Abstract
We have recently reported the creation and initial characterization of the first etiology-based recombinant mouse model of major depressive disorder (MDD). This was achieved by replacing the corresponding mouse DNA sequence with a 6-base DNA sequence from the human CREB1 promoter that is associated with the development of MDD in families identified by probands with recurrent, early-onset MDD. The current study explored whether the desired homologous recombination event at the mouse Creb1 gene that resulted in the creation of the mouse model was also accompanied by insertions of the targeting vector at unintended non-homologous locations in the mouse genome. No evidence of insertions of targeting vector sequence was observed at regions other than the mouse Creb1 gene.
Keywords: Major Depressive Disorder, Genetics, Mouse, Animal Model
INTRODUCTION
Major depressive disorder (MDD) has an estimated lifetime prevalence of about 10% (F/M ratio ~2) and is a leading cause of suffering and disability worldwide [Zubenko et al., 2001; Lopez et al., 2006]. It is also an important contributor to mortality from all causes, including suicide, and has been estimated to be among the costliest medical disorders from an economic perspective [Zubenko et al., 2001; Greenberg et al., 2003]. Twin and adoption studies indicate that genetic factors account for 40% to 70% of the risk of developing MDD [for review see Zubenko et al., 2001]. However, our understanding of the underlying cause of this disorder remains rudimentary, and existing treatments are often ineffective and are commonly associated with poorly tolerated side effects.
A better understanding of the cellular and molecular brain mechanisms that lead to the expression of MDD and the mechanism of action of existing antidepressants seems prerequisite to preventing or reducing the global burden of this major public health problem. Studies of MDD are constrained by numerous factors including the complexity of the brain and our limited understanding of normal brain functioning, the inaccessibility of the brain in living subjects, and the limitations inherent in postmortem studies. The development of a valid animal model for MDD that reflects the brain mechanisms that lead to MDD would significantly accelerate the pace toward achieving these goals.
The laboratory mouse has many features that make it an attractive model organism for the study of human diseases, including their striking similarity to humans in anatomy, physiology, and genetics. We have recently reported the creation and initial characterization of the first etiology-based recombinant mouse model of MDD [Zubenko and Hughes, 2011]. This was achieved by replacing the corresponding mouse DNA sequence with a 6-base DNA sequence from the human CREB1 promoter that is associated with the development of MDD in families identified by probands with recurrent, early-onset MDD (RE-MDD). This congenic mutant C57BL/6NTac mouse line is based on a rare, highly penetrant, pathogenic mutation in the human CREB1 promoter [Zubenko and Hughes, 2010], rather than psychological hypotheses, and it mimics the brain mechanism that leads to MDD in humans, rather than symptoms or antidepressant responsiveness. Nonetheless, in our initial characterization, the mutant mice exhibited several features that were reminiscent of the human disorder, including alterations of brain anatomy, gene expression, behavior, as well as increased infant mortality [Zubenko and Hughes, 2011].
The current study explored whether the desired homologous recombination event at the mouse Creb1 gene that resulted in the creation of the mouse model was also accompanied by insertions of the targeting vector at unintended non-homologous locations in the mouse genome. Such unintended mutagenic events, if they occurred, have the potential to modify the phenotype of the altered Creb1 allele or contribute independent effects that might confound studies of the mouse model. If detected, such unwanted mutations would warrant efforts to exclude them from the mouse line by selective breeding.
MATERIALS AND METHODS
Isolation of DNA from mouse tissues
Each 4 mm mouse tail tip sample was incubated in 150 μl of 100 mM Tris, pH 8.5, 400 mM NaCl, 5 mM EDTA (all Sigma, St. Louis, MO), 0.2% SDS (Gibco, Grand Island, NY), and 0.5 mg/ml Proteinase K (Roche Diagnostics Corp., Indianapolis, IN) for at least 3 hours at 55°C in a shaker incubator (New Brunswick Scientific, Edison, NJ) at 150 rpm. Samples were centrifuged at 12,000 × g for 5 min at room temperature to remove debris. DNA was precipitated from clarified supernatants by the addition of 270 μl of 100% ethanol (Pharmco, Brookfield, CT) followed by gentle inversion to mix, and collected by centrifugation at 12,000 × g for 5 min at room temperature. After incubation at room temperature to remove the ethanol, the samples and were suspended in 150 μl of 1 mM Tris, pH 8.0, 0.1 mM EDTA (Sigma, St. Louis, MO) and solubilized by incubation at 55°C.
PCR amplification of targeting vector regions
Oligonucleotide primers were designed using the software Primer 3, available on-line at URL: http://frodo.wi.mit.edu/primer3/, and were manufactured by Invitrogen Custom Primers (Carlsbad, CA). Each region was amplified using 10 pmole of each oligonucleotide primer pair and Platinum® Taq DNA Polymerase (Invitrogen) according to the manufacturer's instructions. Following an initial denaturation at 94°C for 3 min, amplification was achieved by thirty cycles of denaturing at 94°C for 30 sec, annealing at 65°C for 30 sec and extension at 72°C for 1 min.
