Abstract
5-Azacytidine (5-Aza) induces differentiation of mesenchymal stem cells (MSCs) into cardiomyocytes. However, the underlying mechanisms are not well understood. Our previous work showed that 5-Aza induces human bone marrow-derived MSCs to differentiate into cardiomyocytes. Here, we demonstrated that 5-Aza induced cardiac differentiation of human umbilical cord-derived MSCs (hucMSCs) and explored the potential signaling pathway. Our results showed that hucMSCs had cardiomyocyte phenotypes after 5-Aza treatment. In addition, myogenic cells differentiated from hucMSCs were positive for mRNA and protein of desmin, β-myosin heavy chain, cardiac troponin T, A-type natriuretic peptide, and Nkx2.5. Human diploid lung fibroblasts treated with 5-Aza expressed no cardiac-specific genes. 5-Aza did not induce hucMSCs to differentiate into osteoblasts. Further study revealed that 5-Aza treatment activated extracellular signal related kinases (ERK) in hucMSCs, but protein kinase C showed no response to 5-Aza administration. U0126, a specific inhibitor of ERK, could inhibit 5-Aza-induced expression of cardiac-specific genes and proteins in hucMSCs. Increased phosphorylation of signal transducers and activators of transcription 3, and up-regulation of myocyte enhancer-binding factor-2c and myogenic differentiation antigen in 5-Aza-treated hucMSCs were also suppressed by U0126. Taken together, these results suggested that sustained activation of ERK by 5-Aza contributed to the induction of the differentiation of hucMSCs into cardiomyocytes in vitro.
Introduction
Myocardial disorders such as acute myocardial infarction endanger millions of people and cause a substantial number of deaths each year. Because adult cardiomyocytes are unable to regenerate necrotic tissue to compensate for cardiac dysfunction, massive loss of functional cardiomyocytes leads to myocardial disorder. Mesenchymal stem cells (MSCs) have enormous potential for clinical applications of tissue regeneration, including myocardial regeneration [1]. Several studies have shown that bone marrow-derived MSCs (BMSCs) are a promising therapeutic option for the treatment of heart disease [2,3]. In vitro induction of the differentiation of murine or porcine BMSCs into cardiomyocytes by 5-azacytidine (5-Aza) has been reported [4,5]. Our group reported the ability of adult human BMSCs to differentiate into cardiomyocytes, and to express cardiac-specific genes after treatment with 5-Aza in vitro [6]. To find a new and reliable source of MSCs, we successfully isolated and expanded MSCs from human umbilical cords and confirmed that human umbilical cord-derived MSCs (hucMSCs) possess characteristics similar to BMSCs [7]. Because they are easily isolated and expanded, have the potential for proliferation and multi-differentiation, and are widely available [7,8], hucMSCs have become a promising therapeutic candidate for treating injury to a number of tissues such as heart, liver [9], and kidney [10,11].
Although 5-Aza-induced differentiation of MSCs into cardiomyocytes has been widely studied, the details of the signaling mechanisms remain unclear. GSK-3α and GSK-3β play distinct roles in mediating cardiomyocyte differentiation in murine BMSCs. GSK-3β promotes cardiomyocyte differentiation of MSCs by downregulating β-catenin, whereas GSK-3α inhibits cardiomyocyte differentiation of MSCs through downregulation of c-Jun [12]. Mitogen-activated protein kinases (MAPKs) play important roles in the cellular response to growth factors, cytokines and chemicals, or environmental stress. There are at least 3 distinctly regulated families of MAPKs: extracellular signal related kinases (ERK), Jun amino-terminal kinas, and p38 MAPKs (p38). The activation of ERK is an essential signal in mesoderm differentiation during embryonic development [13]. The ERK pathway is reported to regulate the proliferation and osteogenic differentiation of MSCs [14]. In addition, activated ERK is involved in the differentiation of MSCs into mature adipocytes [14,15]. The ERK signaling pathway is also reported to promote the development of cardiomyocytes derived from embryonic stem cells [16]. Fukuda found that ERK phosphorylation increased in a cardiomyogenic cell line from murine BMSCs after 5-Aza exposure and stimulation with phenylephrine [17].
Studies have explored the ability of hucMSCs to differentiate into cardiomyocytes in vitro, and have evaluated their therapeutic effects on damaged cardiac tissues in vivo [18]. In this study, we investigated the cardiomyocyte differentiation of hucMSCs in response to 5-Aza treatment and the role of the ERK pathway in mediating this process.
