Abstract
Objective
Calcineurin (Cn) and the NFAT family of transcription factors are critical in vascular smooth muscle cell (SMC) development and pathology. Here, we use a genomics approach to identify and validate NFAT gene targets activated during PDGF-BB-induced SMC phenotypic modulation.
Methods & Results
Genome-wide expression arrays were used to identify genes both: 1) differentially activated in response to PDGF-BB and 2) whose differential expression was reduced by both the Cn inhibitor, cyclosporin A (CsA), and the NFAT inhibitor, A-285222. The top 20 pharmacologically sensitive genes were validated by qRT-PCR analysis of PDGF-BB-stimulated SMCs in the presence of Cn/NFAT inhibitors, including the VIVIT peptide. In all experiments, Endothelial Protein C Receptor (EPCR/PROCR) gene activation was reduced. We show that PROCR expression is virtually absent in untreated, quiescent SMCs. PDGF-BB stimulation, however, induces significant PROCR promoter activation and downstream protein expression in a Cn/NFAT-dependent manner. Mutation of a species-conserved, NFAT binding motif significantly attenuates PDGF-BB-induced PROCR promoter activity, thereby distinguishing NFAT as the first PROCR transcriptional activator to date. Moreover, SMC PROCR expression is upregulated in the neointima as early as 7 days following acute vascular injury in rat carotid arteries.
Conclusions
We hereby report PROCR as a novel, NFAT-dependent gene that may be implicated in vascular restenosis and consequent inward remodeling.
Keywords: NFAT, transcription, vascular SMCs, restenosis
INTRODUCTION
Vascular smooth muscle cells (SMC) can undergo phenotypic modulation1, or the process by which a quiescent, contractile SMC alters its expression profile to facilitate cell proliferation, migration, and/or inflammatory cell recruitment (see reviews1,2). SMC phenotypic modulation is a critical event underlying atherosclerosis and in-stent restenosis that involves several signaling cascades, such as the calcineurin/nuclear factor of activated T cells (NFAT) pathway. Calcineurin (Cn), a cytosolic, calcium-dependent phosphatase, activates the NFAT family of transcription factors (NFATc1-c4) for nuclear translocation and subsequent target gene transcription. Cn/NFAT activity contributes to vascular SMC proliferation and migration in response to receptor tyrosine kinase and G-protein-coupled receptor agonists, respectively3,4. In vivo, Cn expression increases in response to acute vascular injury (Supplemental Figure I), and pharmacological inhibition of Cn/NFAT signaling has been shown to significantly reduce neointima formation5. Identifying downstream targets of NFAT activity is therefore important in understanding the role of Cn/NFAT signaling in SMC phenotypic modulation and vascular disease.
Several NFAT target genes, although limited in number, have already been described in vascular SMCs. Many of these genes encode proteins associated with SMC proliferation, migration and inflammation. For example, basal expression of the pro-inflammatory protein, protease-activated receptor 1 (PAR-1), is transcriptionally regulated by NFATc1 in human saphenous vein SMCs6. Knockdown of endogenous NFATc1 also inhibits thrombin-induced interleukin-6 (IL6) expression, supporting previous findings that IL6 is an NFAT target gene7. Gomez and colleagues showed that glucose-induced osteopontin (OPN) expression in intact arteries was reduced with pharmacological inhibitors of Cn/NFAT signaling and in NFATc3-/- mice8. NFATc3 occupancy at the OPN promoter was also demonstrated using chromatin immunoprecipitation (ChIP) assays. Proteins involved in cell cycling have also been identified as NFAT-dependent. In human aortic SMCs, platelet-derived growth factor BB (PDGF-BB)-mediated induction of cyclin D1 was attenuated by inhibitors of Cn/NFAT signaling. ChIP assay using an antibody to NFATc1 also showed enrichment at the cyclin D1 promoter9. The closely-related protein, cyclin A, has also been reported by the same group to be NFATc1-dependent10. Furthermore, we and others have shown that genes upregulated with vascular injury, namely cyclooxygenase-2 (COX2) and vascular cell adhesion molecule-1 (VCAM1), exhibit Cn/NFAT-dependency11-13, supporting the notion that Cn/NFAT signaling is implicated in vascular remodeling.
To expand our understanding of NFAT activity in vascular SMCs, we previously applied an unbiased integrative genomics approach13. Putative target genes were identified using gene expression array datasets in combination with an in silico ‘NFATome’ (a species-conserved list of gene promoters containing at least one NFAT binding site). We identified Down Syndrome Candidate Region 1 (DSCR1) as a novel, Cn/NFAT-dependent, injury-responsive gene in vascular SMCs13. We are still, however, far from understanding the coordinated orchestration of NFAT target gene activation in vascular SMCs.
In this study, we expand our genomics investigation to further identify NFAT target genes involved in SMC phenotypic modulation. We present two additional gene expression arrays where different Cn/NFAT inhibitors, cyclosporin A (CsA)7 and A-28522212,14, were each used to block NFAT activation during PDGF-BB treatment. Here, we report Endothelial Protein C Receptor (EPCR/PROCR) as a novel, Cn/NFAT-dependent gene in vascular SMCs. To our knowledge, NFATc is the first transcription factor that has been validated to transcriptionally regulate PROCR in any cell type. Furthermore, while basal medial PROCR expression in vivo is barely detectable, acute vascular injury results in pronounced neointimal PROCR expression. Our findings, together with the recent identification of functional PROCR in vascular SMCs15, suggest PROCR may facilitate SMC phenotypic modulation and contribute to the pathological events following vascular remodeling.
