMG. In 11 subjects, task- and phase-dependent modulation of H-reflex was examined during standing and walking. For details, see H-reflex measurements of the soleus, MG, and LG below.
H-reflex measurements of the soleus, MG, and LG
For all 24 subjects, the H-reflex and M-wave recruitment curves were obtained from the soleus, MG, and LG simultaneously while the subject stood and maintained a pre-defined level (usually 10–15% of the MVC) of rectified soleus EMG activity (see Electrical stimulation and EMG recording). The stimulus level was increased in steps of 1.25–2.5 mA from the soleus H-reflex threshold to just above the level that was required to elicit the maximum M-wave (Mmax) in all three muscles 3, 28, 29. Generally, ten different stimulus levels were used to obtain the recruitment curve, and four EMG responses were recorded and averaged at each level.
For 19 subjects, the H-reflexes of the soleus, MG, and LG were elicited 225 times (3 blocks of 75 trials) to examine the relation among the three muscles’ H-reflexes. The trial occurred during standing while the soleus EMG activity was maintained within a pre-defined range (i.e. 10–15% MVC) (see EMG recording and electrical stimulation). The stimulus level was set at just above the soleus M-wave threshold. Small adjustments were occasionally needed to maintain the same soleus M-wave amplitude throughout the 225 trials. By doing so, the MG and LG M-wave amplitudes were also maintained, as the M-waves of the three muscles usually changed in very similar ways. The MG and LG M-waves were also monitored throughout these trials.
For 11 subjects, the H-reflexes of the soleus, MG, and LG were measured during standing with various levels of background activity and during walking to assess task- and phase-dependent modulation of the H-reflex. During standing, the subject was instructed to match the soleus background activity to specific target levels (4 to 5 different levels within 5–100% MVC range) while approximately 10 consecutive stimuli were applied at each level (see EMG recording and electrical stimulation). Thus, roughly 50 H-reflexes were recorded from the soleus, MG, and LG. Small adjustments of stimulus current were occasionally made to maintain the same soleus M-wave amplitude. After completing the H-reflex measurement during standing, the subject walked on a horizontal treadmill at his/her comfortable speed (average 0.9 m/s) while the tibial nerve was pseudo-randomly stimulated to elicit the H-reflexes (see Electrical stimulation and EMG recording). Foot switches (Bortec Biomedical, Calgary, Canada) were inserted between the subject’s shoe and the heel to detect heel contact during walking. Several different stimulus levels were used to obtain the H-reflexes with the same M-wave size across different phases of the gait cycle 2, 4, 30 (see Data analysis).
Electrical Stimulation and EMG Recording
Self-adhesive surface Ag-AgCl electrodes (2.2 × 2.2 cm for the cathode and 2.2 × 3.5 cm for the anode; Vermed, Bellows Falls, VT) were placed on the skin over the popliteal fossa to stimulate the tibial nerve using a Grass S48 stimulator (with CCU1 constant current unit and SIU5 stimulus isolation unit; Astro-Med, West Warwick, RI). For the EMG activity recording, a pair of self-adhesive surface Ag-AgCl electrodes (2.2 × 3.5 cm, Vermed, Bellows Falls, VT) was placed longitudinally on the skin over the soleus just below the gastrocnemii with an interelectrode distance of 3 cm. EMG recording electrodes were also placed over the center of the muscle bellies of the MG, LG, and TA. Pairs of electrodes for the soleus, MG, and LG muscles were placed at least 7 cm apart between the muscles to minimize cross-talk. For each subject, the locations of the nerve stimulating electrodes were selected so that the stimulus current eliciting the soleus H-reflex was as low as possible, and the soleus maximum H-reflex (Hmax) and Mmax were as large as possible. Once these locations were selected, the placements of the MG and LG EMG recording electrodes were adjusted slightly to ensure that the lowest H-reflex threshold and the largest Hmax and Mmax amplitudes were achieved in each muscle.
EMG signals were amplified, band-pass filtered (10–1000 Hz), and recorded with a custom-made system and Axoscope (Molecular Devices Inc., Sunnyvale, CA) at 5000 Hz (for standing data) or 2000 Hz (for walking data). During standing, the EMG and nerve stimulus signals were recorded for a period of 200 ms in response to each test stimulus pulse including a prestimulus period of 50 ms. In addition, the soleus EMG signals were rectified, averaged every 100 ms, and shown on the computer screen as a bar graph for visual feedback. If the soleus background EMG was kept in the specified range (i.e., natural standing level, typically 10–15% MVC) for 2 s and if 5 s had passed since the last stimulus (i.e., the minimum interstimulus period was 5 s), a square stimulus pulse with 1 ms of duration was delivered to elicit the H-reflex and M-wave. During the measurement of the standing H-reflexes with various background activity levels, the visual feedback was used to help the subject grade the background EMG activity from 5 to 100% MVC levels. In order to simplify the task, no feedback of the MG and LG background EMG activity was provided. During walking, the EMG activity, heel contact, and nerve stimulation signals were continuously recorded while the H-reflexes were elicited at pseudo-random intervals (i.e., interstimulus interval of 2.5–4.5 s). This was to ensure that the H-reflexes were obtained at various phases throughout the entire gait cycle. No more than one stimulus was delivered per gait cycle, and there was at least one gait cycle without stimulation between stimulated cycles 3, 31, 32.
