Abstract
Injection of the GABAA receptor agonist muscimol into the nucleus accumbens shell (AcbSh) elicits robust feeding in satiated rats, but has no effect on water intake. The current study was designed to examine whether intra-AcbSh muscimol injections influence the intake of ethanol solutions in rats trained to drink using a limited access paradigm. We confirmed that bilateral injections of muscimol (100 ng) into the AcbSh produce large increases in the intake of sucrose solutions and of the chow maintenance diet but found in two independent experiments that these injections potently reduce the intake of a 10% ethanol solution. Furthermore, intra-AcbSh muscimol significantly increased intake of an ethanol-sucrose mixture. These results demonstrate that activating GABAA receptors in the vicinity of the AcbSh can have opposite effects on the intake of different caloric substances and are consistent with the possibility that GABAergic circuits in the AcbSh may play a role in mediating voluntary ethanol intake.
Keywords: Alcohol, GABA, Muscimol, Food Intake, Taste, Calories, Reward
The nucleus accumbens shell (AcbSh) has been implicated as a key component of a neural system involved in the mediation of feeding behavior (Stratford, 2007). Inhibition of neurons in the AcbSh with GABA agonists or glutamate antagonists elicits intense feeding in satiated rats, with short-term intakes that are among the highest reported in the literature (Maldonado-Irizarry et al., 1995; Stratford and Kelley, 1997, 1999). Importantly, inhibition of the AcbSh appears to affect feeding behavior specifically, as such treatments do not increase water intake, non-ingestive gnawing, or locomotor activity when food is present (Stratford et al., 1998; Ward et al., 2000). Furthermore, although inhibiting neurons in the AcbSh does not alter water intake even in water-deprived rats that are primed to drink (Stratford and Kelley, 1997; Stratford et al., 1998), the treatment does increase intake of certain concentrations of sucrose solutions (Basso and Kelley, 1999; Stratford et al., 1998), demonstrating that AcbSh-mediated hyperphagia is not limited to ingestion of solid food or maintenance diets. The AcbSh is believed to play an important role in reward or reinforcement mechanisms (Koob and Bloom, 1988; Robbins and Everitt, 1996), however, the behavioral specificity of the feeding response suggests that a general alteration in reward processing does not underlie the effect. Rather, amino acid-coded circuits in the AcbSh appear to be preferentially involved in the control of food intake.
Several lines of evidence suggest that the AcbSh may also be involved in the control of ethanol intake. For instance, intracerebroventricular or intraperitoneal administration of ethanol increases Fos expression in the AcbSh (Crankshaw et al., 2003; Herring et al., 2004; Ryabinin et al., 1997), and voluntary ingestion of ethanol elicits a number of physiological changes in the AcbSh, including increases in dopamine (Doyon et al., 2003) and opioid levels (Marinelli et al., 2005; Marinelli et al., 2006; Marinelli et al., 2003), expression of CaM IV kinase and CREB phosphorylation (Misra et al., 2001), and changes in neuronal firing rates (Janak et al., 1999) and glucose utilization (Porrino et al., 1998). Additionally, alcohol-preferring rats have higher densities of GABA terminals in the Acb than rats bred for low alcohol consumption (Hwang et al., 1990; McBride et al., 1990).
In the present study, we investigated whether local injections of the GABAA receptor agonist muscimol into the AcbSh, a treatment that potently stimulates food intake, would alter intake of a 10% ethanol solution or a 10% ethanol solution sweetened with 10% sucrose. The effects of the treatment on the intake of lab chow and a 10% sucrose solution were also examined in the same rats as positive controls.