Materials and Methods
HucMSC isolation and culture
Fresh umbilical cords were collected from informed, consenting mothers and processed within the optimal period of 6 h as described [7]. Umbilical cords were rinsed twice in phosphate-buffered saline (PBS) containing penicillin and streptomycin until the cord blood was cleared, and cord vessels were removed. Cords were cut into pieces of 1–3 mm3 and adhered to flasks for 30 min, then floated in low glucose–Dulbecco's modified Eagle's medium (LG-DMEM) containing 10% fetal bovine serum (FBS; Gibco), 1% penicillin and streptomycin. Cord pieces were subsequently incubated at 37°C in humid air with 5% CO2. The medium was changed every 3 days after initial plating. When well-developed colonies of fibroblast-like cells reached 80% confluency, cultures were trypsinized with 0.25% trypsin–ethylenediamine tetraacetic acid (Invitrogen) and passaged into new flasks for further expansion.
Myogenic differentiation of hucMSCs in vitro
Second-passage cells were seeded in 6-well plates at 10,000 cells per well. After 24 h, medium was changed to conditioned medium (LG-DMEM supplemented with 10% FBS and 10 μM 5-Aza). To prevent cell death from prolonged 5-Aza exposure, after induction for 24 h, cells were washed twice with PBS to remove 5-Aza and medium replaced with fresh LG-DMEM containing 10% FBS. Medium was changed every 3 days until the experiment was terminated after 2 weeks. As a negative control cell line, human diploid lung fibroblasts (HFL1s, Shanghai Institutes for Biological Science, CAS) were cultured and treated under the same conditions as hucMSCs, with MEM-α supplemented with 15% FBS. For analysis of the mechanisms involved in the differentiation, U0126 (Promega) was added to the medium until the cells were collected.
Osteogenic differentiation of hucMSCs
The hucMSCs of second passage were cultured in osteogenic medium (0.1 mM dexamethasone, 10 mM b-glycerophosphate, and 50 mM ascorbate-phosphate). All reagents were from Sigma-Aldrich. After 2 weeks, specific histochemical staining for neutrophil alkaline phosphatase (NAP) with NAP staining kit (Sun Bio) was examined in hucMSCs, hucMSCs treated with 5-Aza, and hucMSCs cultured in osteogenic medium.
Transmission electron microscopy
The cells cultured in the presence or absence of 5-Aza were washed twice with PBS, fixed with PBS containing 2.5% glutaraldehyde for 1 h, and embedded in epoxy resin. Ultra-thin sections were cut horizontally to the growing surface. Sections were double-stained in uranylacetate and lead citrate, and were viewed under a transmission electron microscope.
Total RNA isolation and reverse transcription–polymerase chain reaction
Total RNA was extracted with Trizol reagent (Invitrogen) from untreated, 5-Aza-treated, and 5-Aza plus U0126-treated hucMSCs as well as untreated and 5-Aza-treated HFL1s. The cDNAs were synthesized by a reverse transcription kit according to the manufacturer's instruction (Invitrogen). GAPDH served as an internal control. The thermal profile for polymerase chain reaction (PCR) was 94°C for 2 min, followed by 35 cycles of 30 s at 94°C, with 30 s annealing followed by 1 min extension at 72°C. Additional 10-min incubation at 72°C was included after the last cycle. PCR products were size-fractioned by electrophoresis on 1.5% agarose gels. The 8 specific primers were designed as shown in Table 1.
Table 1.
Primer Sequences of Target Genes
| Genes | Primer sequence (5′–3′) | Amplicon size (bp) | Annealing temp (°C) |
|---|---|---|---|
| desmin | For: CCAACAAGAACAACGACG Rev: TGGTATGGACCTCAGAACC |
408 | 60 |
| β-MHC | For: GATCACCAACAACCCCTACG Rev: ATGCAGAGCTGCTCAAAGC |
528 | 60 |
| cTnT | For: AGGCGCTGATTGAGGCTCAC Rev: ATAGATGCTCTGCCACAGC |
416 | 58 |
| Nkx2.5 | For: GGAGAAGACAGAGGCGGACA Rev: ACGCCGAAGTTCACGAAGTT |
525 | 61 |
| ANP | For: ACGCAGACCTGATGGATTT Rev: AGATGACACGAATGCAGCAG |
450 | 60 |
| α-SMA | For: CTGACTGAGCGTGGCTATTC Rev: CCACCGATCCAGACAGAGTA |
452 | 58 |
| FAP | For: ATAGCAGTGGCTCCAGTCTC Rev: GATAAGCCGTGGTTCTGGTC |
278 | 59 |
| GAPDH | For: CGGATTTGGTCGTATTGG Rev: TCAAAGGTGGAGGAGTGG |
445 | 60 |
Immunohistochemistry
Untreated, 5-Aza-treated, and 5-Aza plus U0126-treated hucMSCs were adhered to chamber slides and fixed with methanol for 10 min at −20°C. After washing 3 times with PBS, cells were incubated at 4°C. Diluted mouse polyclonal primary antibodies against desmin (1:100) and cardiac troponin T (cTnT) (1:100) (Boster Bioengineering Company Limited) were incubated with the cells for 1.5 h at 37°C, and incubated with a secondary antibody for 30 min. Slides were visualized using diaminobenzidine (Boster Bioengineering Company Limited) substrate and counterstaining with hematoxylin for microscopic examination.