MVC measurements of the soleus, MG, and LG
The MVC values of the soleus, MG, and LG were determined as the maximum rectified EMG level. The subject stood on both feet with heels slightly raised from the ground, and often shifted body weight onto the tested leg as the level of contraction increased. Also, if necessary, vertical resistance was applied by either pressing down the subject’s shoulders or asking the subject to push up against the hand-rail that was placed in front for balancing. The subject was asked to exert maximum effort to activate the plantarflexors for 5 s. Three MVC measurements were made with short breaks in between. Average EMG activity of the middle 3 s was calculated for the soleus, MG, and LG, and the highest value of the three trials was taken as the muscle’s MVC.
Data analysis
All analyses were done with a custom-written MATLAB program (Mathworks, Natick, MA). To measure the background activity level during standing, rectified EMG activity in the 50-ms prestimulus period was averaged. For the background activity during walking, the EMG data measured during unstimulated steps were averaged and used as the control EMG activity 3, 31, 32. Thus, the background activity of the stimulated step at a certain time in the gait cycle was calculated from the averaged EMG activity during the unstimulated steps at a corresponding time.
The H-reflex and the M-wave amplitudes were measured as the peak-to-peak values in time windows determined for each subject. Typically, for the soleus, a time window of 33–47 ms post-stimulus was used for the H-reflex and 6–23 ms post-stimulus for the M-wave. For the MG the typical windows were 31–42 ms for the H-reflex and 4–20 ms for the M-wave, and for the LG they were 32–43 ms for the H-reflex and 4–20 ms for the M-wave. For all three muscles, the Hmax, Mmax, and Hmax/Mmax ratio were obtained from the recruitment curve measurement. In order to evaluate the MG and LG H-reflex amplitudes during standing in relation to the soleus H-reflex amplitudes, the MG or LG H-reflexes were plotted against the soleus H-reflexes. Then, using linear regression analysis, their correlations were calculated as the coefficient of determination (R2).
To investigate the task- and phase-dependent modulation of the H-reflex, trials with consistent M-wave size were selected for data analysis for each of the three muscles in order to compare between tasks (i.e. standing and walking) or across various phases of the gait cycle at equal stimulus levels 2, 4, 30. Thus, some of the responses with too large or too small M-waves were eliminated from further analyses. The standing data with various background EMG levels were sorted based on the background activity, and the H-reflex amplitude and background activity were averaged every 4–5 trials to yield approximately ten data points for each muscle. For the walking data, the gait cycle was determined using heel contact signals. Then, the entire gait cycle was divided into 12 equal bins, and each muscle’s H-reflex amplitudes and background activity were averaged for each bin 3. Approximately 10 responses were averaged in each bin. To compare the H-reflex amplitudes between the two tasks, the standing and walking H-reflexes were plotted as a function of the background EMG activity for each muscle 1, 4. To evaluate H-reflex modulation during walking, the modulation index [100 × (maximum H-reflex – minimum H-reflex)/maximum H-reflex] 3, 33 was calculated over the gait cycle. For statistical comparisons between muscles and tasks, the background EMG activity was normalized to the MVC value, the H-reflex amplitude was normalized to the Mmax amplitude, and the normalized values were averaged across the subjects. All group average data were presented as mean ± standard error (SE). Statistical differences among the muscles were examined using paired t-tests, if not indicated otherwise. The α level was set at 0.05. To correct the significance level for multiple comparisons, Sidak correction [1-(1-α)1/n] was used to determine the p-value threshold. That is, for comparing among the three triceps muscles (i.e., 3 potential comparisons), we used 1-(1-0.05)1/3 = 0.017 as the threshold for significance.
Results
Hmax and Mmax during standing
Typical examples of H-reflexes and M-waves during standing in the soleus, MG, and LG are shown in Figure 1. The H-reflexes and the M-waves of the three muscles were simultaneously recorded in response to a test stimulus, while the background EMG activity levels of the soleus, MG, and LG remained within a narrow range (i.e., typically 10–13% MVC for the soleus, 3–8% MVC for the MG, and 3–8% MVC for the LG) throughout the measurement.
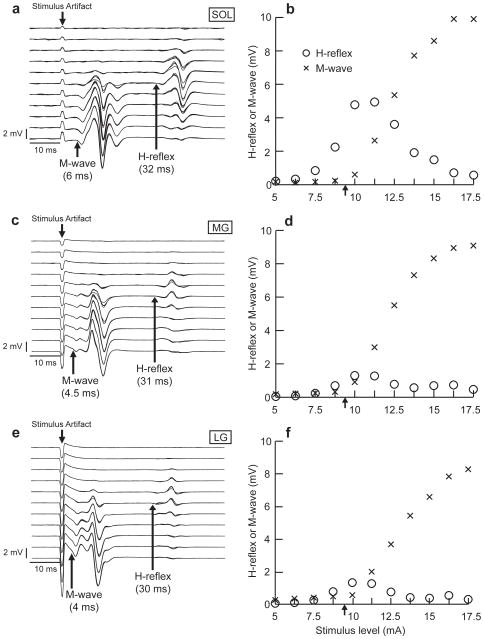
Figure 1.
