Abstract
Both betulinic acid (BA) and mithramycin A (MIT) exhibit potent anti-tumor activity through distinct mechanisms of Sp1 inhibition. However, it is unknown whether a combination of these two compounds results in a synergistic inhibitory effect on pancreatic cancer growth and/or has a therapeutic advantage over gemcitabine. In xenograft mouse models of human pancreatic cancer, treatment with either BA or MIT alone showed dose-dependent antitumor activity, but led to systemic side effects as measured by overall weight loss. Treatment with a nontoxic dose of either compound alone had only marginal antitumor effects. Importantly, combination treatment with nontoxic doses of BA and MIT produced synergistic antitumor activity, including inhibitory effects on cell proliferation, invasion and angiogenesis. The treatment combination also produced less discernible side effects than therapeutic doses of gemcitabine. Moreover, combined treatment of BA and MIT resulted in drastic inhibition of Sp1 recruitment onto Sp1 and VEGF promoters, leading to transcriptional inhibition of both Sp1 and VEGF and downregulation of Sp1 and VEGF protein expression. Ectopic overexpression of Sp1 rendered tumor cells resistant to BA, MIT, and the combination of the two. Overall, our findings argue that Sp1 is important target of BA and MIT and that their combination can produce an enhanced therapeutic response in human pancreatic cancer.
Keywords: Sp1, angiogenesis, VEGF, betulinic acid, mithramycin A
Introduction
Pancreatic cancer is currently the fourth leading cause of cancer-related deaths worldwide. The median survival duration from diagnosis to death is about6 months, and the overall 5-year survival rate is less than 5% (1–3). Pancreatic tumor is highly resistant to chemotherapy and radiation therapy. Surgery resection is still the primary choice when it is feasible (4, 5). A full understanding of the cellular and molecular mechanisms of the development and progression of pancreatic cancer is crucial for identifying new targets of effective treatment modalities for this deadly disease (6–19). Previous studies have demonstrated that Sp1 overexpression play an important role in regulating the expression of vascular endothelial growth factor (VEGF) and angiogenesis in pancreatic cancer (11–19). Also, we have shown that neutralization of VEGF by treatment with bevacizumab (Avastin) leads to feedback activation of Sp1 and subsequent upregulation of expression of VEGF and other factors, leading to Avastin resistance, whereas blockade of Sp1 expression and function sensitizes tumors to Avastin and/or reverses Avastin resistance (20). The synergistic downregulation of Sp1 by tolfenamic acid and mithramycin A (MIT) produced significant anti-tumor activity (21).
Sp1 is a zinc finger transcription factor that is important to the transcription of many cellular and viral genes containing GC boxes in their promoters. Although Sp1 has been perceived to be a basal transcription factor since its discovery, increasing evidence suggests that it regulates a variety of biological functions, including cell survival, growth, and differentiation and tumor development and progression (19, 22–24). Recently it was reported that Sp1 is essential for the epithelial to mesenchymal transformation induced by TGF-β in pancreatic cancer cells (25). The epithelial to mesenchymal transformation plays an important role in pancreatic cancer resistance to chemotherapy as well as other tumor types (26, 27).
A recent study has shown that betulinic acid (BA) inhibits prostate cancer growth through inhibition of specificity protein transcription factors (28). BA is a naturally occurring pentacyclic triterpene that exhibits potent antitumor properties. This anticancer activity has been linked to its ability to directly trigger mitochondrial membrane permeabilization. In contrast to the potent cytotoxicity of BA against a variety of cancer types, non-neoplastic cells as well as normal tissue remain relatively resistant to BA, thus pointing to a therapeutic window. Because agents that exert a direct action on mitochondria may bypass resistance to conventional chemotherapeutics, there is increasing interest to develop such compounds as experimental cancer therapeutics. Thus, mitochondrion-targeted agents such as BA hold great promise as a novel approach to overcome certain forms of drug resistance in human cancers (29–31). Interestingly, MIT inhibits Sp1 activity and has antitumor activities (32, 33). Its major underlying mechanism of action includes a reversible interaction with double-stranded DNA with GC-base specificity and selective regulation of transcription of genes having GC-rich promoter sequences (34–37).
Therefore, MIT and BA appear to have distinct mechanisms of inhibiting Sp1 activity (20, 28). However, it is unknown whether Sp1 is a critical target for the observed antitumor activities of those drugs. Also, it is significant to investigate whether a combination of these two compounds has a synergistic inhibitory effect on Sp1 activity and consequent suppression of pancreatic cancer growth and whether this antitumor activity has any advantages over gemcitabine. In the present study, we sought to address those issues using cell cultures and animal models of pancreatic cancer and also explored their underlying mechanisms.
Materials and Methods
Chemicals and reagents
MIT (1 mg/vial crystal powder; lot 098K4043) was purchased from Sigma Chemical Co. and diluted in sterile water. BA (powder; lot S43559) also was purchased from Sigma Chemical Co. and was mixed with corn oil. In our animal experiments, MIT (0.05–1.50 mg/kg body weight) was administered via intraperitoneal injection twice a week or as indicated, BA (10–40 mg/kg) was administered via oral gavages 3 times a week, and Gemcitabine (Eli Lilly) was administered (75 or 150 mg/kg) intraperitoneally twice a week (10).
Cell lines and culture conditions
The human pancreatic adenocarcinoma cell lines BxPC3 and PANC-1 were purchased from the American Type Culture Collection. FG human pancreatic adenocarcinoma cells were used as reported previously (17). The cell lines were maintained in plastic flasks as adherent monolayersin minimal essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution(Flow Laboratories).
Animals
Female athymic BALB/c nude mice were purchased from The Jackson Laboratory. The mice were housed in laminar flow cabinets under specific pathogen-free conditions and used when they were 8 weeks old. The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and National Institutes of Health.
Matrigel plug assay
A Matrigel plug angiogenesis assay was performed essentially as described previously (38). Matrigel (200 μl) containing 2×106 cells was injected subcutaneously into nude mice (two injection sites per mouse). The Matrigel plugs were recovered from the mice 8 days after injection and carefully stripped of host tissues. After photomicrography, the Matrigel plugs were weighed and homogenized in 1 ml of distilled water and then centrifuged at 10,000 rpm for 5 min. The supernatants were collected for hemoglobin-concentration measurement using Drabkin solution (Sigma Chemical Co.) and a Microplate Manager enzyme-linked immunosorbent assay reader at 540 nm according to the manufacturer’s instructions. The relative hemoglobin concentrations were calculated and further normalized according to the weights of the plugs.