MATERIALS AND METHODS
Cell Culture
Rat aortic SMCs (RASMCs) were plated in base media containing 10% FBS as previously described16. RASMCs were growth-arrested at ~40% confluency using insulin-free, serum-free media. Cells were treated either with vehicle, PDGF-BB (30ng/mL, Upstate), PDGF-BB + Cyclosporin A (3μM, Sigma), or PDGF-BB + A-285222 (10μM, Abbott Labs). Cells treated with either CsA or A-285222 were pretreated with drug alone for 30 minutes prior to PDGF-BB stimulation.
Affymetrix rat expression arrays and analysis
RASMCs were growth arrested for 48 hours prior to PDGF-BB treatment both in the presence and absence of Cn/NFAT inhibitors, as described above. Total RNA was harvested at 3 hrs post-treatment for each of the conditions (n=2 arrays per condition) using an RNeasy Mini Kit spin column (Qiagen). Total RNA was prepared according to manufacturer specifications (Affymetrix Rat Expression Array 230 2.0) at the University of Virginia GeneChip/Microarray Biostatistics Core (GSE19106). Arrays were analyzed as previously described13.
Tissue panel/expression analysis of candidate NFAT-targets
Total RNA was harvested from tissues/cells according to kit specifications (Qiagen). cDNA was synthesized using the iScript cDNA Synthesis Kit (BioRad) and traditional reverse-transcription polymerase chain reaction (RT-PCR) was performed using Taq (Qiagen). Amplicon products were run on a 1% agarose/TAE gel for imaging. Gene-specific RT-PCR primers were designed using Primer3 (MIT), and gene sequences were found for each primer product for validation (Supplemental Table I).
Quantitative RT-PCR
Total RNA and cDNA was prepared from RASMCs as described above. Quantitative RT-PCR analysis (iCycler, BioRad) was performed using SYBR Green, as previously described (CITE). Quantitative RT-PCR on genes of interest were run in biological duplicates (triplicates for t=3hrs) and RNA abundance was normalized to 18S rRNA.
PROCR promoter construct generation
The pGL3-PROCR-WT-luc plasmid construct contains 723 base pairs of the rat PROCR promoter (−701 to +22). To make this construct, 908 base pairs of the wild-type PROCR promoter was originally amplified from rat normal liver genomic DNA (BioChain Institute) using the PROCR-8F (5′-GTGCACTTGTCCTCACAGCA) and PROCR-9R (5′- AAGCTTGAGGGAAGGGTGGAAAGAGA) primers. This amplicon was cloned into the pCR-XL-TOPO vector. A site-directed mutagenesis of the conserved NFAT bindting motif (GGAAA→TTAAA) was performed on the generated pCR-XL-TOPO-PROCR-WT plasmid using customized primers containing the desired mutation (Agilent Technologies). PROCR-9R contains a HindIII linker at the 5′ tail to facilitate sub-cloning. The rat PROCR promoter contains a SacI site at the −701 position. HindIII/SacI restriction digestion released a promoter fragment that was sub-cloned into the pGL3 vector (Promega). The pGL3-PROCR-MUT-luc plasmid was hence generated in parallel with the pGL3-PROCR-WT-luc plasmid. Sequence fidelity was confirmed by sequencing with RVprimer3 and RVprimer4 (Promega) (Supplemental Figure II).
PROCR-luciferase activity measurements
Cells were transfected with the PROCR-WT-luc (or PROCR-MUT-luc) promoter construct 24 hrs prior to treatment using FuGENE6 (Roche). Cells were harvested at designated time points by direct addition of 1X Passive Lysis Buffer (Promega). Luciferase activity was measured on an Omega FLUOstar plate reader (BMG Labtech) and fluorescence units were normalized to total protein.
Adenoviral infection (Ad-CMV-GFP, Ad-VIVIT-GFP)
RASMCs were plated as previously described and growth-arrested for 72 hrs. Cells were transfected with either an empty GFP vector (Viraquest) or a GFP-labeled, VIVIT-expressing adenovirus (courtesy of Harris, TE, Dept. of Pharmacology, University of Virginia). RASMCs were infected with the appropriate adenovirus 24 hours prior to PDGF-BB or 10% FBS stimulation. Total cell number in a representative well was determined in order to infect cells at a consistent Multiplicity of Infection (MOI) of 400 pfu/cell. Cells were treated with the appropriate phenotypic modulatory stimuli for the indicated time duration and harvested accordingly.
PROCR Western blot
Protein lysates were collected by direct addition of a 2X Laemmli Buffer solution. Equal protein volumes were loaded on a 10% SDS-PAGE gel and transferred to nitrocellulose membranes for protein quantification via Odyssey infrared imaging (LI-COR). Membranes were blocked in an Odyssey/PBS (1:1) blocking solution for at least 1 hr. Primary antibodies were diluted in an Odyssey/PBS (1:1) blocking + 0.1% Tween-20 solution and membranes were allowed to incubate at 4°C overnight (anti-PROCR 1:200, Santa Cruz; anti-βtub 1:1000, Cell Signaling). Fluorescently-labeled secondary antibodies were used to detect protein signals on the Odyssey system (IRDye Secondary Antibodies).