METHODS
Subjects
Nineteen male Sprague-Dawley rats (Harlan, Madison, WI) weighing between 305 and 385 g at the time of surgery served as subjects. The rats were housed individually in wire-mesh cages on a 12 h light:12 h dark cycle at a constant room temperature (~ 21° C) with food (Harlan Teklad) and tap water available ad libitum, except as noted below. All experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Test solutions and acquisition of ethanol drinking
The ethanol solution was prepared by diluting 95% ethanol with tap water to yield a 10% ethanol (w/v) solution, the sucrose solution by dissolving sucrose to a concentration of 10% (w/v) in tap water, and the sweetened ethanol solution by dissolving sucrose to a concentration of 10% (w/v) in the 10% ethanol solution. Rats were trained to drink ethanol over a four-week period using a limited-access protocol. During the ethanol drinking acquisition phase, rats were placed in test cages for one hour each day, beginning four hours after the start of the light period, where they were given access to an ethanol solution. The rats were water-deprived for 20 h before the first exposure to encourage sampling of the ethanol solution and neither food nor water was available during the ethanol presentation. The concentration of the ethanol solution was increased from 3% during the first week of acquisition to 6% during the second week and to 10% during the third and fourth weeks. At the end of four weeks, baseline intakes of 10% ethanol had stabilized. The ten rats in Experiment 1 received several exposures to the sucrose solution or lab chow in the test cages interspersed with the ethanol trials, while the nine rats in Experiment 2 received several interspersed exposures to the sucrose or the sweetened ethanol solution. Two rats consistently failed to drink any unsweetened ethanol during the presentations and were excluded from the remainder of the studies.
Surgery
At the end of the four-week ethanol acquisition phase, the rats were anesthetized with sodium pentobarbital (60 mg/kg) and bilateral 26-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted using standard, flat-skull stereotaxic techniques. The guide cannulae were aimed so as to terminate 2.0 mm dorsal to the AcbSh using the following coordinates: anteroposterior: 1.5, mediolateral: ±0.8, and dorsoventral: −6.1 (mm from bregma). The guide cannulae were held in place using stainless steel screws and denture lining material and a stainless steel obturator was inserted into the lumen of each cannula to help maintain patency. Each rat was allowed to recover for at least seven days during which time the daily presentations of ethanol continued.
Intracerebral Injections
In order to acclimate the rats to the test procedure, they were restrained gently, the obturators removed, and a 32-gauge injection cannula, extending 2.0 mm beyond the ventral tip of the guide, was inserted into each guide cannula on three consecutive days. On each day, the obturators were replaced and the rats were placed in the test cages for 60 min with ethanol present. On the final acclimation day, each rat received bilateral 0.25 μl intracerebral injections of sterile 0.15 M saline. On test days, each rat received simultaneous bilateral 0.25 μl injections of 100 ng muscimol or the sterile saline vehicle into the ventromedial AcbSh at a rate of 0.25 μl/min (the dose of muscimol we have found to elicit maximal food intake when administered into the AcbSh (Stratford and Kelley, 1997) and the use of which allows us to maximize comparability with our previous results). After the infusion, the injection cannulae were left in place for an additional 60 seconds in order to minimize leakage up the cannula track. In Experiment 1, the rats then were placed in test cages for one hour with either 10% sucrose, 10% ethanol, or chow available. In Experiment 2, the rats were placed in test cages with either 10% sucrose, 10% ethanol, or a sweetened 10% ethanol solution containing 10% sucrose. In both experiments, the ethanol and sucrose solutions were presented in a counterbalanced order in burettes calibrated to 0.1 ml. Intake of chow was evaluated in Experiment 1 and intake of the sweetened ethanol solution in Experiment 2 after the other tests had been completed. At least 72 hours were allowed between injections.
Histology
After the completion of behavioral testing, each of the rats was deeply anesthetized using sodium pentobarbital and perfused transcardially with 50 ml of a 0.15 M saline solution followed immediately by 500 ml of a 10% buffered formalin solution. The brains were removed and stored in fixative for at least one week, after which they were frozen and 60 μm-thick coronal sections were taken throughout the extent of the AcbSh. The sections were stained with cresyl-violet and the injection sites were examined for placement accuracy and evidence of excessive damage.