Examples of typical standing M-waves and H-reflexes at 11 different stimulus levels (a, c, and e) and conventional recruitment curves (b, d, and f) for the soleus (SOL), MG, and LG of one subject. The H-reflexes and the M-waves of the three muscles were recorded simultaneously in response to a series of test stimuli. Their background activity levels were stable throughout. The stimulus level range was 5–17.5 mA, increasing in steps of 1.25 mA (i.e., 11 different levels). In a, c, and e, stimulus level increases from top to bottom, and the arrows indicate the onsets of the M-wave and H-reflex. In b, d, and f, each data point is an average of four responses at the same stimulus level. The Hmax/Mmax ratios were 0.50, 0.14, and 0.16 for the soleus (Mmax 9.9 mV, Hmax 4.9 mV), MG (Mmax 9.1 mV, Hmax 1.3 mV), and LG (Mmax 8.3 mV, Hmax 1.3 mV), respectively. The arrows in b, d, and f indicate the stimulus levels (i.e., just above M-wave threshold) that were used to study the relationships among the SOL, MG, and LG H-reflexes (Figure 2).
The average Hmax/Mmax ratios (± SE) from 24 subjects for the soleus, MG, and LG were 0.45 (± 0.05), 0.20 (± 0.03), and 0.20 (± 0.03), respectively. The average Hmax/Mmax ratio of the soleus was significantly larger than that of the MG and LG (p<0.01, paired t-test, for both MG and LG). There were no significant differences in the average Mmax among the three muscles (soleus: 8.12 ± 0.58 mV, MG: 6.84 ± 0.56 mV, and LG: 6.47 ± 0.57 mV, paired t-test). The average Hmax of the soleus was significantly larger than that of the MG and LG (p<0.01, paired t-test, for both MG and LG, soleus: 3.33 ± 0.36 mV, MG: 1.14 ± 0.12 mV, and LG: 1.13 ± 0.14 mV).
Figures 1a, c, and e show the H-reflexes and M-waves at 11 different stimulus levels during standing in a typical subject. Four sweeps are superimposed at each stimulus level. Conventional recruitment curves for these responses are shown in Figures 1b, d, and f. These panels show that the absolute H-reflex amplitude of the soleus was much larger than that of the MG and LG at a given stimulus level. In addition, in this subject, the latencies of both the H-reflex and the M-wave were slightly shorter for the MG and LG than for the soleus (for the soleus, MG, and LG, M-wave onset latencies were 6 ms, 4.5 ms, and 4 ms, and H-reflex onset latencies were 32 ms, 31 ms, and 30 ms, respectively), due to the more proximal locations of the MG and LG electrodes compared to the soleus electrodes. These differences in the M-wave and H-reflex latency, shape, and size development in response to the increasing stimulus level among the soleus, MG, and LG also indicate that the signal sources for these EMG channels were different from each other and that cross-talk among the three EMG channels was very small, if present at all.
MG and LG H-reflexes in relation to the soleus H-reflex
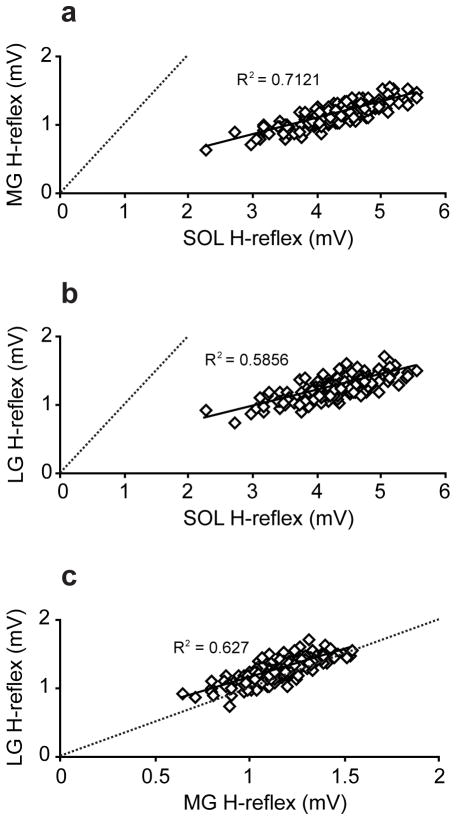
The correlations among the H-reflex amplitudes of the soleus, MG, and LG were examined during standing with consistent background activity (i.e., 10–13 %MVC for the soleus, 3–8 %MVC for the MG, and 3–8 %MVC for the LG) and M-wave amplitude at just above threshold (i.e., typically 3–5 %Mmax for the soleus, 15–20 %Mmax for the MG, and 17–22 %Mmax for the LG). A typical stimulus level used for this measurement is indicated by arrows in Figures 1b, d, and f. Sample correlation plots from one subject are shown in Figure 2a–c. In Figure 2a and b, all data points were below the unity slope lines (i.e. dotted lines), suggesting that the amplitudes of the MG and LG H-reflexes were smaller than that of the soleus. Although there were amplitude differences between the soleus and both gastrocnemii, the H-reflex amplitudes in the three muscles were strongly correlated with each other. Significant linear correlations were present in all pairs in all subjects (p<0.01), and the R2 values (± SE) among all subjects for the soleus-MG, soleus-LG, and MG-LG pair were 0.47 ± 0.06, 0.57 ± 0.05, 0.48 ± 0.06, respectively.