PROCR Immunofluorescence
RASMCs were plated in chamber slides, as previously described, and treated with the indicated conditions. Cells were fixed in a 4% PFA/PBS solution, 6 hours post-stimulation. Cells were permeabilized using a 0.02% Triton-X-100 solution and blocked in 0.1% BSA/PBS for at least 1hr. Primary antibodies were diluted in 0.1% BSA/PBS (anti-PROCR 1:200, Santa Cruz) and cells were allowed to incubate at room temperature for at least 1.5 hrs. Fluorescently-labeled secondary antibodies were used to detect cytosolic PROCR and DAPI stain was subsequently added (ProLong Gold, Invitrogen). Images were taken using a confocal microscope (Olympus).
PROCR Immunohistochemistry
The balloon-injury model was performed on rat carotid arteries, as previously described17. Both the injured carotid arteries and contralateral control arteries were harvested 7, 14, and 21-days post-injury. Paraffin-embedded sections underwent microwave antigen retrieval and incubated with the PROCR antibody, as previously described18.
RESULTS
Whole genome expression analyses identify candidate Cn/NFAT-dependent genes
The overall objective of this study was to identify and validate novel Cn/NFAT-dependent genes involved in vascular SMC phenotypic modulation. A commonly used in vitro model of SMC phenotypic modulation involves stimulation of SMC monoculture with platelet-derived growth factor BB (PDGF-BB)19,20. We previously reported that NFATc transcriptional activity is rapid and transient in response to either PDGF-BB or serum stimulation in rat aortic SMCs 13. In this study, we used a combination of pharmacologic Cn/NFAT inhibitors and whole-genome expression arrays to identify candidate NFAT target genes. RNA for microarray analysis was isolated from SMCs pre-treated with CsA or A-285222 for 30 minutes prior to 3 hours of PDGF-BB stimulation. Differentially upregulated genes were first identified in the PDGF-BB-treated array by comparing expression values to those in the vehicle-treated array. Differentially upregulated genes exhibit at least a 1.5-fold induction and a p-value < 0.1 (Supplemental Figure IIIA, x). Secondly, CsA- or A-285222-treated arrays were independently analyzed to identify genes that were decreased in expression relative to the PDGF-BB-treated array (Supplemental Figure IIIA, y). Differentially downregulated genes were identified in both CsA- and A-285222-treated arrays and subsequently merged to uncover genes whose PDGF-BB-mediated induction was sensitive to both CsA and A-285222 treatment (Supplemental Figure IIIB, z). Genes were ranked by percent reduction in expression as a result of each respective inhibitor (Table). The top 20 sensitive genes were then subjected to rigorous follow-up validation.
Table.
PDGF-BB-induced changes in gene expression are indicated by fold change (PDGF-BB | VEH). Differentially upregulated genes exhibit at least a 1.5-fold induction and a p-value < 0.1. Reduction in differential gene expression as a result of CsA or A-285222 treatment is denoted by percent decrease for each inhibitor (n=2 arrays per condition).
| Gene Symbol | Gene Title; Biologicol Process | Fold Induction (PDGF-BB) |
% Decrease (PDGF-BB + CsA) |
% Decrease (PDGF-BB + A-28S222) |
|---|---|---|---|---|
| Acot2 | acyl-CoA thioesterase 2; very-long-chain fatty acid metabolic process | 2.73 | 70.0 | 80.6 |
| Adss | adenylosucdnate synthetase; nudeotide binding | 1.57 | 65.2 | 72.2 |
| Cacnb2 | calcium channel, voltage-dependent, beta 2 subunit; ion cransport | 9.52 | 59.8 | 46.0 |
| Csnk1g1 | casein kinase 1, gamma 1; protein amino acid phosphorylation | 2.34 | 53.3 | 38.1 |
| Dscr1 | Down syn drome candidate region 1; signal transduction | 3.82 | 89.5 | 84.8 |