Western blotting
Cells were harvested and lysed in RIPA buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.1% SDS, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 1 μg/mL aprotinin, and 1 μg/mL leupeptin). Protein concentration was determined using the BCA assay kit (Pierce). Equal amounts of cell lysates were loaded and separated on a 12% SDS-PAGE gel. Proteins were transferred to polyvinylidene fluoride membranes that were blocked with 5% milk for 1 h. After incubation with the primary antibodies overnight at 4°C, membranes were washed 3 times with tris-buffered saline with 0.05% Tween-20 and challenged with HRP-conjugated goat anti-rabbit, or goat anti-mouse antibodies (dilution 1:2,000), followed by detection with an enhanced chemiluminescent substrate (Millipore) and quantitated using a Molecular Dynamics Densitometer (Sage Creation Science Company Limited) with MD ImageQuant Software. Sources and dilution factors of primary antibodies were: mouse polyclonal anti-pERK (1:1,000; Kang Cheng), anti-cTnT (1:100; Boster Bioengineering Company Limited), rabbit polyclonal anti-tERK (1:1,000; Kang Cheng), anti-myocyte enhancer-binding factor (MEF)-2c (1:200; Sant Cruz), anti-protein kinase C (PKC) and anti-signal transducers and activators of transcription 3 (STAT3) (1:500, Bioworld), anti-myogenic differentiation antigen (MyoD), anti-Nanog and anti-Sox2 (1:500, SAB), and mouse monoclonal anti-GAPDH (1:1,000; Kang Cheng).
Statistical analysis
Data were expressed as means±standard deviation (SD). Statistical analysis was performed to analyze the density of western blotting bands by Student's t-test or analysis of variance using Prism software (Graph Pad). Analysis of variance was used to analyze variance among all groups, and Student's t-test was performed to compare experimental and relative control groups. Statistical P values <0.05 were considered significant.
Results
5-Aza induces morphological changes and primitive myofilaments formation in hucMSCs
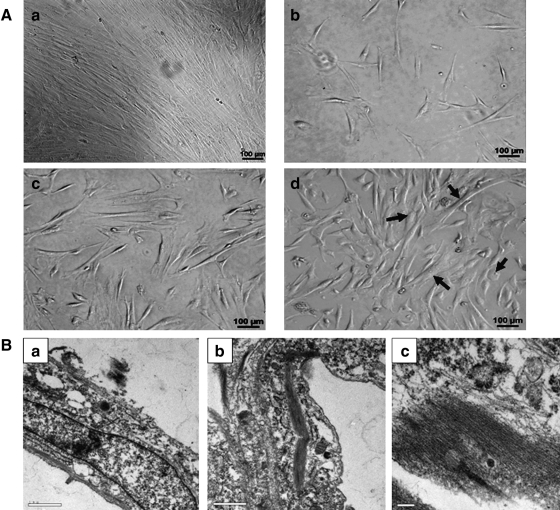
After 5–7 days in primary culture, hucMSCs adhered to a plastic surface, generating a small population of single cells that were spindle-shaped. At 10 to 15 days after initial plating, the cells resembled long, spindle-shaped fibroblasts and began to form colonies. After replating, cells were polygonal or spindly, with long processes. During exposure to 5-Aza, some adherent cells died, and the surviving cells began to proliferate and differentiate. The morphology of the hucMSCs changed, with the remaining adherent cells enlarging and forming a ball-like appearance, or lengthening in one direction and forming a stick-like morphology (Fig. 1A). Transmission electron microscopy photographs are shown in Fig. 1B. Two weeks after induction, numerous primitive myofilaments were evident in the cytoplasm of 5-Aza-treated cells.
FIG. 1.
Morphological changes in 5-Aza-treated hucMSCs. A: (a) Morphology of control hucMSCs. (b–d) Morphology of 5-Aza-treated hucMSCs. (b) 24 h; (c) 6 days; and (d) 2 weeks after induction. Arrow bars show ball-like or stick-like cells; scale bars: 100 μm. B: Transmission electron micrographs of (a) control hucMSCs (magnification:×18,500); (b, c) hucMSCs after 2 weeks of 5-Aza induction (magnification:×18,500,×46,000). 5-Aza, 5-azacytidine; hucMSC, human umbilical cord-derived mesenchymal stem cell.