RESULTS
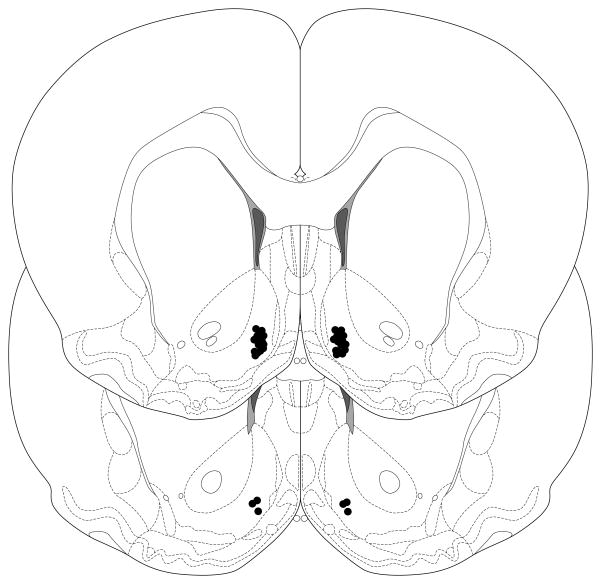
Histological examination showed that all microinjector cannulae terminated in the medial AcbSh at placements similar to those used in our previous studies (e.g., (Stratford, 2005; Stratford and Kelley, 1997; Stratford and Wirtshafter, 2004)). Figure 1 is a schematic illustration of the 17 bilateral AcbSh injection sites.
Fig. 1.
Schematic representation of a typical bilateral AcbSh injection site. (AcbC: nucleus accumbens core; CPu: caudate-putamen)
Mean intakes in Experiment 1 can be seen in Fig. 2 which shows that injections of muscimol into the AcbSh produced a significant decrease in ethanol intake as compared to saline injections (F(1,7) = 45.6; p < .001). In contrast, muscimol injections in these same animals produced significant increases, relative to saline injections, in the intakes of both a 10% sucrose solution (F(1,7) = 35.8; p < .001) and of their regular chow maintenance diet (F(1,7) = 41.9; p < .001)
Fig. 2.
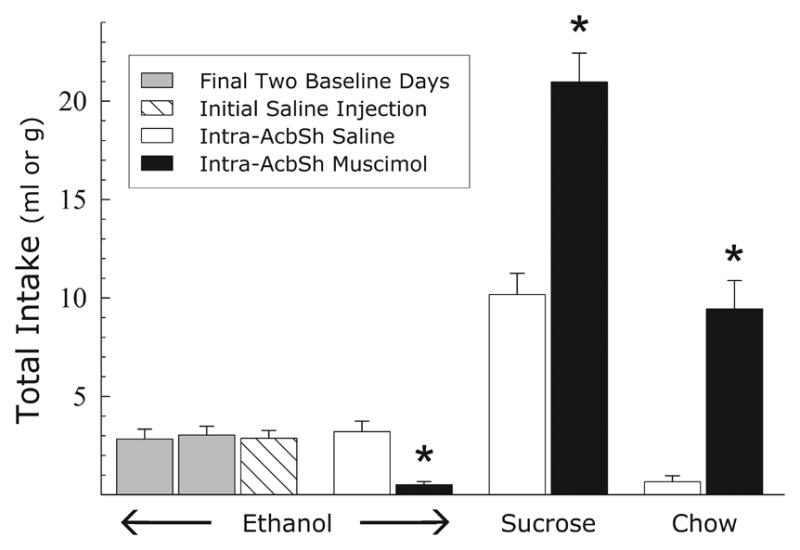
Experiment 1: Effects of intra-AcbSh injections of saline or muscimol (100 ng/side) on the mean 60 min intakes of a 10% ethanol solution, a 10% sucrose solution, or lab chow. * = p < .001 vs. saline injections
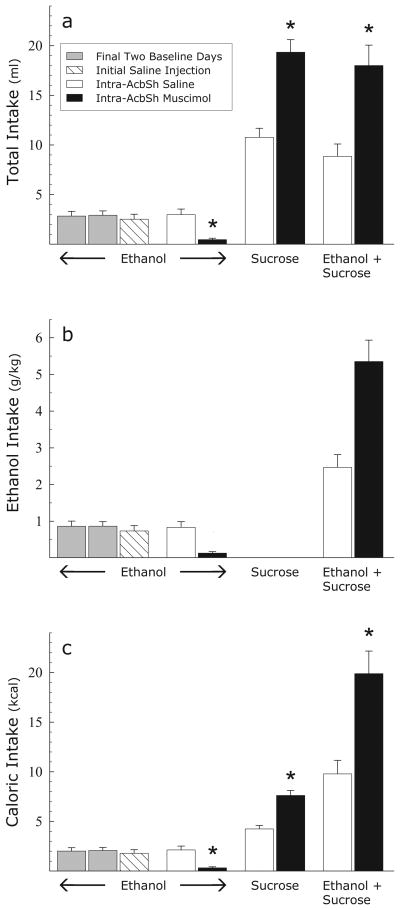
Mean total intakes in Experiment 2 are shown in Fig. 3a (and, for convenience, are expressed as dose of ethanol received in Fig. 3b). Again, intra-AcbSh muscimol resulted in a marked suppression of ethanol intake (F(1,8) = 24.6; p < .001), while producing increases of similar magnitude in the intakes of both the sucrose solution and the sucrose + ethanol mixture. The data for the two sucrose containing solutions were analyzed by means of a 2 × 2 (ethanol × muscimol) repeated measures ANOVA which indicated a significant effect of muscimol (F(1,8) = 26.1, p < .001). The effect of ethanol was also significant (F(1,8) = 7.4; p < .03), reflecting the fact that intakes of the mixture were slightly lower than intakes of the sucrose solution following injections of either saline or muscimol. The ethanol × muscimol interaction (F < 1) was not significant, however, indicating that muscimol produced statistically indistinguishable increases in the intakes of the two solutions. This result is striking because the sweetened ethanol solution was much more calorically dense than the sucrose solution alone. Mean calories consumed are shown in Fig. 3c, where it can be seen that muscimol actually tended to produce a larger increase in caloric intake when animals consumed the sucrose + ethanol mixture than when they consumed the sucrose solution. Caloric intake under the sucrose and sucrose + ethanol conditions was analyzed by means of a 2 × 2 (ethanol × muscimol) repeated measures ANOVA. This analysis demonstrated a significant effect of ethanol (F(1,8) = 110.6; p < .0001) indicating that animals took in significantly more calories while consuming the sucrose + ethanol mixture than the sucrose solution. The effect of muscimol was significant (F(1,8) = 17.0; p < .003) and the effect of the ethanol × muscimol interaction bordered on significance (F(1,8) = 4.9; p < .06), indicating that muscimol tended to produce a larger increase in caloric intake when animals were consuming the mixture than the sucrose solution.
Fig. 3.
Experiment 2: Effects of intra-AcbSh injections of saline or muscimol (100 ng/side) on the 60 min consumption of a 10% ethanol solution, a 10% sucrose solution, or a solution containing 10% ethanol and 10% sucrose expressed as a) mean total volume ingested, b) mean dose of ethanol ingested, and c) mean total caloric intake. * = p < .001 vs. saline injections.