Figure 2.
Correlation of the peak-to-peak H-reflex amplitudes among the soleus (SOL), MG, and LG measured during standing in a single subject (a: SOL-MG, b: SOL-LG, and c: MG-LG). The dotted lines are unity lines, on which amplitudes of two given muscles are the same size. All the data points in a and b were below the unity lines. Although the absolute amplitudes of the MG and LG are much smaller than that of the soleus, they are strongly correlated in a linear fashion. R2 values are shown in each panel.
Task-dependent modulation of the H-reflex
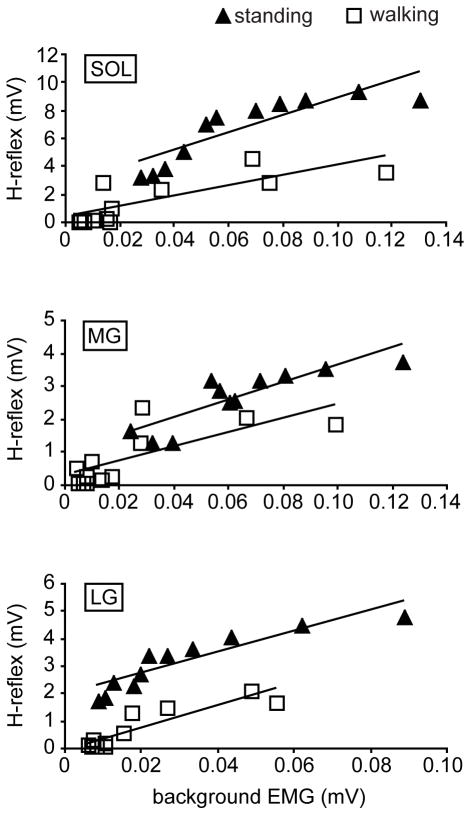
Figure 3 shows the H-reflexes during standing and walking in one subject as a function of the background activity. The group average M-wave amplitudes (± SE) were matched between the tasks: standing vs. walking, 0.60 ± 0.08 mV vs. 0.67 ± 0.08 mV for the soleus (p=0.14, paired t-test), 0.68 ± 0.10 mV vs. 0.66 ± 0.09 mV for the MG (p=0.38), and 0.86 ± 0.23 mV vs. 0.77 ± 0.20 mV for the LG (p=0.14). In Figure 3, the linear regression lines are fitted to aid the H-reflex amplitude comparison between the tasks. R2 values of the fitted lines for the soleus, MG, and LG are 0.74, 0.74, and 0.83, respectively, for standing, and 0.63, 0.55, and 0.82 for walking. As seen in Figure 3, the soleus H-reflex amplitude was greater during standing than during walking, similar to the finding of Capaday and Stein 4. This task-dependent modulation of the reflex amplitude was also seen in the MG and LG.
Figure 3.
The H-reflex amplitudes during walking and standing plotted against the background EMG activity for each muscle in one subject. The M-wave amplitudes were well-matched between the two tasks. The H-reflex amplitudes at a given EMG level were larger during standing than during walking in all three muscles.
For the statistical comparison between the tasks, the background EMG activity was normalized using the MVC value, and the H-reflex amplitude was normalized using the Mmax value. Since there were relatively few data points above the 60% MVC during walking, the data from background EMG levels below 60% MVC were used for the statistical comparison between the tasks 3. Also, because there was little or no H-reflex observed near 0% MVC during walking (i.e., swing phase) due to phase-dependent modulation, in order to evaluate the extent of task-dependent modulation, H-reflexes below 20% MVC were not included in this part of analysis. Thus, the_average H-reflex amplitudes (normalized to Mmax) for background EMG levels of 20–60% MVC were calculated for each subject. The mean values among all subjects are shown in Figure 4. The H-reflex amplitude was significantly larger during standing than during walking in all three muscles (p<0.01 in the soleus and MG, p<0.05 in LG, paired t-test). The relative size difference between the tasks (i.e. walking amplitude/standing amplitude ratio) was calculated for each muscle and for each subject. The group average ratios (± SE) for the soleus, MG, and LG were 0.64 ± 0.08, 0.49 ± 0.06, and 0.71 ± 0.10, respectively, which did not differ significantly from each other (p=0.17 for soleus vs. MG, p=0.61 for soleus vs. LG, and p=0.07 for MG vs. LG).
Figure 4.