5-Aza promotes cardiac-specific gene and protein expression and inhibits stem cell-associated protein expression
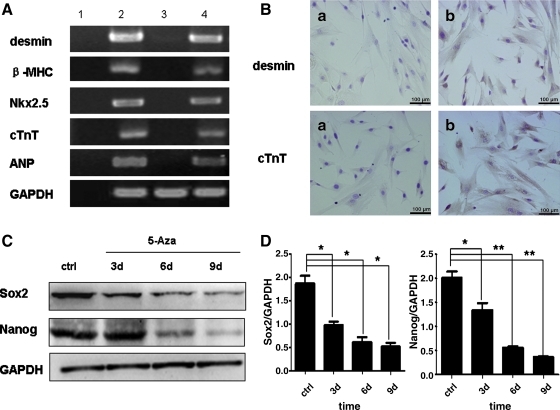
Total RNA from induced and noninduced cells was isolated and analyzed by RT-PCR. No expression of cardiac-specific genes was detected in untreated hucMSCs, but 5-Aza treatment strongly induced expression of Nkx2.5, one of the earliest cardiac markers, and β-myosin heavy chain (β-MHC), a contractile protein, as well as the cardiomyocyte-related genes cTnT and A-type natriuretic peptide (ANP). Desmin, an early marker of myogenic differentiation, was barely detectable in the control group but highly expressed in induced hucMSCs (Fig. 2A). We confirmed the expression of cardiomyocyte-related proteins by immunohistochemical staining. As shown in Fig. 2B, hucMSCs consistently stained positively for desmin and cTnT after 2 weeks of 5-Aza induction. We also found that the expression of stem cell-associated proteins Sox2 and Nanog time-dependently decreased with 5-Aza induction (Fig. 2C, D). Collectively, these results indicated that hucMSCs underwent cardiomyocyte differentiation in response to 5-Aza treatment.
FIG. 2.
5-Aza-induced cardiac-specific genes and proteins in hucMSCs. A: Expression of desmin, β-MHC, Nkx2.5, cTnT, and ANP genes were detected by RT-PCR in control and induced hucMSCs. (Lane 1, blank; Lane 2, human cardiomyocytes; Lane 3, control hucMSCs; Lane 4, hucMSCs treated with 5-Aza for 24 h, then cultured for 2 weeks) B: Immunohistochemical staining for desmin and cTnT. (a) Control hucMSCs; (b) hucMSCs treated with 5-Aza for 24 h and cultured for 2 weeks after removal of 5-Aza; scale bars: 100 μm. C: Expression of Sox2 and Nanog proteins in control versus induced hucMSCs by western blotting. D: Density analysis of Western blotting bands. *P<0.05 and **P<0.01, compared to the relative control group (n=3). β-MHC, β-myosin heavy chain; ANP, A-type natriuretic peptide; PCR, polymerase chain reaction.
Analysis of 5-Aza specificity
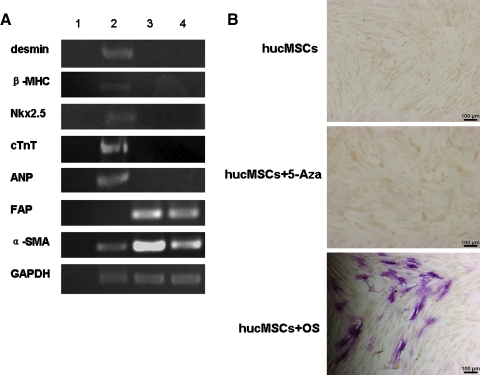
HFL1s were treated with 5-Aza under the same conditions as above and the expression of mRNA was analyzed by RT-PCR. Both untreated and treated HFL1s expressed fibroblast markers such as α-smooth muscle actin and fibroblast activation protein (FAP), but negative for desmin, β-MHC, cTnT, ANP, and Nkx2.5 (Fig. 3A). HucMSCs in osteogenic inductive medium became NAP-positive, with the appearance of red deposits in the cytoplasm after 2 weeks. In contrast, hucMSCs and hucMSCs treated with 5-Aza did not form osteoblasts (Fig. 3B).
FIG. 3.