Tumor cell invasion/migration assay
BxPC-3, FG or PANC-1 cells were pretreated for 12 hrs with 2.5–10 μM of BA or DMSO (ctrl). Cells from each group were trypsinized and 2–5×104 cells of each group were re-suspended and seeded in the upper of modified Boyden chambers with Matrigel-coated membrane. DMEM with 10% FBS was used as chemoattractant. For each cell line, 750 μl of respective conditioned media was added into the lower chamber. After 24–48 hours incubation, invasive cells which had moved through the Matrigel membrane were stained, counted and photographed under a microscope (×200 magnification).
Gene expression analyses
For Western blot, whole-cell lysates were prepared from human pancreatic cancer cell lines and tumor tissue specimens (17). Standard Western blotting was performed using polyclonal rabbit antibodies against human Sp1, VEGF, Survivin, uPAR and Ki67 (Santa Cruz Biotechnology) and an anti-rabbit IgG antibody, which was a horseradish peroxidase-linked F(ab')2 fragment obtained from a donkey (Amersham). Equal protein-specimen loading was monitored by probing the same membrane filter with an antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (17). The probe proteins were detected using the Amersham enhanced chemiluminescence system according to the manufacturer’s instructions. For quantitative real-time PCR (qPCR), total RNA was reversely transcribted into cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The cDNA products were used in qPCR analyses of gene expression using PCR primer and probe sets custom-designed or purchased from Applied Biosystems (Carlsbad, CA) and relative RNA-expression calculations were performed using a commercially available software programs (SDS version 1.2; Applied Biosystems) (18).
Immunohistochemical analysis and quantification of tumor MVD
Tissue sections were prepared and processed for immunostaining using specific antibodies against CD31, Sp1, and VEGF and appropriate secondary antibodies. The levels of gene expression and quantification of tumor MVD were evaluated as described previously (20).
Sp1 and VEGF promoter constructs and analysis of Sp1 and VEGF promoter activity
The minimal Sp1 and VEGF promoter reporters in pGL3 luciferase constructs were generated and used as described previously (17, 20). To examine transcriptional regulation of the Sp1 and VEGF promoters by BA and MIT, PANC-1 cells were seeded to about 80% confluence in six-well plates (in triplicate) and transiently transfected with 0.6 μg of minimal Sp1 or VEGF reporter plasmids and 12 ng of Renilla reporter plasmids as indicated in each experiment using Lipofectamine (Invitrogen) according to the manufacturer’s instructions. The reporter luciferase activity was measured 48 h later using a luciferase assay kit (Promega). Promoter activity was normalized according to the protein concentration as described previously (17, 20).
Chromatin immunoprecipitation
Chromatin was prepared from pancreatic cancer cells as described previously (20). A chromatin immunoprecipitation (ChIP) assay was performed using a Chromatin Immunoprecipitation Assay Kit (Upstate) according to the manufacturer’s instructions. Briefly, DNA cross-binding proteins were cross-linked with DNA and lysed in sodium dodecyl sulfate lysis buffer. The lysate was sonicated to shear DNA to 200–500 bp. After preclearing with a salmon sperm DNA/protein A agarose 50% slurry for 30 min at 4°C, chromatin specimens were immunoprecipitated overnight with no antibody or an anti-Sp1 antibody (PEP2). The region from -224 to -53 bp of the Sp1 promoter was amplified using the following primers: sense, 5’-caggcacgcaacttagtc-3’; antisense, 5’-gtaaggaggagggagcag-3’. The region from -272 to +18 bp of the VEGF promoter was amplified using the following primers: sense, 5’-ccgcgggcgcgtgtctctgg-3’; antisense, 5’-tgccccaagcctccgcgatcctc-3’. Polymerase chain reaction (PCR) products were separated on a 1.5% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light.
Statistical analysis
All in vivo experiments used 5 mice per group and were repeated at least once with similar results; one representative experiment was presented. The in vitro cytotoxicity experiments have been performed in triplicate for each and every time points and concentrations. The significance of the in vitro data was determined using the Student t-test (two-tailed), whereas the significance of the in vivo data was determined using the two-tailed Mann-Whitney U test. P levels of ≤0.05 and <0.01 were deemed statistically significant (*) and highly significant (#), respectively.
Results
Antitumor effects of BA and MIT in xenograft mouse models of human pancreatic cancer
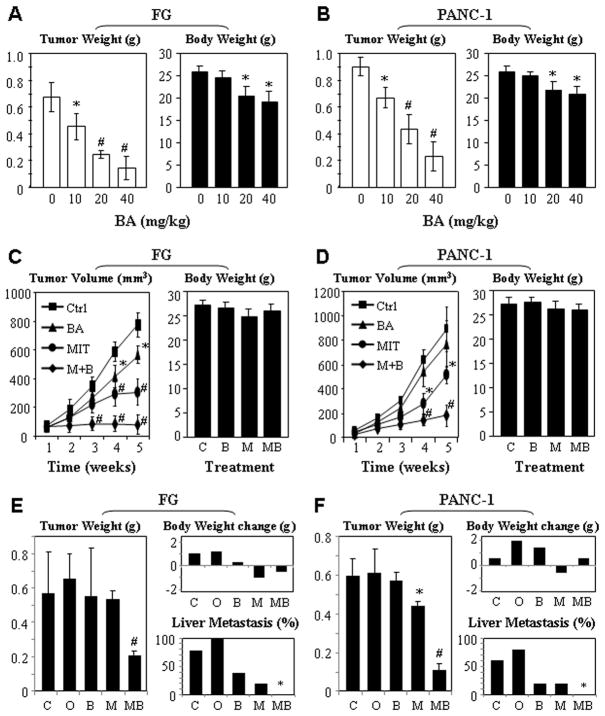
Previous studies have demonstrated that Sp1 activity be essential for VEGF expression and that VEGF plays a major role in pancreatic tumor angiogenesis (17, 39, 40). Treatment with both BA and MIT can downregulate Sp1, VEGF, and VEGF receptor expression (28, 41). However, whether these two drugs interact synergistically in regulating Sp1 activity and pancreatic tumor growth is unknown. In our previous studies, we have already demonstrated a dose-dependent antitumor activity of MIT (21). In this first set of experiments, we treated FG and PANC-1 xenograft tumors in nude mice with different doses of BA three times a week. BA produced dose-dependent antitumor activities in both FG and PANC-1 models, while the mice’s body weights decreased in a dose-dependent manner (Fig. 1A & 1B).