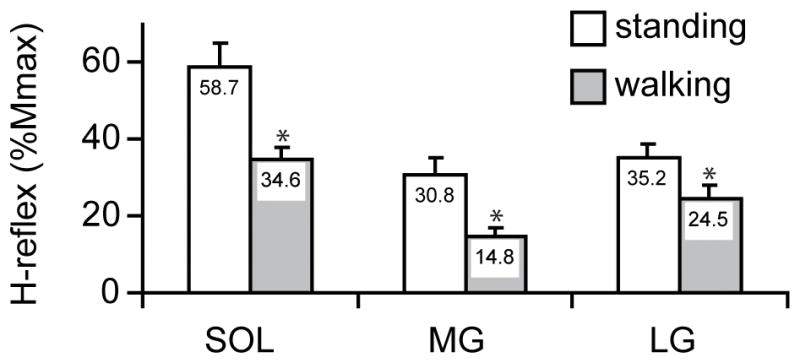
The mean H-reflex amplitudes (normalized using the Mmax amplitude) in the background activity range of 20–60% MVC averaged among all subjects (n=11) for each task. *: The H-reflex amplitudes during walking were significantly smaller than those during standing in all three muscles [p<0.01 in the soleus (SOL) and MG, p<0.05 in LG, paired t-test]. The relative size differences between the tasks were not statistically different among the three muscles.
Phase-dependent modulation of the H-reflex
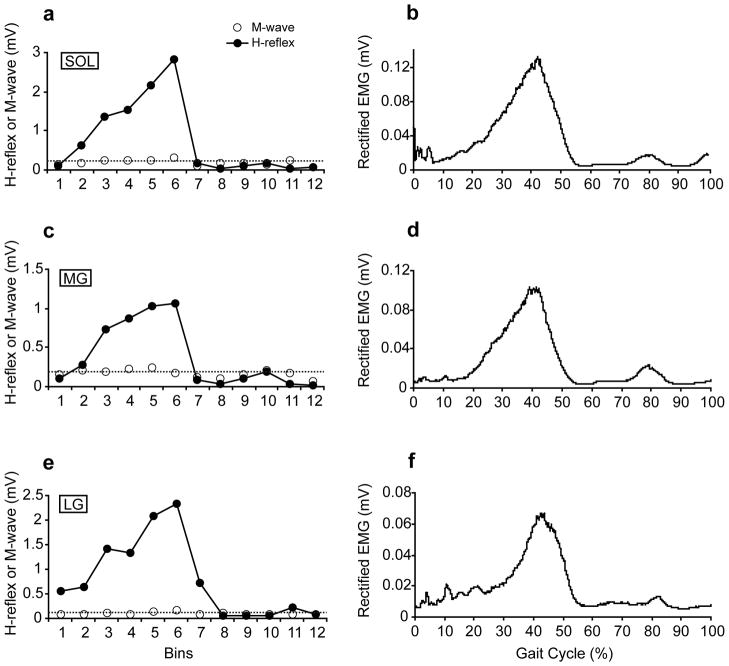
Figure 5 shows H-reflex modulation during walking as a function of the gait cycle phases as well as the locomotor EMG activity in the soleus, MG, and LG in one subject. The locomotor EMG activity for each muscle was obtained by averaging 73 unstimulated steps. The M-wave amplitudes were consistent throughout the gait cycle as indicated by dotted lines. The average M-wave amplitudes showed no significant changes throughout the 12 bins (p=0.12 for soleus, p=0.86 for MG, and p=0.10 for LG, one-way repeated measures ANOVA). Similar to the previous studies 2–4, 30, the soleus H-reflex amplitude was low at heel-contact, gradually increased to its maximum value toward late stance phase, and rapidly decreased at the beginning of the swing phase. This strong phase-dependent modulation was also seen in the MG and LG. Indeed, average modulation indices (± SE) of the three muscles among all subjects were high (98.3 ± 0.3 for the soleus, 96.1 ± 0.9 for the MG, and 96.1 ± 0.7 for the LG), indicating that the H-reflexes in each muscle are highly modulated during walking based on gait cycle phase. The modulation indices for the MG and LG were statistically smaller than that for the soleus (p<0.01 for both MG and LG, paired t-test), probably due to smaller H-reflex amplitudes in the MG and LG. Patterns of the EMG activity and H-reflex modulation were similar during locomotion in each muscle (i.e., high in the late stance phase and silent during the swing phase).
Figure 5.
The H-reflex and M-wave amplitudes during walking as a function of a gait cycle (a, c, and e) as well as EMG activity during walking (b, d, and f) in one subject. a, c, e) The gait cycle (from heel contact to the next heel contact) was divided into 12 equal bins and the reflexes elicited in the same bin were averaged together. The M-wave amplitudes (dotted lines) were kept consistent throughout the gait cycle for each muscle. The H-reflex amplitudes were highly modulated between phases in all muscles. The modulation indices for this subject were 98.9, 97.6, and 97.4 for the soleus, MG, and LG, respectively. b, d, f) The locomotor EMG activity for each muscle was obtained by averaging 73 unstimulated steps. The locomotor EMG activity and the amplitude modulation of the H-reflex were similar in each muscle.