Analysis of 5-Aza specificity. A: Expression of desmin, β-MHC, Nkx2.5, cTnT, ANP, α-SMA, and FAP genes by RT-PCR in control versus induced HFL1s (Lane 1, blank; Lane 2, human cardiomyocytes; Lane 3, control HFL1s; Lane 4, HFL1s treated with 5-Aza). B: neutrophil alkaline phosphatase expression at 2 weeks in untreated hucMSCs, hucMSCs treated with 5-Aza, and hucMSCs cultured in osteogenic medium; scale bars: 100 μm. HFL1s, human diploid lung fibroblasts; cTnT, cardiac troponin T; α-SMA, α-smooth muscle actin.
5-Aza activates ERK phosphorylation in hucMSCs
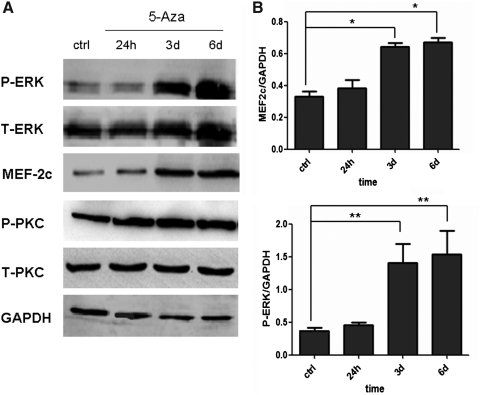
To identify the signaling pathways involved in 5-Aza-induced myogenesis in hucMSCs, we examined the status of phosphorylated ERK and PKC after 5-Aza treatment. Western blotting revealed that 5-Aza induced time-dependent ERK phosphorylation in hucMSCs (Fig. 4A). At 6 days after induction, the level of phosphorylated ERK in 5-Aza-treated hucMSCs was about 4-fold higher than in the control group (Fig. 4B). Further, at 6 days after induction, the level of MEF-2c protein in induced hucMSCs also increased, and was 2-fold higher than control hucMSCs. However, the level of phosphorylated PKC showed no obvious change after 5-Aza treatment.
FIG. 4.
5-Aza induced prolonged ERK phosphorylation in hucMSCs. A: HucMSCs were treated with 5-Aza for 24 h and cultured for indicated times (24 h, 3 days, and 6 days) and analyzed by Western blotting for T-ERK, P-ERK, MEF-2c, T-PKC, and P-PKC. B: Density analysis of Western blotting bands. *P<0.05 and **P<0.01, compared to the relative control group (n=3). PKC, protein kinase C; ERK, extracellular signal related kinases; MEF, myocyte enhancer-binding factor.
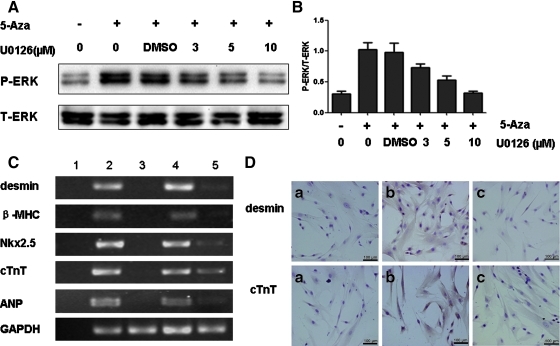
ERK inhibitor blocks 5-Aza-induced myogenic differentiation of hucMSCs
To further investigate the impact of ERK activation on 5-Aza-induced cardiomyocyte differentiation of hucMSCs, we blocked 5-Aza-induced phosphorylation of ERK in hucMSCs with U0126, a specific ERK inhibitor. We found that the 5-Aza-induced increase in ERK phosphorylation was reduced in a dose-dependent manner when cells were treated with U0126 (Fig. 5A, B). Treatment with U0126 (10 μM) strongly reversed the 5-Aza-induced expression of myogenic and cardiac-specific genes including desmin, β-MHC, Nkx2.5, cTnT, and ANP (Fig. 5C). Immunohistochemical analysis further revealed that the positivity of desmin and cTnT was substantially reduced after treatment with U0126 for 2 weeks (Fig. 5D).
FIG. 5.
Blockade of ERK activation led to inhibition of 5-Aza-induced hucMSCs myogenesis. A: HucMSCs were treated with 5-Aza in the presence or absence of indicated U0126 concentrations for 6 days. ERK phosphorylation was examined by Western blotting. B: Density analysis of Western blotting bands (n=2). C: HucMSCs were treated with U0126 (10 μM) for 2 weeks. The mRNA levels of desmin, β-MHC, ANP, Nkx2.5, and cTnT were determined by RT-PCR (Lane 1, blank; Lane 2, human cardiomyocytes; Lane 3, control hucMSCs; Lane 4, hucMSCs treated with 5-Aza; Lane 5, hucMSCs treated with 5-Aza and U0126). D: Immunohistochemical staining for desmin and cTnT. (a) Control hucMSCs; (b) hucMSCs treated with 5-Aza; (c) hucMSCs treated with 5-Aza and U0126; scale bars: 100 μm.