| Ousp1 | dual specificity phosphatase 1; protein tyrosine phosphatase activity | 3.60 | 72.5 | 80.1 |
| Fam126a | family with sequence sim ilarity 126, member A; --- | 1.94 | 66.4 | 47.8 |
| Fzd7 | frizzled homolog 7 (Orosoph ila); cell surface receptor linked signal transduction | 1.95 | 53.4 | 49.2 |
| Gla | galactosidase, alpha; carbohydrate metabolic process | 2.08 | 63.0 | 52.5 |
| Hspa1a/b | heat shock 70kD protein l A, heat shock 70kD protein 1B; mRNA catabolic process | 1.56 | 88.7 | 99.6 |
| Kif5c | kinesin fam ily member 5C; organelle organization | 1.68 | 91.4 | 91.6 |
| Mgatl | mannoside acetylglucosaminyltransferase 1; carbohydrate metabolic process | 3.06 | 77.2 | 64.8 |
| Mobkl2a | MOB1, Mps One Binder kinase activator-like 2B (yeast); --- | 2.81 | 58.6 | 51.7 |
| Otud1 | OUT domain contain ing 1; G-protein coupled receptor protein signaling pathway | 2.34 | 65.1 | 41.2 |
| Pdlim5 | POZ and LIM domain 5; --- | 1.86 | 50.3 | 53.8 |
| Procr | protein C receptor; immune response | 33.28 | 56.0 | 56.7 |
| Rapgef2 | Rap guanine nucleotide exchange factor (GEF) 2; MAPKKK cascade | 2.11 | 56.1 | 53.2 |
| Rbm14 | RNA binding motif protein 14; DNA replication | 2.37 | 85.5 | 53.3 |
| Rras2 | related RAS viral (r-ras) oncogene homolog 2; intracellular protein transport | 1.78 | 63.0 | 58.7 |
| Vegfc | vascular endothelial growth factor C; angiogenesis | 1.68 | 53.5 | 44.3 |
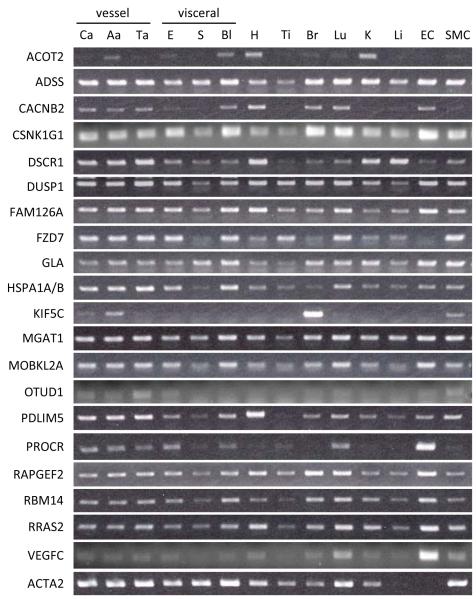
Expression analysis of candidate NFAT-regulated genes in rat tissues and cells
While the NFAT transcription factor was originally described in T cells, NFAT signaling has been shown to exist in almost every cell type. We therefore examined mRNA expression levels of the 20 NFAT target gene candidates in a panel of normal rat tissues and rat vascular cell lines (Figure 1). Most genes were expressed in three different blood vessels (carotid artery, abdominal aorta, thoracic aorta), aortic endothelial cells (EC), and aortic SMCs. Some genes, such as DSCR1, DUSP1 and PDLIM5, were expressed at higher levels in blood vessels than in visceral smooth muscle. Originally described in ECs21, PROCR was identified in the array as a PDGF-BB-regulated gene in SMCs, but as shown here, is highly expressed in ECs and barely detectable in quiescent SMCs.
Figure 1. Expression analysis of candidate NFAT-regulated genes in rat tissues and cells.
Agarose gel electrophoresis was performed for PCR amplicon products. Expression levels of each NFAT candidate gene differs depending on tissue and cell type. carotid artery (Ca), abdominal aorta (Aa), thoracic aorta (Ta), esophagus (E), stomach (S), bladder (Bl), heart (H), tibialis anterior (Ti), brain (Br), lung (Lu), kidney (K), liver (Li), aortic endothelial cell (EC), aortic smooth muscle cell (SMC)
PROCR transcriptional activity is dependent on Cn/NFAT signaling
The top 20 candidate NFAT target genes underwent two different follow-up tests for Cn/NFAT dependency. First, Cn/NFAT dependence was investigated using CsA or A-285222 at 10 time points across 24 hours to visualize the expression profile of each gene. A second method of validation involved use of an adenovirus expressing the VIVIT peptide, an inhibitor of NFAT signaling. Endogenous NFAT activation requires binding of Cn to a conserved NFAT regulatory domain containing the PxIxIT consensus sequence22,23. The VIVIT peptide has a higher affinity for the regulatory domain compared to Cn, thereby impairing NFAT activation without obstruction of other Cn-dependent downstream activity24. Experiments using the VIVIT peptide were completed only for the first 3 hours given that the identified genes by whole genome expression analyses were upregulated within 3 hours of PDGF-BB stimulation.