Discussion
The purpose of this study was to examine the H-reflexes of the MG and LG in humans, in comparison to the well-known soleus H-reflex. The H-reflexes of the soleus, MG, and LG were measured during standing and walking to examine the phase- and task-dependency of the reflex amplitudes. Although the Hmax/Mmax ratios were significantly different between the soleus and the gastrocnemii, the H-reflex amplitudes in response to single test stimuli were strongly correlated among the three muscles during standing. The MG and LG H-reflex amplitudes were modulated depending on the task (i.e., standing vs. walking) and on the phase of the gait cycle. The pattern of the modulation was similar to that seen with the soleus H-reflex.
The Hmax/Mmax ratios of the MG and LG were smaller than that of the soleus
The Hmax amplitudes calculated from the recruitment curves of the three muscles were significantly different: the soleus Hmax was larger than the MG or LG Hmax. While there were no statistical differences in the Mmax of all three muscles, the Hmax/Mmax ratio of the soleus was significantly larger than that of the MG or LG. Those results are consistent with previous animal data34. Several possible explanations can be given regarding what make the Hmax/Mmax ratios different between the soleus and the gastrocnemii. First, the soleus and the gastrocnemii have different compositions of muscle fiber types. Type I (“slow”) muscle fibers are predominant in the soleus (70–90%) whereas type I and II (“fast”) muscle fibers are equally common (50% for both types) in the gastrocnemii 12–14. Thus, motoneurons that innervate the soleus and the gastrocnemii have different properties such as axon diameter and cell size, which will greatly influence the recruitment of H-reflex and M-wave at different stimulus levels in each muscle 35. During the recruitment curve measurement, the motoneurons are recruited into the H-reflex activation by the Ia afferent volley from the smallest to largest in an orderly manner in accordance with the size principle 36 while the order of the recruitment is reversed for the M-wave (i.e., from the largest motoneurons to the smallest motoneurons) as electrical stimulation first activates axons with larger diameters 37. Thus, as the stimulus level increases, the reflex activation of relatively large motoneurons would theoretically be occluded by the antidromic motor volley on the same motor axons 37–39 and/or the refractoriness of the motoneurons due to the antidromic efferent conduction 40, thereby contributing to the falling part of the H-reflex recruitment curve. Indeed, in the human soleus, activation of slow-twitch fibers (i.e., innervated by smaller motoneurons with smaller axon diameters) is mainly responsible for the H-reflex 41. The fact that the soleus motoneuron pool contains more small motoneurons to be recruited into the H-reflex than the MG or LG pools would, at least partially, explain the smaller Hmax amplitudes of the MG and LG when compared to that of the soleus 12–14, 37–40. Second, it is known that the EPSPs evoked by Ia afferents from a wide variety of muscles (i.e., homonymous and heteronymous inputs) are larger in soleus motoneurons than in MG and LG motoneurons in cats 24, 25, 42 and non-human primates 26, 43. This would explain partially why the H-reflex amplitudes of the MG and LG were smaller than that of the soleus (see Figure 1). In addition, in cats, the number of muscle spindles is greater in the soleus than in the MG 22. If the same uneven distribution of spindles exists in humans, the number of Ia afferents is presumably smaller in the gastrocnemii than in the soleus, possibly leading to less homonymous Ia excitation of the motoneuron at a given stimulus level in the gastrocnemii when compared to the soleus. However, the counts of the muscle spindles in cats are not consistent between studies 22, 23, and human data are unavailable. Other factors that affect the H-reflex amplitude, such as presynaptic inhibition and postsynaptic inhibition 44–46, may differently influence H-reflex recruitment (i.e., Hmax and Hmax/Mmax ratio) between the soleus and gastrocnemii (see Discussion by Schieppati 38). For example, presynaptic inhibition can alter synaptic transmission in a monosynaptic reflex pathway, and this inhibitory mechanism can selectively affect different afferent terminals from the same muscle 47. However, to our knowledge, no study has reported differences in the influence of presynaptic inhibition among the shared Ia innervations of human soleus, MG, and LG muscles.
The amplitudes of the MG and LG H-reflexes co-vary with soleus H- reflex amplitude
In this study, the level of electrical stimulation for eliciting the H-reflex in all three muscles was set at just above the M-wave threshold during standing (Figure 2). Thus, the reflexes of all three muscles were elicited in the ascending part of the recruitment curve (i.e., before the Hmax level, see arrows in Figure 1b, d, and f). Despite the smaller amplitudes of the MG and LG H-reflexes compared to the soleus, the H-reflex amplitudes of the three muscles were strongly positively correlated with each other. This suggests that, although there are several mechanical and physiological differences between the soleus and the gastrocnemii 11, 15–18, 20, the excitability of the H-reflex pathway is modulated in similar ways among the three muscles.
The MG and LG H-reflexes are task- and phase-dependently modulated similarly to the soleus H-reflex
The excitability of the H-reflex pathway is similarly modulated between the soleus and gastrocnemii not only during standing (as discussed above) but also during walking. Although the H-reflex amplitudes of the MG and LG were smaller than that of the soleus during standing and walking, the standing H-reflexes were larger than the walking H-reflexes in all three muscles (Figure 4). The extent of this task-dependent modulation (indicated by the walking reflex/standing reflex ratio) was not significantly different among the three muscles, which suggests that the H-reflexes were similarly modulated between standing and walking among the three muscles. In addition, the modulation indices during walking showed high values for all three muscles (>96%), indicating that the H-reflex amplitudes of all three muscles are similarly modulated with gait cycle phase.