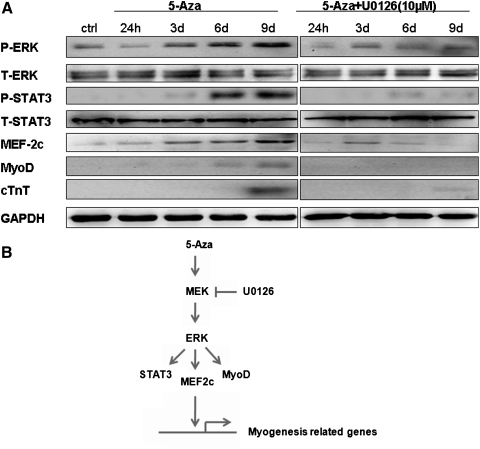
U0126 inhibits 5-Aza-induced STAT3 phosphorylation, MEF-2c, and MyoD up-regulation
A time-course analysis was performed to determine ERK activation and the expression of other myogenesis-related proteins during U0126 treatment. The increased expression of phosphorylated ERK and MEF-2c following 5-Aza treatment was inhibited by U0126. Western blotting showed that the level of phosphorylated STAT3 greatly increased at 6 days after 5-Aza treatment, and this increase was almost completely abrogated by U0126 treatment. Similarly, the up-regulation of MyoD and cTnT following 5-Aza induction were reversed by U0126 (Fig. 6A).
FIG. 6.
U0126 treatment inhibits STAT3 phosphorylation, MEF-2c, and MyoD up-regulation in response to 5-Aza. (A) 5-Aza induced up-regulation of phosphorylated STAT3, MEF-2c, and MyoD that was blocked by U0126 (10 μM). (B) Schematic model of 5-Aza-induced cardiac differentiation of hucMSCs through the ERK pathway. MyoD, myogenic differentiation antigen; STAT3, signal transducers and activators of transcription 3.
Discussion
MSCs are considered a promising therapeutic tool for diseases that include heart failure, kidney injury, and myasthenia gravis. In recent years, human umbilical cord has become an attractive source of MSCs for tissue regeneration because they do not have ethical considerations [19,20]. Panepucci et al. compared the gene expression patterns of hucMSCs and BMSCs, and found that a group of genes associated with osteogenesis was more expressed in BMSCs, while hucMSCs had a higher expression of genes related to matrix remodeling by promoting angiogenesis and metalloproteinases [21]. Work by Kadivar and colleagues suggests that hucMSCs may be a better source of seed cells for cardiomyocytes and more applicable in cellular cardiomyoplasty [22]. As a result, MSCs from different sources may tend to differentiate into particular types of tissues that are beneficial for specifically targeted therapies.
Reports on the effect of 5-Aza on stem cells have been contradictory. First, Makino et al. reported that MSCs could differentiate into cardiomyocytes [4]. Following this report, several studies showed that after exposure to 5-Aza, BMSCs could differentiate into cardiomyocytes [6,17]. However, 5-Aza treatment also failed to induce a cardiomyogenic phenotype in MSCs in vitro [23]. In this study, we confirmed that hucMSCs differentiate into cardiomyocytes in vitro in response to 5-Aza treatment. Morphological results showed spindle-shaped cells that gradually increased in size during 5-Aza induction. After 2 weeks in culture, primitive myofilaments were detected in cytoplasm under a transmission electron microscope. The changes in morphology may be associated with expression of cytoskeleton-maintaining proteins [4]. The myogenic cells that differentiated from hucMSCs in our study had some morphological and ultrastructural similarities with cardiomyocytes. Notably, the 5-Aza-induced cells expressed myogenic and cardiac-specific genes including desmin, β-MHC, Nkx2.5, cTnT, and ANP. Expression of desmin and cTnT proteins was also seen in the cytoplasm of induced hucMSCs by immunohistochemical analysis. The expression of stem cell-associated proteins Sox2 and Nanog decreased in 5-Aza-induced cells. Previously, 5-Aza was reported to cause phenotype changes in some cell types by activating muscle gene expression [24]. Treatment with 5-Aza also caused fibroblasts to convert phenotypically to adipocytes and muscle cells [25]. Our results showed that HFL1s that were treated with 5-Aza did not express myogenic or cardiac-specific genes, but assays for markers of fibroblasts, such as α-smooth muscle actin and FAP, were positive. Osteogenic differentiation was not induced in hucMSCs by 5-Aza. These differences in results might be because of the cell types, induction time, drug doses, or means of administration.