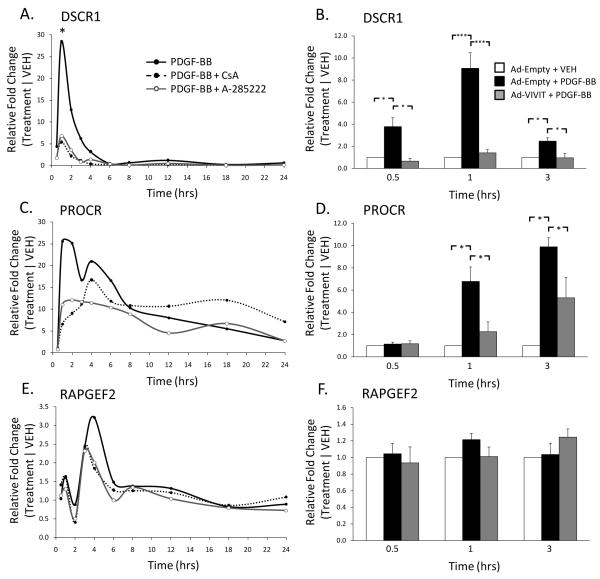
We recently identified DSCR1 as a novel Cn/NFAT-dependent mediator of vascular SMC phenotypic modulation13, thus serving as a positive control in this study (Table). Validation experiments in our previous approach utilized identical pharmacological inhibitors. DSCR1 expression in response to PDGF-BB was rapid and transient, peaking at 1 hour (~28-fold) with significant inhibition in the presence of either CsA or A-285222 (Figure 2A, taken from 13). Likewise, DSCR1 expression was upregulated at 0.5, 1, and 3 hours following PDGF-BB stimulation, with significant attenuation in the presence of the VIVIT peptide (Figure 2B). Use of the VIVIT peptide, herein, further demonstrates the NFAT-dependent property of DSCR1 expression in vascular SMCs. The expression pattern of DSCR1 at the 3 hour time point in Figure 2B is similar, but not as robust as the 1 hour response, perhaps due to the transient nature of DSCR1 expression. Adenoviral-treated cells also appear to exhibit an attenuation in peak mRNA fold change values (Figures 2B, 2D, 2F compared to Figures 2A, 2C, 2E), an experimental artifact that has been observed in previously published studies. DSCR1 and PROCR, however, maintain NFAT-dependency as trends are sustained regardless of the inhibitor used.
Figure 2. PROCR mRNA expression is dependent on Cn/NFAT signaling.
DSCR1 (A, from(13)) and PROCR (C) mRNA expression is upregulated in response to PDGF-BB stimulation (30ng/mL). Transcriptional activation, however, is attenuated upon treatment with either CsA (3μM) or A-285222 (10μM). Similar results are seen with use of the VIVIT peptide; DSCR1 and PROCR expression is inhibited at 0.5, 1, 3 hrs (B) and 1, 3 hrs (D) upon treatment, respectively. PDGF-BB induced RAPGEF2 gene activation does not appear to be Cn/NFAT-dependent (E, F). Representative graph shown for A, C, E. Target mRNA levels are normalized to the 18S housekeeping gene. Treatment conditions are normalized to respective vehicle conditions. (n≥3, *p<0.05, ***p<0.001)
The 20 NFAT target genes presented in the Table were subject to both validation procedures, including use of the VIVIT peptide (Supplemental Figure IV). PROCR mRNA was upregulated ~25-fold as early as 1 hour following PDGF-BB stimulation and attenuated by treatment with either CsA or A-285222 (Figure 2C). Use of the VIVIT peptide together with PDGF-BB significantly reduced PROCR mRNA expression, emphasizing the NFAT-dependence of PROCR gene expression (Figure 2D). On the other hand, ~35% of the 20 candidate target genes did not meet the follow-up criteria for Cn/NFAT dependency. For example, RAPGEF2 was identified by array analyses to be Cn/NFAT-dependent (Table), but in vitro validation shows that PDGF-BB-induced RAPGEF2 expression is not dependent on Cn/NFAT activity (Figure 2E). In addition, RAPGEF2 mRNA expression did not appear to change in response to the VIVIT peptide (Figure 2F), indicating Cn/NFAT signaling is not required for RAPGEF2 expression in vascular SMCs. Despite the presence of false positives in our genomics approach, several observations are of interest. For example, many of the candidate NFAT targets exhibit PDGF-BB-induced gene expression at time points earlier than 3 hours of treatment (Supplemental Figure IV). Furthermore, several of these target genes demonstrate significant attenuation in PDGF-BB-induced signal with the VIVIT peptide at the 1-hour time point, suggesting peak NFAT transcriptional activity occurs earlier than predicted. Importantly, microarray-derived biological responses undoubtedly require subsequent in vitro validation given the relative abundance of false-positives identified in our follow-up studies.
In this study, we chose to follow up on the PROCR gene because: 1) PROCR was only recently identified as being expressed in vascular SMCs15, and 2) transcription factors that regulate PROCR expression have not be clearly identified in any cell type.