DISCUSSION
The results of these two independent experiments demonstrate clearly that injecting muscimol into the medial AcbSh significantly reduces intake of a 10% ethanol solution by rats in a limited-access paradigm, suggesting that GABAergic circuits in this region play a role in voluntary ethanol intake under some circumstances. In striking contrast, intra-AcbSh muscimol greatly increases intake of chow, a 10% sucrose solution, and even a 10% ethanol solution when it is sweetened with sucrose. The ability of AcbSh muscimol to increase intake of chow, sucrose, or a sucrose/ethanol mixture demonstrates that rats receiving AcbSh muscimol injections are fully capable of intense, focused feeding behavior and that the suppression of ethanol intake by intra-AcbSh muscimol cannot be attributed to treatment-induced malaise, interference with fine motor movements, or elicitation of competing behaviors that are incompatible with ingestion. It is possible that muscimol injections in the AcbSh affect two different neural mechanisms, one of which acts to increase intakes of sucrose solutions and chow, and another to suppress intake of ethanol. Alternatively, muscimol may act on a single mechanism that differentially affects the intakes of various substances. It should be noted that the AcbSh region has been carefully mapped for the effects of muscimol on feeding behavior (Basso and Kelley, 1999; Reynolds and Berridge, 2001; Stratford and Kelley, 1997) and that in the current study, muscimol was infused into the region of the AcbSh shown to be the most sensitive to the hyperphagic effects of this drug; further studies will be needed to determine whether the suppressive effects on ethanol intake are similarly located, or whether the accumbens core might also play a role, as is suggested by some observations (Hodge et al., 1995; Hyytia and Koob, 1995). Whichever explanation is correct, the current observations substantially extend our knowledge of the syndrome produced by muscimol injections into the AcbSh.
The relation between effects on the intake of ethanol and of other foods is rather complex and a variety of patterns have been observed after manipulations of sites outside of the accumbens. Some treatments, such as chemical inhibition of the median raphe nucleus (Tomkins et al., 1994; Wirtshafter, 2001), electrical stimulation of the lateral hypothalamus (Atrens et al., 1983; Wayner et al., 1971) or intra-ventricular injections of melanin-concentrating hormone (Duncan et al., 2005) have been reported to increase intakes of both sucrose and ethanol solutions. Although comparison of these results with those of the current study must be approached with caution, these differing patterns of effects suggest that intra-AcbSh muscimol may be affecting ingestive behavior in a different manner than do these other treatments. Other manipulations, however, have been shown to exert effects that are similar to those observed here. For example, intraventricular injections of neuropeptide Y (NPY) increase food intake but, under some conditions, actually decrease intake of ethanol (Gilpin et al., 2008; Thorsell, 2008). NPY has been shown to play a role in mediating the effects of intra-AcbSh muscimol on food intake (Stratford and Wirtshafter, 2004) and it is possible that this peptide may also be involved in the influence of the AcbSh on ethanol ingestion.
The finding that muscimol injections into the AcbSh produce opposite effects on ethanol and sucrose intake provides evidence against any model that suggests that inhibition of this region produces its actions through a “nonspecific” mechanism not related to the specific properties of the ingestate. For example, it has been suggested that inhibition of the AcbSh simply disinhibits “feeding specific motor pattern controllers” (Kelley et al., 2005), but it is difficult to see how such a mechanism could account for the opposite effects of this treatment on the licking of ethanol and sucrose. Similarly, evidence linking the accumbens to “reward” mechanisms immediately suggests the possibility that AcbSh-mediated hyperphagia might result from an increase in the “reward value” of ingestants. However, it is again difficult to see how such an action could produce opposite effects on the intakes of sucrose and ethanol solutions, given that both solutions are presumably “rewarding”, as indexed by voluntary ingestion. Indeed, the fact that inhibiting AcbSh neurons increases intake of food, but not of water, even when rats are water deprived (Stratford, 2007; Stratford et al., 1998), strongly suggests that muscimol-induced hyperphagia cannot simply be the result of alterations in the general processing of “reward value”.