Although the activation of the PKC signaling pathway is reported to be involved in myogenesis [26], we found no significant change in total PKC or phosphorylated PKC in 5-Aza-treated hucMSCs. This indicated that PKC activation was not the major mechanism responsible for 5-Aza-induced hucMSC differentiation into cardiomyocytes. Several previous studies found that ERK frequently showed a transient increase or decrease, or in some cases, a sustained effect that could last several days. The results depend on the stimuli, its concentration, and cell type [27]. In our study, ERK phosphorylation was unchanged at 24 h after 5-Aza induction, but the level of ERK increased remarkably at 3 days after induction and was sustained at a high level even 9 days after induction. These results indicate that ERK phosphorylation after 5-Aza treatment was not transient and could be involved in the differentiation of hucMSCs to cardiomyocytes in vitro.
The function of the ERK signaling pathway in myogenesis is still controversial. Tortorella et al. reported that ERK activation repressed transcription and blocked the activity of muscle-specific genes, leading to enhanced myotube formation in C2C12 myoblast cells [28]. However, Kim et al. demonstrated that HRG-b1 promoted the development of cardiomyocytes by activation of ERK [16]. These differences may result from the cell types and treatments used. Here, we used U0126 to determine the specificity of the ERK signaling pathway in mediating 5-Aza-induced myogenesis of hucMSCs. We found that U0126 significantly inhibited ERK phosphorylation and expression of cardiac-specific genes and proteins induced by 5-Aza. This demonstrated that the ERK pathway at least partially participates in 5-Aza-induced myogenesis of hucMSCs. As a transcriptional factor, STAT3 plays a central role in the regulation of growth, differentiation, and survival in many types of cells [29]. STAT3, which is also regulated by ERK signaling pathway [30,31], was chosen as another target to examine the effect of the ERK pathway in 5-Aza-induced myogenesis. Recently, STAT3 was found to directly interact with MyoD to induce myogenic differentiation [32]. MyoD is known to regulate myogenic differentiation and MEF-2c, a member of the MEF2 family, is crucial for the activation of cardiac-specific embryonic genes [33]. MEF2 directly interacts with members of the MRFs (myogenic regulatory factor) (i.e., MyoD, Myf5, and MRF4) to synergistically activate many muscle-specific genes [34,35]. In our study, we found an increase in STAT3 phosphorylation, as well as up-regulation of MEF-2c and MyoD induced by 5-Aza treatment, which was notably inhibited by U0126. Further studies are needed to understand how 5-Aza activates ERK and the detailed interactions between its downstream targets (Fig. 5B).
In conclusion, our findings suggest that 5-Aza can induce hucMSCs to differentiate into cardiomyocytes in vitro, and that sustained ERK phosphorylation at least partially contributes to this process. Our work provides a new source of MSCs for cardiac tissue engineering and a potential mechanism mediated by 5-Aza that induces cardiac differentiation of MSCs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 30840053 and 81000181), Jiangsu Province's Outstanding Medical Academic Leader Program (Grant no. LJ200614), the Natural Science Foundation of Jiangsu Province (Grant no. BE2010703, BK2007092, and BK2008232), the Sci-tech InnovationTeam and Talents of Jiangsu University (Grant no.2008-018-02), and Foundation of the Jiangsu Province for transfer of scientific and technological achievements (Grant no. BA2009124). We thank International Science Editing Compuscript Ltd for revision of the manuscript.
Author Disclosure Statement
No competing financial interest exists.