Figure 1.
Dose-dependent antitumor effects of BA and MIT in xenograft models of human pancreatic cancer. Dose response: FG (A) and PANC-1 (B) cells were injected into the pancreases of nude mice (n=5). Ten days after tumor injections, the mice were treated with different doses of BA (10, 20, and 40 mg/kg) via oral gavages three times a week. The tumors were weighed 45 days after tumor cell injection (left panels); the mice were weighed at the same time and columns, mean weights; bars, standard deviations (right panels). Synergistic antitumor effect in ectopic models: FG (C) and PANC-1 (D) cells were injected into the subcutis of nude mice (n=5). When tumors reached around 4 mm in diameter, the animals received MIT (0.05 mg/kg) via intraperitoneal injection twice a week and BA (10 mg/kg). Tumor volumes were measured every week until the mice were killed 45 days after tumor cell injection (left panels); the mice were weighed at the time of termination of experiments (right panels). Synergistic antitumor effect in orthotopic models: FG (E) and PANC-1 (F) cells were injected into the pancreases of nude mice (n=5). Ten days after tumor cell injections, the animals received MIT (0.05 mg/kg) via intraperitoneal injection twice a week and BA (10 mg/kg) via oral gavage three times a week. The mice were killed 45 days after tumor cell injection; both tumors (left panels) and the mice (upper right panels) were weighed, and hepatic metastases were determined (lower right panels). *P<0.05 and #P<0.01 as compared to respective controls (two tailed student t test). C, Control; O, Corn oil; B, BA; M, MIT; MB, MIT+BA.
Next, we treated FG and PANC-1 xenograft tumors in nude mice with nontoxic doses of MIT (0.05 mg/kg), BA (10 mg/kg), or both. We found that BA and MIT alone had marginal antitumor activity. In contrast, the combination of MIT and BA had significant antitumor activity in both FG and PANC-1 models. Furthermore, treatment with low doses of BA and MIT produced synergistic antitumor activity without any significant systemic side effects as indicated by a lack of significant weight loss (Fig. 1C &. 1D). Similar results were obtained in orthotopic models (Fig. 1E &. 1F). Therefore, combination administration of low doses of MIT and BA has a significant therapeutic benefit for pancreatic cancer. This notion was further confirmed using both ectopic and orthotopic models of FG (Fig. S1) and PANC-1 (Fig. S2) cells. Specifically, we found that the doses of Gemcitabine that produced significant antitumor activities also led to more discernible losses of animal body weights than the combination of BA and MIT.
Synergistic cytotoxicity of BA and MIT in human pancreatic cell lines in vitro
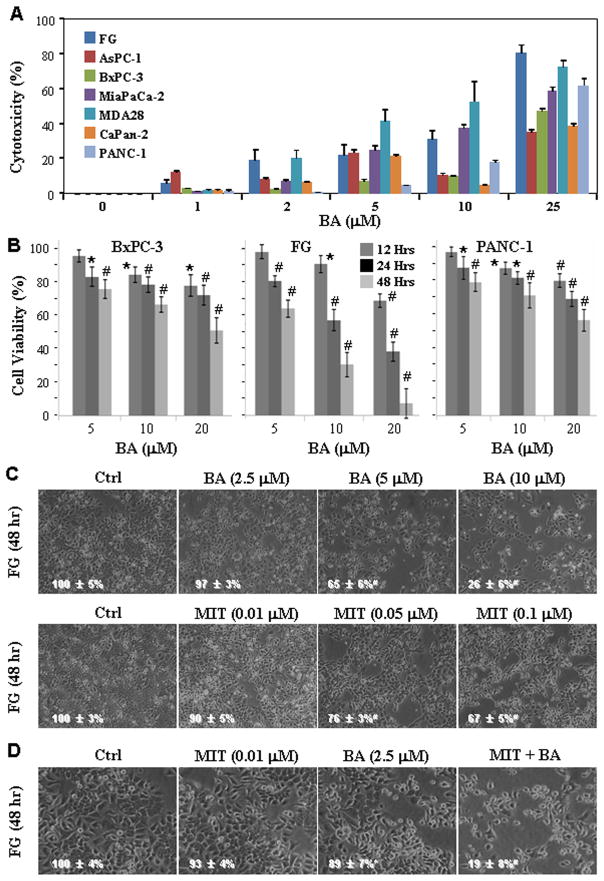
To assess the direct cytotoxicity of BA and MIT, we treated various pancreatic cancer cell lines with BA for 48 h (Fig. 2A); and BxPC-3, FG, and PANC-1 cells with BA for 12, 24 or 48 h (Fig. 2B). Inhibition of cell proliferation was assessed using an MTT assay. We found that BA produced concentration-dependent cytotoxicity and FG cells exhibited the highest sensitivity to BA-mediated cytotoxicity. FG cells were then treated with different concentrations of BA or MIT. Both drugs exhibited concentration-dependent cytotoxicity (Fig. 2C). We then optimized the drug concentrations so that neither agent alone had an extensive cytotoxic effect. Under this condition, the combination of BA and MIT had substantial cytotoxic effects (Fig. 2D). The combination treatment with BA and MIT revealed a synergistic effect of cytotoxicity (data not shown). Similar results were obtained from using PANC-1 cells (Fig. S3).
Figure 2.
Synergistic effect of treatment with BA and MIT on inhibition of pancreatic cancer cell proliferation. A, Various pancreatic cancer cell lines were treated with BA at concentrations ranging from 1 to 25μM for 48 h. Inhibition of cell proliferation was assessed using an MTT assay. B, BxPC-3, FG, and PANC-1 cells were treated with BA at concentrations ranging from 5, 10, and 20μM for 12, 24 or 48 h. Inhibition of cell proliferation was assessed using an MTT assay. C, FG cells were treated with BA at concentrations ranging from 2.5, 5, and 10μM or MIT at concentrations ranging from 0.01, 0.05, and 0.10μM for 48 h. Cell cultures were photographed before assessing cell proliferation using an MTT assay (inserted number represented percent viability ± SD). D, FG cells were treated with 2.5μM BA, 0.01 μM MIT or both for 48 h. Cell cultures were photographed before assessing cell proliferation using an MTT assay (inserted number represented percent viability ± SD). *P<0.05 and #P<0.01 (two-tailed student t test).