The present data present an interesting paradox in that muscimol injections into the AcbSh elicit opposite effects on the ingestion of two solutions that rats consume voluntarily. Sucrose and ethanol solutions differ in a number of properties with the most salient relating to the unique pharmacological properties of ethanol and to the differing tastes of the solutions. Therefore, it is reasonable to expect that the differential effects on intake may be related to involvement of the AcbSh in the response to one, or both, of these factors.
The mean baseline intake of 10% ethanol in the current study was 0.84 g/kg (Fig. 3b), a magnitude shown to result in blood alcohol levels sufficient to alter brain glucose utilization (Porrino et al., 1998) and motor activity (Gill et al., 1986; Linseman, 1987), indicating that even the relatively small amounts consumed here can exert direct effects on the brain. It is possible that the suppressive effects of intra-AcbSh muscimol on ethanol intake may have resulted from an alteration of these effects, but two considerations argue against this view. First, muscimol administration reduced intake of the 10% ethanol solution to such low levels that it is likely ingestion terminated in most rats before enough ethanol was consumed to exert a postingestive pharmacological effect. Secondly, the addition of ethanol to the sucrose solution did not attenuate the ability of muscimol to increase intake, even though under these conditions animals consumed much more ethanol than they did when presented with the unsweetened ethanol solution. If inactivation of the AcbSh exaggerated some postingestive effect of ethanol, one would expect muscimol would differentially alter the intake of the sweetened ethanol and the sucrose solutions. In fact, muscimol produced almost identical increases in the intakes of both solutions, even though muscimol treated rats consuming the ethanol/sucrose mixture ingested considerably more ethanol (mean = 5.02 g/kg, Fig. 3b) than is required to induce a conditioned taste aversion (Thiele et al., 1996). These results also suggest that even though ethanol ingestion alters transmitter release (Doyon et al., 2005; Marinelli et al., 2005; Marinelli et al., 2006; Marinelli et al., 2003; Piepponen et al., 2002) and neuronal activity (Crankshaw et al., 2003; Herring et al., 2004; Janak et al., 1999; Porrino et al., 1998) in the AcbSh, AcbSh-mediated changes in ethanol intake cannot be explained by a simple feedback mechanism based on such physiological effects.
Although the magnitude of the muscimol response was not altered, addition of ethanol to the sucrose solution did result in very small, but statistically significant, reductions in intake following intra-AcbSh injections of either saline or muscimol. These decreases may have reflected alterations in either the taste of the solution or in the number of calories it contained. The inability of ethanol to modify the effects of muscimol on sucrose intake, despite a near tripling of the caloric density of the solution, suggests that the caloric contribution of the ethanol does not play a role in determining the magnitude of the muscimol response under these test conditions. This observation poses difficulties for the theory that GABA receptors in the accumbens are involved primarily in mediating the effects of calories on ingestion (Basso and Kelley, 1999). It is worth noting in this context that the 10% ethanol solution examined here is the only caloric diet tested to date that is not consumed more avidly after muscimol injections in the AcbSh. It is possible that although rats in long-term studies can respond to the calories obtained from ethanol (Richter, 1941), the relatively low baseline intakes in the current experiments may have limited the ability of the animals to learn to identify the ethanol solutions as a significant source of calories.
A possible explanation for the differential effects of AcbSh muscimol on sucrose and ethanol intakes might be that intra-AcbSh muscimol increases the effects of taste on intake regardless of the valence of these effects. In other words, muscimol might amplify both the appetitive and aversive properties of the tastes and thereby increase the intakes of sweet solutions while reducing the intakes of bitter solutions. A 10% ethanol solution has both sweet and bitter taste components (Di Lorenzo et al., 1986; Kiefer et al., 1990; Lawrence and Kiefer, 1987) and the bitter taste of ethanol appears to be an important contributing factor in determining how much an animal will ingest (Hyytia and Sinclair, 1993; Sinclair et al., 1992). The idea that intra-AcbSh muscimol sensitizes rats to bitter tastes is supported by our observation that this treatment also decreases intake of concentrated saccharin solutions (Stratford and Wirtshafter, 2007) which, like ethanol solutions, have both sweet and bitter taste components (Dess, 1993). Our current finding that muscimol increases intakes of an ethanol/sucrose mixture may then reflect the ability of sucrose to mask the aversive components of ethanol’s flavor.