PROCR promoter activation is Cn/NFAT-dependent
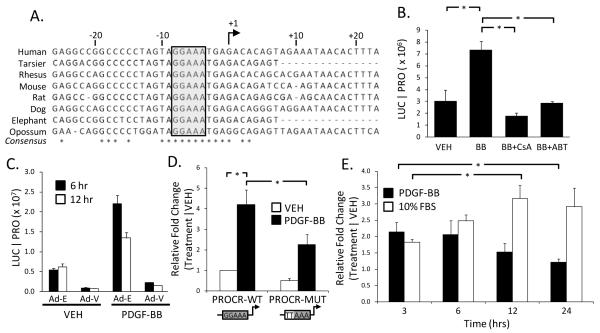
The PROCR promoter contains one NFAT binding site 8 base pairs upstream of the transcriptional start site. Bioinformatic analyses showed that this particular NFAT site is conserved across 8 species, ranging from human to opossum (Figure 3A). In order to measure PROCR promoter activity, 723 base pairs of the rat PROCR promoter was cloned upstream of the firefly luciferase gene in a plasmid vector (PROCR-WT-luc). SMCs were transfected with the PROCR-WT-luc plasmid construct and subsequently treated with PDGF-BB. PROCR promoter activity increased ~7-fold in response to 6 hours of PDGF-BB treatment and was significantly reduced when treated in the presence of either CsA or A-285222 (Figure 3B). We also tested PROCR-WT-luc activity in the presence of the VIVIT peptide. PROCR promoter activity was completely blocked following expression of the VIVIT peptide (Figure 3C). VIVIT expression also lowered basal PROCR-WT-luc activity. A point mutation was introduced into the NFAT binding site (GGAAA→TTAAA, Supp Figure 2) to determine if the identified NFAT motif is necessary for optimal PROCR promoter activation. Mutation of the NFAT site resulted in significant reduction of PDGF-BB-induced PROCR promoter activity, suggesting NFAT is necessary for PROCR expression (Figure 3D). The presence of ~50% activity despite the introduced mutation suggests other transcription factors (i.e. Sp1, refer to Discussion) may be involved in activation of PROCR expression in smooth muscle. SMCs were also treated with PDGF-BB or 10% FBS over a span of 24 hours to compare the effects of differing phenotypic modulatory stimuli on PROCR promoter activity. Interestingly, the PROCR promoter is activated relatively early in response to PDGF-BB stimulation, peaking at 3 hours (~2.1-fold) whereas stimulation with 10% FBS resulted in a delayed response with peak promoter activity occurring at 12 hours (Figure 3E). These findings suggest that the window of PROCR promoter activation is differentially regulated by different stimuli and signaling pathways.
Figure 3. PROCR promoter activation is Cn/NFAT-dependent.
(A) Sequence alignment of the PROCR promoter identifies a species-conserved, NFAT binding site. (B) Use of the PROCR-WT-luc promoter reporter construct indicates activity 6 hours post-PDGF-BB stimulation. Simultaneous treatment with either CsA or A-285222, however, inhibits PROCR promoter activation. (C) Use of an adenoviral-mediated VIVIT peptide represses PDGF-BB-induced PROCR promoter activity (Ad-E=Ad-Empty; Ad-V=Ad-VIVIT). (D) Mutation of the NFAT binding site (GGAAA→TTAAA) significantly attenuates PDGF-BB-induced PROCR promoter activity 6 hours post-stimulation. (E) The PROCR promoter exhibits a differential response depending on the phenotypic modulatory stimuli. Species promoter sequences retrieved from the UCSC genome browser. Luciferase output normalized to total protein. Treatment conditions normalized to vehicle. (n≥3, *p<0.05)
PROCR protein expression is Cn/NFAT-dependent
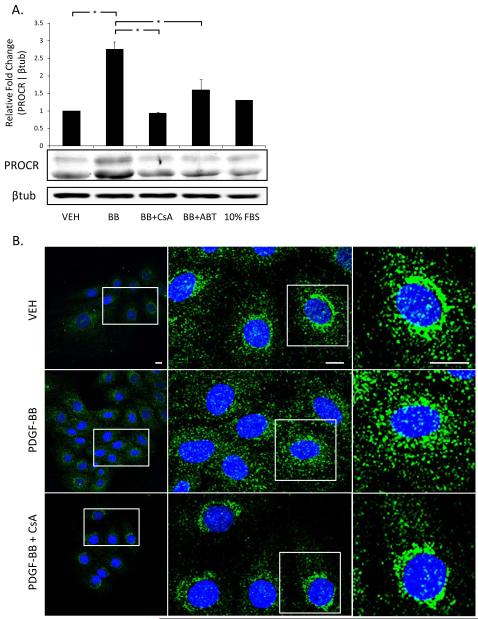
PROCR protein expression was measured to test NFAT-dependency at the translational level. PROCR protein levels increased ~2.5-fold in response to 6 hours of PDGF-BB treatment when normalized to vehicle-treated samples. PROCR protein expression was significantly attenuated following treatment with either CsA or A-285222 (Figures 4A). Treatment with 10% FBS did not show a pronounced increase in PROCR protein expression. It is likely that this observation is a result of differing signaling mechanisms that may be involved in PROCR activation, where PDGF-BB stimulation results in a relatively quicker response compared to 10% FBS treatment. This trend was also observed at the promoter level in Figure 3E.
Figure 4. Pharmacological inhibition of Cn/NFAT signaling blocks PROCR protein expression and cytoplasmic patterning.
(A) PROCR protein expression is upregulated 6 hours post-PDGF-BB stimulation, and significantly attenuated when treated with Cn/NFAT inhibitors, as indicated by densitometry analysis. PROCR levels normalized to respective beta-tubulin levels. (B) Immunofluorescence images of PROCR localization in rat aortic SMCs exhibit changes in distribution with PDGF-BB treatment (6hrs). Scale bars represent 10μm. Representative blots/images shown. (n≥3, *p<0.05)
We also investigated PROCR localization in response to PDGF-BB stimulation by immunofluorescence microscopy. As shown in Figure 4B, PROCR is localized near the periphery of the nucleus in untreated SMCs, similar to intracellular PROCR staining in vascular endothelial cells25. PDGF-BB stimulation for 6 hours induced a punctate redistribution of PROCR from the perinuclear region toward the cytoplasm. Pre-treatment with CsA inhibited the PDGF-BB-induced cytoplasmic redistribution as evidenced by restoration of the perinuclear pattern seen with vehicle controls. While our immunofluorescence may not visually reflect the protein changes seen via Western blot, it is possible this discrepancy is due to the fact that Figure 3B reflects a single plane of the Z-axis.