Synergistic effect of treatment with BA and MIT on inhibition of pancreatic cancer cell migration and invasion
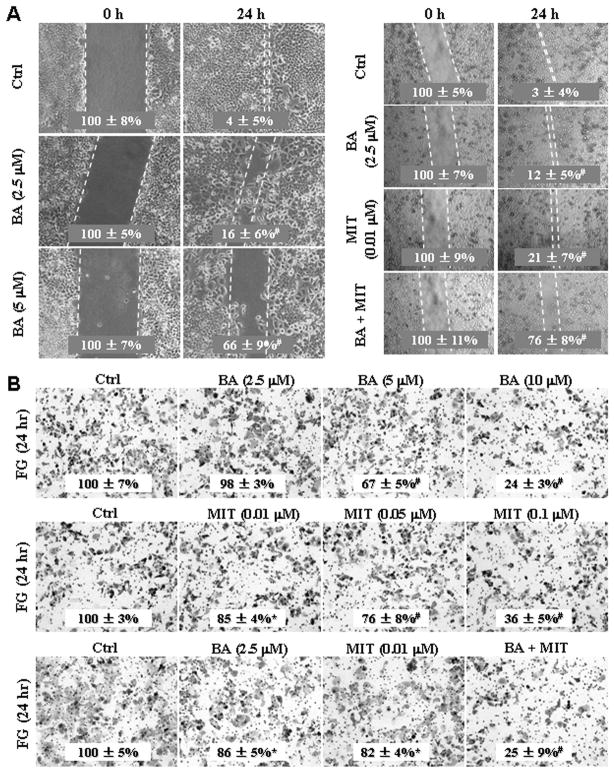
FG cell cultures in triplicate were pretreated with BA (0, 2.5 or 5μM), or pretreated with 2.5μM BA, 0.01 μM MIT or both for 24 h, the cultures were wounded by scratching and maintained for additional 24 h. Cell cultures were photographed and cell migration was assessed by measuring gap sizes (Fig. 3A). FG cells were treated with BA at concentrations ranging from 2.5, 5, and 10μM or MIT at concentrations ranging from 0.01, 0.05, and 0.10μM for 24 h, or FG cells were treated with 2.5μM BA, 0.01 μM MIT or both for 24 h. Representative photomicrographs of tumor cell invaded through Matrigel were taken, while the numbers of invasive cells that penetrated through Matrigel-coated filter were counted in 15 random fields identified within the lower surface of the filters and expressed as % of control (Fig. 3B). Similar results were obtained using PANC-1 cells (Fig. S4).
Figure 3.
Synergistic effect of treatment with BA and MIT on inhibition of pancreatic cancer cell migration and invasion. A, FG cells in triplicate were pretreated with BA at concentrations ranging from 2.5, or 5μM, or pretreated with 2.5μM BA, 0.01 μM MIT or both for 24 h, the cultures were wounded by scratching and maintained for additional 24 h. Cell cultures were photographed and cell migration was assessed by measuring gap sizes (inserted number represented percent area of gap ± SD). B, FG cells were treated with BA at concentrations ranging from 2.5, 5, and 10μM or MIT at concentrations ranging from 0.01, 0.05, and 0.10μM for 24 h, or FG cells were treated with 2.5μM BA, 0.01 μM MIT or both for 24 h. Representative tumor cell invaded through Matrigel were photographed, while the numbers of invasive cells that penetrated through Matrigel-coated filter were counted in 15 random fields identified within the lower surface of the filters and expressed as % of control (inserted numbers). Data represents mean ± SD of triplicates. *P<0.05 and #P<0.01 (two-tailed student t test).
Antiangiogenic effects of BA and MIT in vitro
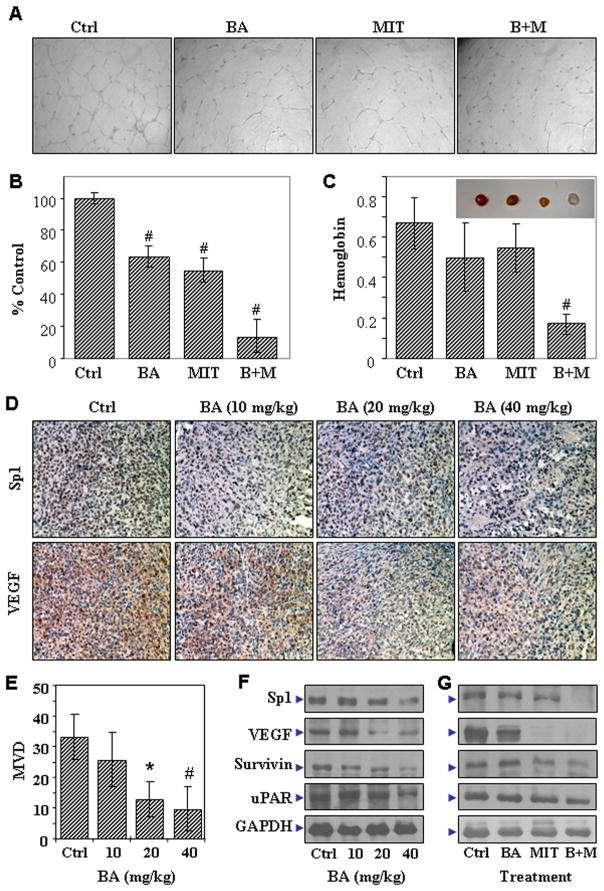
We treated FG cells with 2.5μM BA and/or 0.01μM MIT. Once Sp1 expression downregulation was confirmed by Western blot analysis, we then used an endothelial cell tube formation assay to determine the angiogenic potential of the supernatants of the FG cells. We assessed the degree of tube formation as the percentage of cell surface area versus the total surface area. We obtained representative photomicrographs of tube formation by human umbilical vein endothelial cells in the supernatants in situ (Fig. 4A). Treatment with MIT and/or BA reduced the capacity of supernatants of the FG cells to stimulate tube formation by endothelial cells compared with that of supernatants of control FG cells (Fig. 4B). We confirmed this impaired angiogenic potential using an in vivo Matrigel plug assay (Fig. 4C). Our data suggested that treatment with MIT and/or BA impaired the angiogenic potential of FG cells.