The fact that the MG and LG H-reflexes are task- and phase-dependently modulated in ways similar to the soleus H-reflex supports the hypothesis that the soleus, MG, and LG H-reflex pathways function as one synergistic unit during walking. As suggested by Capaday et al.1 and Edamura et al.2, lowering the reflex gain from standing to walking (i.e., task-dependent modulation) would ensure that the stretch reflex pathways (or net motor outputs) are not saturated during walking so that those muscles can contribute to locomotor activity. Also, during walking, activation of the soleus, MG, and LG motoneurons by Ia excitatory inputs (i.e., stretch reflex activity) in the stance phase would contribute to upward and forward propulsion of the body 4, while suppression of such reflex activity in the swing phase would prevent it from opposing ankle dorsiflexion (i.e., phase-dependent modulation) 4.
While synergistic modulation of the H-reflex between the soleus and gastrocnemii exists during walking, the activity of other reflex pathways may be modulated differently. For instance, Duysens et al.20 found facilitation of the crossed reflex activity in the soleus by contralateral sural or posterior tibial nerve stimulation during the early stance phase, while it was suppressed in the MG. It was suggested that this selective recruitment of the soleus is functionally relevant, because activation of the slow, monoarticular soleus would be effective in this period when the ankle plantar flexors are in eccentric contraction to brake dorsiflexion as the body moves forward. On the other hand, activation of the MG, a biarticular muscle, could cause excessive yield by flexing the knee joint.
During walking, not only the Ia afferents, but also Ib, II, and cutaneous afferents contribute to the appropriate activation of the soleus 48–52. Modulation of the activity of other spinal reflex pathways in the gastrocnemii has yet to be studied.
In summary, the H-reflex amplitudes of the MG and LG are smaller than that of the soleus. Despite these amplitude differences and the functional differences previously reported, the soleus, MG, and LG H-reflexes are similarly modulated between tasks (i.e., standing vs. walking) and within a task (i.e., across the gait cycle). These results support the hypothesis that the soleus, MG, and LG H-reflex pathways function as one synergistic unit during walking.
Soleus H-reflex amplitude is also modulated between walking and running (Capaday and Stein 1 and Edamura et al. 2, see also Ferris et al. 53). Whether the synergistic task- and phase-dependent modulation of the H-reflex among the soleus, MG, and LG extends to running remains to be determined.
Acknowledgments
We thank Dr. Jonathan Carp for helpful comments on the manuscript and Dr. Dennis McFarland and Ms. Briana Abel for advice and assistance in data collection and analysis. This work was supported in part by the New York State Spinal Cord Injury Research Trust (C023685(AKT) and C020932(XYC&RLS)) and the National Institutes of Health (NS069551(AKT), NS22189(JRW), and NS061823 (JRW&XYC)).
Abbreviations
- EMG
electromyography
- EPSP
excitatory post-synaptic potential
- LG
lateral gastrocnemius
- MG
medial gastrocnemius
- MVC
maximum voluntary contraction
- SE
standard error
- TA
tibialis anterior
References
- 1.Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11:420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 4.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JF, Whelan PJ. Neural mechanisms that contribute to cyclical modulation of the soleus H-reflex in walking in humans. Exp Brain Res. 1993;95:547–556. doi: 10.1007/BF00227148. [DOI] [PubMed] [Google Scholar]
- 6.Moritani T, Oddsson L, Thorstensson A. Differences in modulation of the gastrocnemius and soleus H-reflexes during hopping in man. Acta Physiol Scand. 1990;138:575–576. doi: 10.1111/j.1748-1716.1990.tb08886.x. [DOI] [PubMed] [Google Scholar]
- 7.Dyhre-Poulsen P, Simonsen EB, Voigt M. Dynamic control of muscle stiffness and H reflex modulation during hopping and jumping in man. J Physiol. 1991;437:287–304. doi: 10.1113/jphysiol.1991.sp018596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonagh MJ, Duncan A. Interaction of pre-programmed control and natural stretch reflexes in human landing movements. J Physiol. 2002;544:985–994. doi: 10.1113/jphysiol.2002.024844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinniger GJ, Nordlund M, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray MP, Guten GN, Baldwin JM, Gardner GM. A comparison of plantar flexion torque with and without the triceps surae. Acta Orthop Scand. 1976;47:122–124. doi: 10.3109/17453677608998984. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 13.Gollnick PD, Sjodin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 1974;348:247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- 15.Ochs RM, Smith JL, Edgerton VR. Fatigue characteristics of human gastrocnemius and soleus muscles. Electromyogr Clin Neurophysiol. 1977;17:297–306. [PubMed] [Google Scholar]
- 16.Vandervoort AA, McComas AJ. A comparison of the contractile properties of the human gastrocnemius and soleus muscles. Eur J Appl Physiol Occup Physiol. 1983;51:435–440. doi: 10.1007/BF00429079. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J, Nightingale A. Electromyography of muscles of posture: leg muscles in males. J Physiol. 1952;117:484–491. doi: 10.1113/jphysiol.1952.sp004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell KM, Biggs NL, Blanton PL, Lehr RP. Electromyographic investigation of the relative activity among four components of the triceps surae. Am J Phys Med. 1973;52:30–41. [PubMed] [Google Scholar]