In fact, the nucleus accumbens appears to be well suited for processing taste information with sucrose- and quinine-sensitive neurons found in both the core and the shell subregions of the nucleus (Roitman et al., 2005). In addition, electrophysiological data obtained from rats voluntarily consuming a sucrose solution shows that taste is encoded in one population of accumbens neurons, while activity in a separate population is related to the initiation, and perhaps maintenance, of feeding (Taha and Fields, 2005). The presence of both taste-sensitive cells and cells related to the initiation of feeding suggests that one of the functions of the Acb may be to integrate taste detection and feeding behavior. Taken together, the results of these studies are consistent with the hypothesis that inhibiting AcbSh output neurons drives feeding behavior (Stratford and Kelley, 1997) and recommends the AcbSh as a candidate substrate through which both sweet and bitter tastes are able to influence consumption.
In contrast to the present findings, injections of the μ-opioid agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) into shell/core boundary region have been reported to increase intakes of both food and ethanol (Zhang and Kelley, 2002), suggesting that DAMGO and muscimol may be producing their effects in the Acb through actions on distinct cell populations. The notion that the nucleus accumbens may contain several circuits that differentially influence intake of ethanol and other foods is supported by several other observations. For example, intra-Acb injections of orexin have been reported to increase intake of food, but not ethanol (Schneider et al., 2007), whereas the opposite pattern has been seen following injections of sulpiride (Levy et al., 1991).
The downstream pathways through which the AcbSh may alter alcohol ingestion have yet to be identified. However, it has been shown that the AcbSh mediates activity in several brain regions that have been implicated in the control of ethanol intake (Stratford, 2005; Stratford and Kelley, 1999), including the ventral pallidum (Harvey et al., 2002), central nucleus of the amygdala (McBride, 2002), and the paraventricular hypothalamic nucleus (Hodge et al., 1996; Schneider et al., 2007). Furthermore, the AcbSh controls food intake, in part, by mediating activity in the lateral hypothalamus (Maldonado-Irizarry et al., 1995; Stratford, 2005; Stratford and Kelley, 1999), another brain region known to participate in the control of ethanol intake (Schneider et al., 2007; Wayner, 2002; Wayner et al., 1971). The ability of the AcbSh to control activity in these brain regions makes them likely candidates as components in the functional circuit through which the AcbSh effects changes in ethanol intake.
Acknowledgments
This publication is based upon work supported by grants 0641943 from the National Science Foundation, R01DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases, and R03DA020802 from the National Institute for Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atrens DM, Marfaing-Jallat P, Le Magnen J. Ethanol preference following hypothalamic stimulation: relation to stimulation parameters and energy balance. Pharmacol Biochem Behav. 1983;19(4):571–575. doi: 10.1016/0091-3057(83)90329-5. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113(2):324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Crankshaw DL, Briggs JE, Olszewski PK, Shi Q, Grace MK, Billington CJ, Levine AS. Effects of intracerebroventricular ethanol on ingestive behavior and induction of c-Fos immunoreactivity in selected brain regions. Physiol Behav. 2003;79(1):113–120. doi: 10.1016/s0031-9384(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Dess NK. Saccharin’s aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3(1):55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27(10):1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29(6):958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10(4):457–462. doi: 10.1111/j.1530-0277.1986.tb05124.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y suppresses ethanol drinking in ethanol-abstinent, but not non-ethanol-abstinent, Wistar rats. Alcohol. 2008;42(7):541–551. doi: 10.1016/j.alcohol.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22(9):3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol Clin Exp Res. 2004;28(4):588–597. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19(6):1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Slawecki CJ, Aiken AS. Norepinephrine and serotonin receptors in the paraventricular nucleus interactively modulate ethanol consumption. Alcohol Clin Exp Res. 1996;20(9):1669–1674. doi: 10.1111/j.1530-0277.1996.tb01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Lumeng L, Wu JY, Li TK. Increased number of GABAergic terminals in the nucleus accumbens is associated with alcohol preference in rats. Alcohol Clin Exp Res. 1990;14(4):503–507. doi: 10.1111/j.1530-0277.1990.