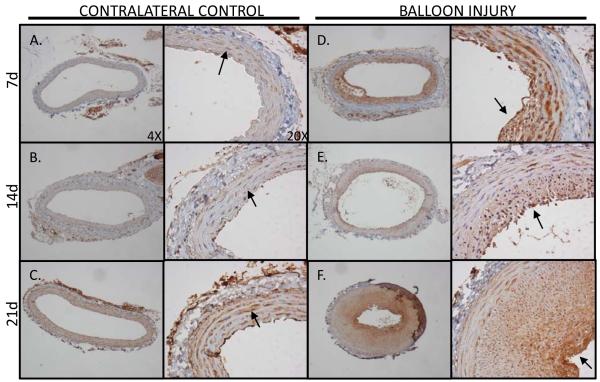
PROCR expression is upregulated in response to acute carotid artery balloon injury
In vitro experimentation clearly demonstrated the Cn/NFAT-dependent regulation of PROCR at the promoter, transcriptional, and translational level. Previous studies demonstrate Cn/NFAT signaling modulates injury-induced neointima formation5. Immunohistological staining of PROCR was performed on balloon-injured rat carotid arteries to determine how PROCR expression changed as a function of time post-injury, as previously described26,27 (Figure 5). Both injured and contralateral control arteries were harvested at 7, 14, and 21 days post-injury. Positive PROCR staining in the media of contralateral arteries supports the recent finding that vascular SMCs express PROCR15, although at a relatively low level. In balloon-injured arteries, staining patterns indicate that PROCR expression is upregulated with injury. Visual assessment of PROCR intensity suggests PROCR induction occurs as early as 7 days post-injury and continues to be highly expressed in neointimal SMCs thereafter (Figures 5D-F). Endothelial cell expression of PROCR cannot be seen in the injured artery samples as this procedure removes the endothelium at the time of injury. The contralateral control artery, however, exhibits endothelial staining with maximal intensity occurring at 21 days post-injury. A cross-section of an ApoE-/- mouse aorta was also used as a positive control for endothelial PROCR expression in intact tissue (Supplemental Figure V). Interestingly, the 21d contralateral control exhibits increased PROCR expression in vascular SMCs relative to the 7 day control artery (Figure 5C). It is possible that balloon-injury in the opposing carotid artery elevates blood thrombin levels as a result of vascular injury. Thrombin has also been shown to activate NFAT signaling6,28, and also induce PROCR promoter activity in vascular SMCs (Supplemental Figure VI).
Figure 5. Acute vascular injury induces neointimal PROCR expression.
Immunohistological staining of PROCR was performed in balloon-injured rat carotid arteries. PROCR staining in contralateral control carotid arteries show positive staining in vascular SMCs (A, B, C; arrows). In injured arteries, PROCR expression is visibly upregulated in the neointima at 7 (D, arrow), 14 (E, arrow), and 21 (F, arrow) days post-injury compared to contralateral controls.
DISCUSSION
In this study, we employ whole genome array analysis and pharmacological reagents to identify PROCR as a potential Cn/NFAT-dependent gene in vascular smooth muscle. We corroborate the only report to date that vascular SMCs do express the protein C receptor (EPCR/PROCR)15. More importantly, we validate our informatics approach and are the first to report Cn/NFAT signaling as a regulator of PROCR activation. We show PDGF-BB stimulation induced PROCR expression in a Cn/NFAT-dependent manner at both the transcriptional and translational levels. Mutation of a highly conserved NFAT binding motif significantly attenuated PROCR promoter activation, supporting the NFAT-dependent property of PROCR activity. In addition, PROCR expression is upregulated in vivo as a result of acute vascular injury, highlighting the potential role of PROCR in vessel restenosis.
Until the recent detection of PROCR in vascular SMCs, PROCR was believed to be expressed predominantly in endothelial cells (EC, EPCR). Studies to date on PROCR transcription focus primarily on the mechanisms of cell-specificity during developmental cell differentiation rather than identification of transcription factor modules operating at the PROCR promoter. One group identified multiple Specificity protein 1 (Sp1) consensus sequences at the PROCR promoter. Electrophoretic mobility shift assays (EMSA) and promoter reporter assays in ECs demonstrate binding of Sp1 to the PROCR promoter and direct contribution to reporter gene expression, respectively29. However, no single Sp1 binding site was found critical for PROCR promoter activity, as site mutations did not result in significant loss of reporter gene activity29. Interestingly, PDGF-BB stimulation increases Sp1 expression in cultured vascular SMCs30, and balloon angioplasty induces neointimal Sp1 expression in vivo31,32. Furthermore, Sp1 has been shown to suppress classic SMC contractile genes, namely SM22α and SMMHC30. Sp1 and NFAT may both be necessary for optimal PROCR expression, as crosstalk between Sp1 and NFAT proteins have been observed in activation of other genes33,34.