- 19.Fitzpatrick RC, Gorman RB, Burke D, Gandevia SC. Postural proprioceptive reflexes in standing human subjects: bandwidth of response and transmission characteristics. J Physiol. 1992;458:69–83. doi: 10.1113/jphysiol.1992.sp019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duysens J, Tax AA, van der Doelen B, Trippel M, Dietz V. Selective activation of human soleus or gastrocnemius in reflex responses during walking and running. Exp Brain Res. 1991;87:193–204. doi: 10.1007/BF00228520. [DOI] [PubMed] [Google Scholar]
- 21.Levy R. The relative importance of the gastrocnemius and soleus muscles in the ankle jerk of man. J Neurol Neurosurg Psychiatry. 1963;26:148–150. doi: 10.1136/jnnp.26.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagbarth KE, Wohlfart G. The number of muscle-spindles in certain muscles in cat in relation to the composition of the muscle nerves. Acta Anat (Basel) 1952;15:85–104. doi: 10.1159/000140738. [DOI] [PubMed] [Google Scholar]
- 23.Swett JE, Eldred E. Distribution and numbers of stretch rceptors in medial gastrocnemius and soleus muscles of the cat. Anat Rec. 1960;137:453–460. doi: 10.1002/ar.1091370405. [DOI] [PubMed] [Google Scholar]
- 24.Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JG, Mendell LM. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976;39:679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- 26.Carp JS. Monosynaptic EPSPs in primate lumbar motoneurons. J Neurophysiol. 1993;70:1585–1592. doi: 10.1152/jn.1993.70.4.1585. [DOI] [PubMed] [Google Scholar]
- 27.Nichols TR. The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J Physiol. 1989;410:463–477. doi: 10.1113/jphysiol.1989.sp017544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zehr EP, Stein RB. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res. 1999;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]
- 29.Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci. 2009;29:5784–5792. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res. 1990;83:22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- 31.Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- 32.Kido A, Tanaka N, Stein RB. Spinal reciprocal inhibition in human locomotion. J Appl Physiol. 2004;96:1969–1977. doi: 10.1152/japplphysiol.01060.2003. [DOI] [PubMed] [Google Scholar]
- 33.Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol. 2001;537:1033–1045. doi: 10.1111/j.1469-7793.2001.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messina C, Cotrufo R. Different excitability of type 1 and type 2 alpha-motoneurons. The recruitment curve of H- and M-responses in slow and fast muscles of rabbits. J Neurol Sci. 1976;28:57–63. doi: 10.1016/0022-510x(76)90047-2. [DOI] [PubMed] [Google Scholar]
- 35.Henneman E, Mendell LM. Funtional Organization of Motoneuron Pool and Inputs. In: Brooks VB, editor. Handbook of Physiology. Baltimore: Williams and Wilkins; 1981. pp. 423–507. Section I: The Nervous System, Volume II: Motor Control, Part I. [Google Scholar]
- 36.Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 37.Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 38.Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 39.Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb GL, Agarwal GC. Extinction of the Hoffmann reflex by antidromic conduction. Electroencephalogr Clin Neurophysiol. 1976;41:19–24. doi: 10.1016/0013-4694(76)90211-x. [DOI] [PubMed] [Google Scholar]
- 41.Buchthal F, Schmalbruch H. Contraction times of twitches evoked by H-reflexes. Acta Physiol Scand. 1970;80:378–382. doi: 10.1111/j.1748-1716.1970.tb04801.x. [DOI] [PubMed] [Google Scholar]
- 42.Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971;34:171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- 43.Hongo T, Lundberg A, Phillips CG, Thompson RF. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci. 1984;221:261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- 44.Capaday C, Stein RB. A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods. 1987;21:91–104. doi: 10.1016/0165-0270(87)90107-5. [DOI] [PubMed] [Google Scholar]
- 45.Capaday C, Stein RB. The effects of postsynaptic inhibition on the monosynaptic reflex of the cat at different levels of motoneuron pool activity. Exp Brain Res. 1989;77:577–584. doi: 10.1007/BF00249610. [DOI] [PubMed] [Google Scholar]
- 46.Stein RB. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–544. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 47.Rudomin P, Jimenez I, Quevedo J. Selectivity of the presynaptic control of synapic effectiveness of group I afferents in the mammalian spinal cord. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic inhibition and neural control. New York: Oxford University Press; 1998. pp. 282–302. [Google Scholar]
- 48.Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]
- 49.Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res. 2006;173:713–723. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- 50.Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol. 2005;93:167–177. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- 51.Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferris DP, Aagaard P, Simonsen EB, Farley CT, Dyhre-Poulsen P. Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J Physiol. 2001;530:167–180. doi: 10.1111/j.1469-7793.2001.0167m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]