Figure 4.
Effect of treatment with BA and MIT on the FG cell angiogenic phenotype. Culture supernatants were harvested from FG cells treated with 0.01μM MIT, 2.5 μM BA, or both. The angiogenic potential of the supernatants was determined using an endothelial cell tube formation assay. A, Representative tube formation in the supernatants were photographed in situ. B, The degrees of tube formation were assessed as the percentage of cell surface area versus total surface area. Control cell cultures were given arbitrary percentage values of 100. C, Matrigel (200 μl) containing 2×106 untreated FG cells or FG cells treated with 2.5 μM BA, 0.01μM MIT, or both was used as described in Materials and Methods (Insert: recovered representative Matrigel plugs from corresponding groups). D–F, The FG tumors from mice receiving dissolvent (Ctrl, Corn Oil) or different doses of BA treatment (described in Fig. 1A) were collected and processed for gene expression analysis by immunostaining of VEGF and Sp1 expression (D), quantitation of tumor angiogenesis by microvessel counting (E), and confirmation of gene expression by Western blot analysis (F). G, The FG tumors from mice receiving dissolvent (Ctrl, Corn Oil) or treatment of BA, MIT or both (described in Fig. 1C) were collected and processed for gene expression analysis by Western blot analysis. *P<0.05 and #P<0.01 as compared to respective controls (two tailed student t test).
Effects of treatment with BA and MIT on Sp1 and VEGF expression and MVD in vivo
To determine the molecular basis for the antitumor effect of treatment of pancreatic cancer with BA, we performed immunohistochemical staining on tissue sections harvested from in Fig. 1A. Treatment with BA decreased expression of Sp1 and its downstream molecules in FG tumors in a dose-dependent manner (Fig. 4D). Also, as indicated by CD31 staining, tumor MVDs were inhibited in a dose-dependent manner (Fig. 4E). The immunostaining results were further confirmed by using Western blot analysis (Fig. 4F).
To determine the molecular basis for the synergistic effect of treatment of pancreatic cancer with BA and MIT, we performed Western blot analysis using total protein lysates extracted from the FG tumor specimens collected from mice that received treatment with PBS, BA, MIT, or both BA and MIT as shown in Fig. 1C. As shown in Fig. 4G, expression of Sp1 and its downstream targets were downregulated by treatment with the combination of BA and MIT (Fig. S5). Similar results were obtained from using PANC-1 cells (Fig. S6). These results suggested that the synergistic antitumor activity of the combination of BA and MIT occur through not only an antiangiogenic effect but also direct inhibition of tumor-cell proliferation.
BA and MIT inhibited the recruitment of Sp1 onto the Sp1 and VEGF promoters and suppressed Sp1 and VEGF protein expression in human pancreatic cancer cells in vitro
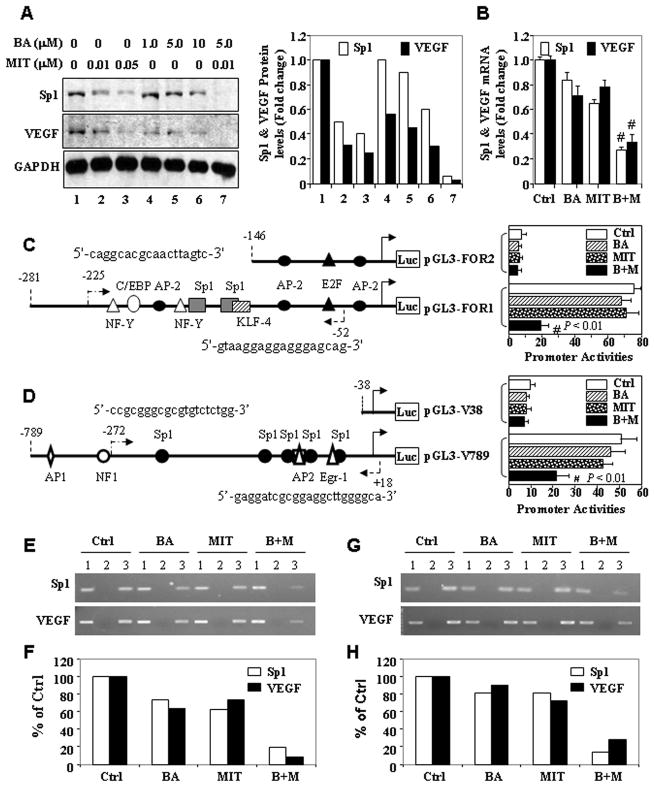
To further confirm the impact of treatment with BA and MIT on gene expression in pancreatic cancer cells, we incubated FG cells in a medium alone or a medium containing MIT (0, 0.01, or 0.05 μM) and/or BA (1, 5, or 10 μM). Sp1 protein expression in the cells was downregulated in a dose-dependent manner after 24 h of treatment with BA and MIT as single agents in vitro as determined by Western blot analysis and its respective quantitation by densitometry (Fig. 5A).
Figure 5.
Treatment with BA and MIT downregulates Sp1 expression in vitro. A, FG cells were incubated in a medium alone or a medium containing different concentrations of BA and/or MIT for 24 h. Total protein lysates were harvested from the cell cultures, and the level of Sp1 and VEGF protein expression was determined using Western blot analysis. Equal protein-specimen loading was monitored by probing the same membrane filter with an anti-GAPDH antibody and changes in gene expression levels were quantitated (A). Total RNA was harvested for qRT-PCR analysis of both Sp1 and VEGF mRNA (B). Sp1 (C) and VEGF (D) promoter reporter constructs were transfected into PANC-1 cells in triplicate and incubated for 12 h. The cells were then incubated for another 24 h in a medium alone or a medium containing 2.5 μM BA, 0.01 μM MIT, or both. Total protein lysates were harvested from the cell cultures for measurement of Sp1 promoter activity using a luciferase assay kit. The relative Sp1 promoter activities in treated groups were expressed as the fold changes from that in their respective control groups. FG (E & F) and PANC-1 (G & H) cells were incubated in vitro in a medium alone or a medium containing 2.5 μM BA, 0.01 μM MIT or both for 24 h and chromatin was extracted from the cells. The ChIP assay was performed using a specific anti-Sp1 antibody and oligonucleotides flanking the VEGF and Sp1 promoter regions containing Sp1-binding sites. The nucleotide positions and sequences of PCR forward and reverse primers flanking those sites in ChIP assay were shown in C and D. *P<0.05 and #P<0.01 (two tailed Student t test). Lane 1, input chromatin DNA; lane 2, chromatin DNA with a control IgG; lane 3, chromatin DNA with an anti-Sp1 antibody. Ctrl, control; M+B, MIT plus BA. Quantitative data were also presented (F & H).