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283(1–3):151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Sinclair JD. Oral etonitazene and cocaine consumption by AA, ANA and Wistar rats. Psychopharmacology (Berl) 1993;111(4):409–414. doi: 10.1007/BF02253529. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817(1–2):172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7(2):115–120. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Lawrence G, Kiefer S. Generalization of specific taste aversions to alcohol in the rat. Chem Senses. 1987;12:591–599. [Google Scholar]
- Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- Linseman MA. Alcohol consumption in free-feeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology (Berl) 1987;92(2):254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29(10):1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30(6):982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169(1):60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71(3):509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, dopamine and GABA involvement in alcohol drinking of selectively bred rats. Alcohol. 1990;7(3):199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Misra K, Roy A, Pandey SC. Effects of voluntary ethanol intake on the expression of Ca(2+)/calmodulin-dependent protein kinase IV and on CREB expression and phosphorylation in the rat nucleus accumbens. Neuroreport. 2001;12(18):4133–4137. doi: 10.1097/00001756-200112210-00054. [DOI] [PubMed] [Google Scholar]
- Piepponen TP, Kiianmaa K, Ahtee L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol Biochem Behav. 2002;74(1):21–30. doi: 10.1016/s0091-3057(02)00937-1. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Whitlow CT, Samson HH. Effects of the self-administration of ethanol and ethanol/sucrose on rates of local cerebral glucose utilization in rats. Brain Res. 1998;791(1–2):18–26. doi: 10.1016/s0006-8993(97)01519-9. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21(9):3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Alcohol as a food. Q J Stud Alcohol. 1941;1:650–662. [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45(4):587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2(1):32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31(11):1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9(2):155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Stratford TR. The nucleus accumbens shell as a model of integrative subcortical forebrain systems regulating food intake. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight: Integrative Systems and the Development of Anti-Obesity Drugs. London: Elsevier; 2007. pp. 27–65. [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley AE. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. Neuroreport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Activation of GABAA receptors in the nucleus accumbens shell elicits opposite effects on consumption of sucrose and saccharin solutions. Soc Neurosci Abstr 2007 [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25(5):1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20(6):1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann N Y Acad Sci. 2008;1148:136–140. doi: 10.1196/annals.1410.083. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Median and dorsal raphe injections of the 5-HT1A agonist, 8-OH- DPAT, and the GABAA agonist, muscimol, increase voluntary ethanol intake in Wistar rats. Neuropharmacology. 1994;33:349–358. doi: 10.1016/0028-3908(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Ward BO, Somerville EM, Clifton PG. Intraaccumbens baclofen selectively enhances feeding behavior in the rat. Physiol Behav. 2000;68(4):463–468. doi: 10.1016/s0031-9384(99)00197-3. [DOI] [PubMed] [Google Scholar]
- Wayner MJ. Craving for alcohol in the rat: adjunctive behavior and the lateral hypothalamus. Pharmacol Biochem Behav. 2002;73(1):27–43. doi: 10.1016/s0091-3057(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Carey RJ, Nolley D. Ethanol drinking elicited during electrical stimulation of the lateral hypothalamus. Physiol Behav. 1971;7(5):793–795. doi: 10.1016/0031-9384(71)90152-1. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D. The control of ingestive behavior by the median raphe nucleus. Appetite. 2001;36(1):99–105. doi: 10.1006/appe.2000.0373. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159(4):415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]