Electrophoresis and Visualization of PCR Amplicons
Fifteen μl of each PCR product was resolved on a 1.75% agarose gel (Invitrogen), along with a 100 bp PCR Molecular Ruler (Bio-Rad, Hercules, CA) as a size standard. Each gel was stained with ethidium bromide (International Biotechnologies, Inc., New Haven, CT), and the results were visualized and documented using a UVP MultiDoc-It™ Imaging System (Upland, CA).
RESULTS
The targeting vector that was used for the creation of the mutant mouse line is shown in Figure 1, and was produced by modifying pNTK [Mortensen, 2008] as previously described [Zubenko and Hughes, 2011]. The genomes of 78 mice from the mutant mouse colony established at the Jackson Laboratory were evaluated for the presence of three bacterial regions of the targeting vector by PCR amplification using the primer pairs listed in Table 1. These 78 mice (F2 generation) were the offspring of 32 heterozygous breeding units (30 pairs, 2 trios) that included 32 (16 male, 16 female) wild type (WT) mice, 32 (16 male, 16 female) heterozygous mice, and 14 (8 male, 6 female) mice that were homozygous for the altered Creb1 promoter. The initial phenotypic characterization of these groups has been previously reported [Zubenko and Hughes, 2011].
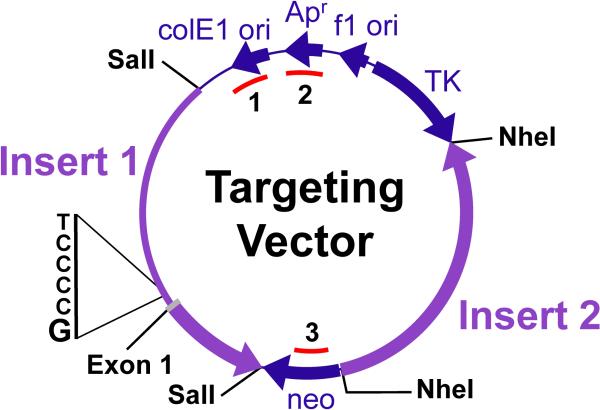
Figure 1.
Targeting Vector Diagram. The targeting vector consists of two sequential fragments of mouse DNA (Insert 1 and Insert 2) cloned into the multiple cloning sites that flank the neo gene (indicated by SalI and NheI) of plasmid pNTK. The thick arrows pointing clockwise show the positions of the selectable marker genes neo and TK. The thin line at the top containing three arrows pointing counter-clockwise is the location of the vector backbone that encodes the functions needed for propagation of the plasmid in bacteria. The thick arrows pointing counter-clockwise within Inserts 1 and 2 indicate the transcribed region of the Creb1 gene cloned into the plasmid. The positions of exon 1 and the replaced bases within the promoter region (5'-TCCCG-3', plus strand) are indicated. The three regions interrogated by PCR amplification are identified by arcs inside of the vector circle (1 = targeting vector backbone region 1; 2 = targeting vector backbone region 2; 3 = neo gene region).
Table 1.
PCR Primer Pairs Used to Genotype Three Regions of the Targeting Vector
| Region | Forward Primer | Reverse Primer |
|---|---|---|
| TVB1 | CGG TAT CAG CTC ACT CAA AGG | GCC TAC ATA CCT CGC TCT GC |
| TVB2 | CAT CCA TAG TTG CCT GAC TCC | TAT GTG GCG CGG TAT TAT CC |
| neo | AGT GAC AAC GTC GAG CAC AG | GGG CTG CAG GAA TTC TAC C |
TVB1, targeting vector backbone region 1; TVB2, targeting vector backbone region 2; neo, neomycin resistance gene sequence.
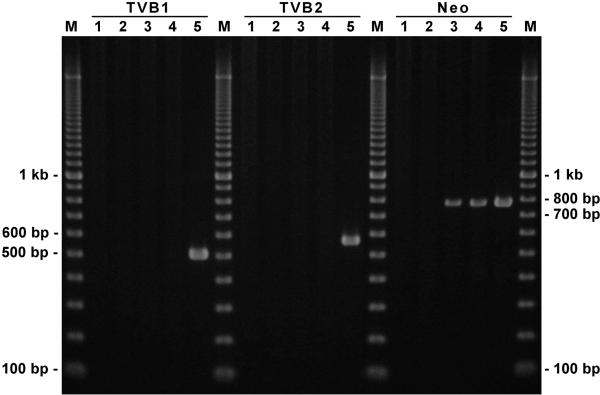
The PCR amplification results obtained for all mice of the same Creb1 genotype were indistinguishable. Representative results of the interrogation of the three Creb1 genotypes for the presence of three bacterial regions of the targeting vector by PCR amplification are shown in Figure 2. No evidence for amplification of the three bacterial regions from the targeting vector (TVB1, TVB2 and neo) was observed using genomic DNA from the negative control or WT F2 samples, while the targeting vector DNA used as a positive control produced all three amplicons of the expected sizes. Likewise, there was no evidence for amplification of the of the two targeting vector backbone regions in heterozygous or homozygous carriers of the mutant Creb1 promoter. Since the neo gene is present within the first Creb1 intron of the mouse model by design, amplification of the neo region was observed in both the heterozygous and homozygous mice, as expected.