Next, we determined whether treatment with BA and/or MIT regulated Sp1 and VEGF expression at the transcriptional level. FG cells were treated in a medium alone or a medium containing 2.5 μM BA or 0.01 μM MIT or both. Treatment with BA or MIT at the given dose resulted in low levels of suppression of Sp1 and VEGF mRNA, whereas treatment with the combination of BA and MIT significantly suppressed mRNA expression (Fig. 5B). Moreover, we transfected Sp1 and VEGF promoter reporter constructs into FG cells and then incubated them in a medium alone or a medium containing 5 μM BA or 0.01 μM MIT. In vitro, treatment with BA or MIT at the given dose resulted in low levels of suppression of Sp1 and VEGF promoter activity, whereas treatment with the combination of BA and MIT significantly suppressed this activity. However, further deletion of Sp1-binding sites eliminated the ability of MIT to suppress Sp1 (Fig. 5C) and VEGF promoter activity (Fig. 5D).
Finally, we performed a ChIP assay using chromatin extracted from FG and PANC-1 cells. Treatment with BA or MIT at the given dose had a minor effect on inhibition of Sp1 recruitment to its own promoter and the VEGF promoter, whereas treatment with BA combined with MIT at the same dose significantly decreased Sp1 recruitment to these two promoters in both FG (Fig. 5E) and PANC-1 cells (Fig. 5G). These results suggested that treatment with BA and MIT at low doses resulted in insignificant transcriptional suppression of Sp1 and VEGF mRNA transcription activated by Sp1, whereas treatment with BA combined with MIT at the same doses produced synergistic transcriptional suppression of Sp1 and VEGF transcription.
Overexpression of Sp1 renders pancreatic cancer cells resistance to BA cytotoxicity
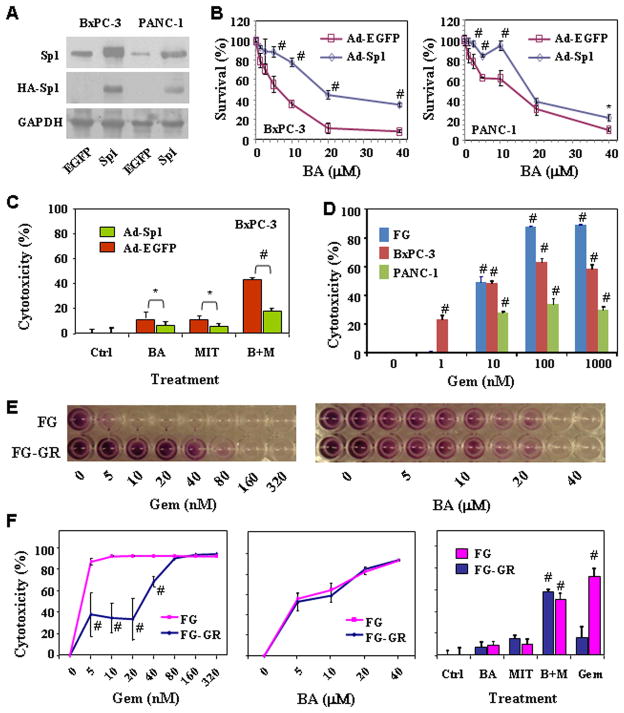
To determine whether Sp1 is a key target of BA, we evaluated the effects of ectopic Sp1 overexpression on BA-mediated cytotoxicity in both BxPC-3 and PANC-1 cells. The ectopic Sp1 protein expression was determined by Western blot using anti-HA antibody (for exogenous Sp1 protein) and anti-Sp1 antibody (for total Sp1 protein) (Fig. 6A). Clearly, the ectopic Sp1 overexpression led to resistance to BA in both BxPC-3 and PANC-1 cells (Fig. 6B) and also to the combination of BA and MIT (Fig. 6C).
Figure 6.
Influence of Sp1 expression on cytotoxicity in vitro. BxPC-3 and PANC-1 cells were treated with Ad-EGFP or Ad-Sp1 (10 MOI) for 6 hours, and cultures were incubated for additional 18 h. The cells were either harvested for analysis of Sp1 expression by using Western blot (A) or plated in 96-well plates and treated with different concentrations of BA (B) or with 2.5 μM BA, 0.01 μM MIT or both (C) for additional 24 h before cell viability determination by MTT assay. D, BxPC-3, FG, and PANC-1 cells were treated with gemcitabine (“Gem”, 0 – 1000 nM) for 48 h. Inhibition of cell proliferation was assessed using an MTT assay. E & F, FG and FG-GR (gemcitabine-resistant variant) cells were treated with gemcitabine (0 – 320 nM) or BA (0 – 40 μM) for 72 h or treated with 2.5 μM BA, 0.01 μM MIT or both for 48 h. Treatment with 10 nM of gemcitabine was used as a control. Inhibition of cell proliferation was assessed using an MTT assay. *P<0.05 and #P<0.01 as compared to respective controls (two tailed student t test).
BA inhibited the growth of gemcitabine-resistant FG cells
In the last set of experiments, we determined whether pancreatic cancer cells cross-resist to both BA and gemcitabine. FG, BxPC-3, and PANC-1 cells were treated with gemcitabine (0–1000 nM) and cytotoxicity was determined by MTT assay. We found the sensitivities to gemcitabine (Fig. 6D) were correlated with that to BA among the three cell lines (Fig. 2A). We then established a gemcitabine-resistant FG cell line, FG-GR, by incubating the FG cells with increasing concentrations of gemcitabine for a period of 3 months. As shown in Fig. 6E & 6F, FG cells were highly sensitive to gemcitabine as compared with FG-GR cells, whereas both FG and FG-GR cells remain similarly sensitive to BA or with the combination of lower levels of both BA and MIT.