References
- 1.Hare JM. Chaparro SV. Cardiac regeneration and stem cell therapy. Curr Opin Organ Transplant. 2008;13:536–542. doi: 10.1097/MOT.0b013e32830fdfc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyahara Y. Nagaya N. Kataoka M. Yanagawa B. Tanaka K. Hao H. Ishino K. Ishida H. Shimizu T. Kangawa K. Sano S. Okano T. Kitamura S. Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 3.Obradović S. Rusović S. Balint B. Ristić-Andelkov A. Romanović R. Baskot B. Vojvodić D. Gligić B. Autologous bone marrow-derived progenitor cell transplantation for myocardial regeneration after acute infarction. Vojnosanit Pregl. 2004;61:519–529. doi: 10.2298/vsp0405519o. [DOI] [PubMed] [Google Scholar]
- 4.Makino S. Fukuda K. Miyoshi S. Konishi F. Kodama H. Pan J. Sano M. Takahashi T. Hori S. Abe H. Hata J. Umezawa A. Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscoso I. Centeno A. López E. Rodriguez-Barbosa JI. Santamarina I. Filgueira P. Sánchez MJ. Domínguez-Perles R. Peñuelas-Rivas G. Domenech N. Differentiation “in vitro” of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transplant Proc. 2005;37:481–482. doi: 10.1016/j.transproceed.2004.12.247. [DOI] [PubMed] [Google Scholar]
- 6.Xu W. Zhang X. Qian H. Zhu W. Sun X. Hu J. Zhou H. Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 7.Qiao C. Xu W. Zhu W. Hu J. Qian H. Yin Q. Jiang R. Yan Y. Mao F. Yang H. Wang X. Chen Y. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kestendjieva S. Kyurkchiev D. Tsvetkova G. Mehandjiev T. Dimitrov A. Nikolov A. Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. 2008;32:724–732. doi: 10.1016/j.cellbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Yan Y. Xu W. Qian H. Si Y. Zhu W. Cao H. Zhou H. Mao F. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356–365. doi: 10.1111/j.1478-3231.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y. Qian H. Zhu W. Zhang X. Yan Y. Ye S. Peng X. Li W. Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2010;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 11.Cao H. Qian H. Xu W. Zhu W. Zhang X. Chen Y. Wang M. Yan Y. Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–732. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 12.Cho J. Rameshwar P. Sadoshima J. Distinct roles of glycogen synthase kinase (GSK)-3alpha and GSK-3β in mediating cardiomyocyte differentiation in murine bone marrow-derived mesenchymal stem cells. J Biol Chem. 2009;284:36647–36658. doi: 10.1074/jbc.M109.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y. Li W. Wu J. Germann UA. Su MS. Kuida K. Boucher DM. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal RK. Jaiswal N. Bruder SP. Mbalaviele G. Marshak DR. Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 15.Sale EM. Atkinson PG. Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HS. Cho JW. Hidaka K. Morisaki T. Activation of MEK–ERK by heregulin-b1 promotes the development of cardiomyocytes derived from ES cells. Biochem Biophys Res Commun. 2007;361:732–738. doi: 10.1016/j.bbrc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K. Regeneration of cardiomyocytes from bone marrow: use of mesenchymal stem cell for cardiovascular tissue engineering. Cytotechnology. 2003;41:165–175. doi: 10.1023/A:1024882908173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu KH. Mo XM. Zhou B. Lu SH. Yang SG. Liu YL. Han ZC. Cardiac potential of stem cells from whole human umbilical cord tissue. J Cell Biochem. 2009;107:926–932. doi: 10.1002/jcb.22193. [DOI] [PubMed] [Google Scholar]
- 19.Wang HS. Hung SC. Peng ST. Huang CC. Wei HM. Guo YJ. Fu YS. Lai MC. Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 20.Weiss ML. Medicetty S. Bledsoe AR. Rachakatla RS. Choi M. Merchav S. Luo Y. Rao MS. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 21.Panepucci RA. Siufi JL. Silva WA., Jr Proto-Siquiera R. Neder L. Orellana M. Rocha V. Covas DT. Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 22.Kadivar M. Khatami S. Mortazavi Y. Shokrgozar MA. Taghikhani M. Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y. Song J. Liu W. Wan Y. Chen X. Hu C. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res. 2003;58:460–468. doi: 10.1016/s0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CP. Blau HM. 5-Azacytidine permits gene activation in a previously noninducible cell type. Cell. 1985;40:417–424. doi: 10.1016/0092-8674(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SM. Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- 26.Capiati DA. Limbozzi F. Téllez-Iñón MT. Boland RL. Evidence on the participation of protein kinase C alpha in the proliferation of cultured myoblasts. J Cell Biochem. 1999;74:292–300. [PubMed] [Google Scholar]
- 27.Shaul YD. Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Bio phys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Tortorella LL. Milasincic DJ. Pilch PF. Critical proliferation independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- 29.Hirano T. Ishihara K. Hibi M. Roles of STAT3 in mediating the cell growth,differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 30.Chung J. Uchida E. Grammer TC. Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haq R. Halupa A. Beattie BK. Mason JM. Zanke BW. Barber DL. Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J Biol Chem. 2002;277:17359–17366. doi: 10.1074/jbc.M201842200. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y. Xu Y. Li W. Wang G. Song Y. Yang G. Han X. Du Z. Sun L. Ma K. STAT3 induces muscle stem cell differentiation by interaction with myoD. Cytokine. 2009;46:137–141. doi: 10.1016/j.cyto.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Harvey RP. Seeking a regulatory roadmap for heart morphogenesis. Semin Cell Dev Biol. 1999;10:99–107. doi: 10.1006/scdb.1998.0277. [DOI] [PubMed] [Google Scholar]
- 34.Molkentin JD. Black BL. Martin JF. Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 35.Black BL. Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]