Figure 2.
Electropherogram showing the products of PCR amplification of targeting vector sequences from genomes of recombinant mice. Two targeting vector backbone regions (TVB1, TVB2) and the neo gene region were amplified by PCR, as described in the Materials and Methods. No evidence for amplification of any of the three regions was visible in the negative control or WT samples, while the positive control lanes contained bands of the expected size: TVB1 = 518 bp, TVB2 = 588 bp, neo = 780 bp. Likewise, there is no evidence for amplification of the two targeting vector backbone regions in heterozygous or homozygous carriers of the mutated Creb1 promoter region. Since the neo gene is present within the first Creb1 intron of the mouse model by design, amplification of the neo region is seen in both the heterozygous and homozygous mice, as expected. Products of PCR amplification were loaded in the following lanes: M, 100 bp PCR Molecular Ruler; 1, C57BL/6J mouse (negative control); 2, WT F2 mouse; 3, heterozygous F2 mouse; 4, homozygous F2 mouse; 5, targeting vector (positive control).
DISCUSSION
We have recently reported the creation and initial characterization of the first etiology-based recombinant mouse model of MDD [Zubenko and Hughes, 2011]. This was achieved by replacing the corresponding mouse DNA sequence with a 6-base DNA sequence from the human CREB1 promoter that is associated with the development of MDD in families identified by probands with a severe, familial subtype of MDD (RE-MDD). The goal of this study was to explore whether the homologous recombination event at the mouse Creb1 gene within the embryonic stem cells used to construct this mouse line was also accompanied by one or more insertions of the targeting vector at unintended non-homologous locations in the mouse genome.
Several features of the genome-wide screen enhanced the sensitivity for detecting insertions of the targeting vector sequences at non-homologous sites in the mouse genome. Probing for three distinct bacterial sequences limited the fragments of the targeting vector that could have escaped detection. This approach also protected against failed amplification that could have resulted from the rearrangement of a particular template sequence that was no longer PCR competent. Such events would occur if the insertion of the targeting vector occurred by recombination within a probed template region. The neo gene was specifically included among the three probe regions that were tested because its expression was required for selection of the recombinant C57BL/6NTac ES cells used to construct the mutant mouse. It seemed conceivable that multiple insertions of the neo gene could have increased the likelihood for a recombinant ES cell clone to emerge from our selection of recombinant neomycin-resistant cells used for the construction of the mutant mouse. Yet no evidence of the presence of the neo gene at locations other than intron 1 of the modified Creb1 allele was detected by PCR amplification.
The results exclude the insertion of the complete targeting vector in the mouse genome, as well as fragments of the targeting vector that include regions 1 or 2. Insertions of targeting vector fragments containing the neo gene (region 3) but lacking regions 1 and 2 can also be excluded from genome of the mutant mouse line, except for potential insertions that are tightly linked to Creb1. This latter inference was drawn from the observation that none of the 32 WT F2 progeny of the heterozygous breeding pairs contained the neo sequence, as determined by PCR amplification. These potentially mutagenic events have been excluded as possible confounding genetic factors in characterization of the mouse model.
It becomes increasingly challenging to perform a genome-wide screen for progressively smaller cryptic insertions, and becomes unfeasible at a level of resolution approaching the single base pair. However, sequential backcrossing to the congenic C57BL/6NTac parent strain should eliminate any remaining cryptic variation that is unlinked to the mutant Creb1 allele of the recombinant mouse model of MDD.
ACKNOWLEDGMENTS
This work was supported by NIH grant application MH47346 (GSZ, PI), the Provost's Fund for Research Development, University of Pittsburgh (GSZ), the Shane Richard Brown Fund, University of Pittsburgh, the Office of the Senior Vice Chancellor for the Health Sciences, University of Pittsburgh, and Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
REFERENCES
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: How did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Measuring the global burden of disease and risk factors, 1990–2001. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CL, editors. Global burden of disease and risk factors. The World Bank and Oxford University Press; New York: 2006. pp. 1–13. [Google Scholar]
- Mortensen R. Production of a heterozygous mutant cell line by homologous recombination (single knockout) Curr Protoc Mol Biol. 2008;S82:23.5.1–23.5.11. doi: 10.1002/0471142727.mb2305s82. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Hughes HB., III Effects of the A(–115)G variant on CREB1 promoter activity in two brain cell lines: Interactions with gonadal steroids. Am J Med Genet Part B. 2010;153B:1365–1372. doi: 10.1002/ajmg.b.31133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Hughes HB., III Replacement of homologous mouse DNA sequence with pathogenic 6-base human CREB1 promoter sequence creates murine model of major depressive disorder. Am J Med Genet Part B. 2011;156:517–531. doi: 10.1002/ajmg.b.31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Zubenko WN, Spiker DG, Giles DE, Kaplan BB. The malignancy of recurrent, early-onset major depression: A family study. Am J Med Genet Part B. 2001;105(8):690–699. doi: 10.1002/ajmg.1554. [DOI] [PubMed] [Google Scholar]