Discussion
BA and its derivatives have significant antitumor activities to various tumor types (28, 31, 42). Of particular interest is its direct and relatively selective cytotoxic effect on various tumor cells vs. normal or non-neoplastic cells (42). Our current study showed that an increasing level of antitumor activities appear to be accompanied by increasing systemic side-effects as reflected by an increasing loss of body weight. Interestingly, FG and PANC-1 tumor cells exhibited quite different sensitivities to BA-mediated cytotoxicity in vitro, while we observed very similar in vivo antitumor activities in the animal models at similar doses of BA. These results suggested that the mechanisms for its antitumor activities might not solely due to BA’s direct cytotoxic effects. Therefore, exploration of other mechanisms underlying its antitumor activities should help enhance therapeutic index of BA-based cancer therapy, i.e., increased tumor suppression and decreased systemic side-effects. Indeed, BA has significant anti-angiogenesis and anti-invasion activities other than anti-proliferation activity. More importantly, a combination of BA and MIT has a synergistic antitumor activity and exhibit less discernible side effect than gemcitabine does, and Sp1 is an important target.
Sp1/VEGF pathway is important to pancreatic tumor angiogenesis and its targeted inhibition suppresses pancreatic tumor growth in mouse models (17, 20, 23, 43). We have shown that MIT could downregulate Sp1 expression through direct interference of Sp1 auto-upregulation (21, 32). However, as the important regulator of VEGF, Sp1 protein is of high stability and abundance, which prevent MIT from rapidly downregulating Sp1 protein level in pancreatic cancer cells. A relative long treatment time and high dosage of MIT are required to downregulate Sp1 protein, thus increasing the possibility of systemic side effects (21). Interestingly, recent studies have indicated that BA and its derivatives promote the degradation of Sp1 proteins (28, 44). In the present study, we demonstrated that BA promoted Sp1 protein downregulation, which is consistent with a previous finding (28). More importantly, combined treatment with MIT and BA, neither of which has significant effects on Sp1 protein level, substantially downregulated Sp1 protein expression and suppressed angiogenesis, which was consistent with the synergistic antitumor effect in our mouse models.
Significantly, we have shown that BA has a strong inhibitory effect on pancreatic cancer cell migration and invasion. This effect might not be simply due to its cytotoxicity, because BA at nontoxic concentrations suppressed tumor cell invasion. Furthermore, BA sensitizes pancreatic cancer cells to MIT treatment. One of the potential underlying mechanisms appears to be a rapid downregulation of Sp1 protein expression. This notion is further supported by our findings, showing that BA produced dose-dependent suppression of uPAR in vitro and xenograft tumors. The uPA and uPAR are known downstream target genes of Sp1 and play an important role in adhesion, migration and invasion of pancreatic cancer cells (45). Given that uncontrolled growth and extensive invasion and metastasis are hallmarks of pancreatic cancer (46), our results suggest a novel and significant mechanism underlying the antitumor activities of BA and its derivatives. Besides uPA and uPAR, other potential targets of BA treatment warrant further investigation.
BA produces antitumor activity in combination with other chemotherapeutic drugs or radiation therapy (47). For example, BA sensitizes drug-resistant colon cancer cells and esophageal squamous carcinoma cells to 5-fluorouracil, irinotecan, and oxaliplatin (31, 48). While the underlying mechanisms remain to be determined, prior studies have indicated that BA induces apoptosis through intrinsic pathway independent of p53 and the Fas-FasL extrinsic pathway (29, 49, 50). Other possible targets for BA antitumor activity include aminopeptidase N or topoisomerase, suggesting BA produces broad anticancer effects and sensitizes others chemo- and radio-therapy through different molecular targets. In contrast, MIT induces apoptosis at least in part by stimulating the expression of apoptosis-inducing ligand, Fas ligand and TNF-α in tumor cells and by preventing p53-mediated transcriptional activation (36, 37). Therefore, MIT and BA have distinct mechanisms to induce apoptosis, which might be the underlying basis for their synergistic antitumor activity. However, our current study also suggested an additional mechanism for apoptosis induction by both MIT and BA. It was reported that BA-based treatment activates Sp protein degradation and inhibits its downstream target Survivin expression in cancer cells, which plays an important role in tumor cell resistance to apoptosis (28). Our results showed that BA inhibits tumor-cell growth in vitro and that this effect is synergistic with that of MIT. Consistently, BA and MIT synergistically downregulated Sp1 and Survivin protein expression. Likewise, a synergistic downregulation of Sp1 may also cause downregualtion of VEGF and uPAR, hence suppression of pancreatic cancer angiogenesis and invasion. Therefore, it is likely that an accelerated downregulation of Sp1 and it major target genes including VEGF, Survivin and uPAR be an important mechanism for antitumor activities of BA and its derivatives. Finally, ectopic overexpression of Sp1 rendered tumor cells resistance to BA, MIT, and their combination, strongly suggested that Sp1 is crucial for the antitumor activities observed.
In summary, we investigated the antitumor activity of natural product BA and its underlying mechanisms of actions in pancreatic cancer models. Besides its expected antiproliferation activity, BA exhibited strong anti-angiogenesis and anti-invasion abilities in pancreatic cancer. Our experimental results further indicated that Sp1 was one of the important targets for BA-mediated protein degradation and MIT-mediated transcriptional repression. Furthermore, our findings that a combination of BA and MIT at low doses effectively downregulated the expression of Sp1 and its downstream targets including VEGF, uPAR and Survivin and produced synergistic antitumor effects with an enhanced therapeutic index strongly suggested that a combined use of drugs having distinct mechanisms of action could potentially benefit cancer patients. Given that the combination of BA and MIT, and their combinations with gemcitabine, appear to be less toxic than gemcitabine alone, the treatment with such combinations in clinical studies is a rational step forward in the development of effective targeted therapies for pancreatic cancer as well as other cancers. Further investigations into the mechanisms for enhanced therapeutic index of such combinations are clearly warranted.
Supplementary Material
Acknowledgments
We thank Don Norwood for editorial comments. The work is supported in part by Pancreatic Cancer SPORE Grant 1P20-CA101936-01-PP4 and grant 5R01-CA129956 from the National Cancer Institute, National Institutes of Health, and Hogan Foundation (to K. Xie) and M. D. Anderson Cancer Center Institutional Start-up Funds (to D.Z. Chang).
References
- 1.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Kindler HL. Pancreatic cancer: an update. Current oncology reports. 2007;9:170–6. doi: 10.1007/s11912-007-0018-z. [DOI] [PubMed] [Google Scholar]
- 3.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. The British journal of surgery. 2004;91:586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 4.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Annals of surgery. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Annals of surgery. 1997;225:621–33. doi: 10.1097/00000658-199705000-00018. discussion 33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie K, Wei D, Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev. 2006;17:147–56. doi: 10.1016/j.cytogfr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Hruban RH, Maitra A, Schulick R, et al. Emerging molecular biology of pancreatic cancer. Gastrointest Cancer Res. 2008;2:S10–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Whipple C, Korc M. Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks Arch Surg. 2008;393:901–10. doi: 10.1007/s00423-008-0280-z. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–5. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Seminars in cancer biology. 2004;14:123–30. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 13.Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312:522–6. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer. 1998;34:337–40. doi: 10.1016/s0959-8049(97)10068-5. [DOI] [PubMed] [Google Scholar]
- 15.Couvelard A, O'Toole D, Leek R, et al. Expression of hypoxia-inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas. Histopathology. 2005;46:668–76. doi: 10.1111/j.1365-2559.2005.02160.x. [DOI] [PubMed] [Google Scholar]
- 16.Hotz HG, Hines OJ, Masood R, et al. VEGF antisense therapy inhibits tumor growth and improves survival in experimental pancreatic cancer. Surgery. 2005;137:192–9. doi: 10.1016/j.surg.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–54. [PubMed] [Google Scholar]
- 18.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–8. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 19.Abdelrahim M, Smith R, 3rd, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–9. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Zhang J, Wei D, et al. Molecular basis of the synergistic antiangiogenic activity of bevacizumab and mithramycin A. Cancer Res. 2007;67:4878–85. doi: 10.1158/0008-5472.CAN-06-3494. [DOI] [PubMed] [Google Scholar]
- 21.Jia Z, Gao Y, Wang L, et al. Combined treatment of pancreatic cancer with mithramycin A and tolfenamic acid promotes Sp1 degradation and synergistic antitumor activity. Cancer Res. 2010;70:1111–9. doi: 10.1158/0008-5472.CAN-09-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 23.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–48. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Wei D, Huang S, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80. [PubMed] [Google Scholar]
- 25.Jungert K, Buck A, von Wichert G, et al. Sp1 is required for transforming growth factor-beta-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 2007;67:1563–70. doi: 10.1158/0008-5472.CAN-06-1670. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64:489–500. [PMC free article] [PubMed] [Google Scholar]
- 27.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature cell biology. 2009;11:1487–95. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 28.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–23. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 29.Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug discovery today. 2009;14:885–90. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Eder-Czembirek C, Erovic BM, Czembirek C, et al. Betulinic Acid a Radiosensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines. Strahlenther Onkol. 2010 doi: 10.1007/s00066-010-2069-6. [DOI] [PubMed] [Google Scholar]
- 31.Jung GR, Kim KJ, Choi CH, et al. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol. 2007;101:277–85. doi: 10.1111/j.1742-7843.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- 32.Majee S, Dasgupta D, Chakrabarti A. Interaction of the DNA-binding antitumor antibiotics, chromomycin and mithramycin with erythroid spectrin. European journal of biochemistry / FEBS. 1999;260:619–26. doi: 10.1046/j.1432-1327.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 33.Remsing LL, Bahadori HR, Carbone GM, McGuffie EM, Catapano CV, Rohr J. Inhibition of c-src transcription by mithramycin: structure-activity relationships of biosynthetically produced mithramycin analogues using the c-src promoter as target. Biochemistry. 2003;42:8313–24. doi: 10.1021/bi034091z. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee S, Zaman K, Ryu H, Conforto A, Ratan RR. Sequence-selective DNA binding drugs mithramycin A and chromomycin A3 are potent inhibitors of neuronal apoptosis induced by oxidative stress and DNA damage in cortical neurons. Ann Neurol. 2001;49:345–54. [PubMed] [Google Scholar]
- 35.Blume SW, Snyder RC, Ray R, Thomas S, Koller CA, Miller DM. Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J Clin Invest. 1991;88:1613–21. doi: 10.1172/JCI115474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duverger V, Murphy AM, Sheehan D, et al. The anticancer drug mithramycin A sensitises tumour cells to apoptosis induced by tumour necrosis factor (TNF) Br J Cancer. 2004;90:2025–31. doi: 10.1038/sj.bjc.6601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koutsodontis G, Kardassis D. Inhibition of p53-mediated transcriptional responses by mithramycin A. Oncogene. 2004;23:9190–200. doi: 10.1038/sj.onc.1208141. [DOI] [PubMed] [Google Scholar]
- 38.Passaniti A, Taylor RM, Pili R, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–28. [PubMed] [Google Scholar]
- 39.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science (New York, NY. 1989;246:1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 40.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (New York, NY. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 41.Abdelrahim M, Baker CH, Abbruzzese JL, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer research. 2007;67:3286–94. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 42.Pisha E, Chai H, Lee IS, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nature medicine. 1995;1:1046–51. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 43.Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1648–52. doi: 10.1158/1055-9965.EPI-07-2791. [DOI] [PubMed] [Google Scholar]
- 44.Chintharlapalli S, Papineni S, Liu S, et al. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis. 2007;28:2337–46. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 45.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 46.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by betulinic Acid. Neoplasia. 2005;7:162–70. doi: 10.1593/neo.04442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamai H, Sawada N, Yoshida T, et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer. 2009;125:952–60. doi: 10.1002/ijc.24433. [DOI] [PubMed] [Google Scholar]
- 49.Rieber M, Rieber MS. Correspondence re: S. Fulda et al., Betulinic acid triggers CD95 (Apo1/Fas)-and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]; Cancer Res. 1998;58:5876–7. [PubMed] [Google Scholar]
- 50.Kessler JH, Mullauer FB, de Roo GM, Medema JP. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007;251:132–45. doi: 10.1016/j.canlet.2006.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.