With regard to biological function, PROCR has been almost exclusively studied in the context of endothelial barrier protection and blood coagulation. Although the functional role of PROCR in vascular SMCs is virtually unknown, research in endothelial biology provides several clues as to what may be occurring in vascular SMCs. The discovery of PROCR in ECs uncovered a novel plasma membrane receptor critical for the protective qualities of activated protein C (APC) in a variety of diseases, including tumor metastasis35, myocardial infarction/reperfusion injury36, and severe sepsis37. In 2000, a group using a primate model of E.coli-mediated septic shock found that binding of APC to PROCR was vital in the host defense against E.coli; inhibition of APC-PROCR binding altered a normally tolerable response to E.coli into a lethal response38. The mechanism of protection against E.coli-induced sepsis by APC, however, was yet to be determined. In 2002, Riewald et al. demonstrated that APC-bound PROCR activated downstream protease-activated receptor 1 (PAR-1) signaling in ECs, providing a mechanism behind PROCR-dependent APC-mediated sepsis protection39. In support of this finding, an in vivo study demonstrated that APC reduces vascular permeability through PAR-1 in mice40. PAR-1 is better known as a thrombin receptor, whose interaction with thrombin results in endothelial barrier dysfunction41. The identification of PAR-1 activation via APC-bound PROCR thus became a paradoxical observation: How does PAR-1, a prototypical thrombin receptor, also mediate the endothelial barrier protective effects of APC? Intriguingly, vascular SMCs have also been shown to not only express functional PROCR, but also bind APC for downstream PAR-1 activation and SMC proliferation, suggesting this signaling axis is implicated in SMC phenotypic modulation15.
A recent study found that APC-mediated endothelial barrier protection occurs through crosstalk of PAR-1 and the sphingosine-1-phosphate (S1P) signaling pathway. Barrier protection by APC occurs through PAR-1-dependent upregulation of sphingosine kinase 1 (SK1, a kinase required for generation of S1P) and binding of de novo S1P to the S1P1 receptor in ECs42,43. S1P, a bioactive phospholipid, has been demonstrated to reorganize the endothelial cytoskeleton and increase cellular adhesion through S1P1, resulting in decreased endothelial permeability44. We have shown that vascular SMCs differentially express S1P1, S1P2, and S1P3 in response to acute vascular injury17. In addition, unpublished data from our group indicate PDGF-BB-stimulated SMCs induce SK1 expression in vitro while in vivo, SK1 kinase activity and S1P levels are increased as a result of acute vascular injury (unpublished). Upregulation of PROCR (herein), PAR-145, S1P117, and SK1 in response to vascular injury suggests APC-PROCR-dependent PAR-1 signaling could be a major contributor to the S1P levels found in atherosclerosis and in-stent restenosis46. S1P may also exert its effects through S1P1 in vascular SMCs, but exhibit biological outcomes unique from those observed in ECs. APC-mediated PAR-1 signaling, while protective in ECs, may promote SMC phenotypic modulation for inward vessel remodeling. Cross-talk between Cn/NFAT, S1P, and PAR1 signaling pathways also leads us toward an interactive, coordinated, and more robust model of Cn/NFAT signaling in SMCs.
APC-PROCR-dependent PAR-1 activation has also been shown to increase monocyte chemoattractant protein-1 (MCP-1/CCL2) expression in ECs39. MCP-1 is a major regulatory chemokine secreted by several cell types, including vascular SMCs, and is known to mediate monocyte/macrophage migration and infiltration47,48. Hence, MCP-1 is a major determinant in atherosclerotic plaque formation, as evidenced by two well-known atherosclerotic mouse models, LDLR-/- and ApoE-/- mice49,50. Given the similarities in signaling mechanisms between ECs and vascular SMCs thus far, it is possible that upregulation of APC-PROCR-dependent PAR-1 activation will also lead to upregulation of MCP-1 in vascular SMCs. PROCR has also been found to exist in a soluble form with full ligand-binding capability51. Interestingly, soluble PROCR can bind to activated neutrophils through proteinase-3 and partial dependence on neutrophil-expressed Mac-1 integrin protein52. This discovery suggests PROCR is involved, not only as a signaling factor/receptor, but also as an adhesion molecule. Furthermore, inhibition of APC binding to PROCR is correlated with intense neutrophil influx into tissues38, suggesting PROCR is somehow involved in leukocyte trafficking. Monocyte/macrophage infiltration into the vessel medial layer is a characteristic event of atherosclerotic plaque development, where Mac-1 is often used as a marker for macrophage involvement53,54. The proposed increase in MCP-1 generation together with increased PROCR expression could be a synergistic attempt by vascular SMCs for monocyte recruitment. Further experimentation, however, is critical in addressing these hypotheses and to elucidate the function of PROCR in vascular SMCs.
In summary, the data presented herein demonstrates the value of a high-throughput, whole genome approach in identification of novel transcriptional targets and importantly, follow-up in vitro validation studies. Above all, we show for the first time that PROCR expression in vascular SMCs is dependent on Cn/NFAT signaling and upregulated in response to acute vascular injury. This is the first study to validate a transcription factor that regulates PROCR in any cell type. While our current understanding of PROCR function in vascular SMCs is truly at its infancy, it is evident that PROCR may have significant implications in SMC phenotypic modulation and vascular disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Themistoclis Karaoli and Melissa Bevard for their technical help. The authors would also like to thank Abbott Laboratories for providing the A-285222 compound.
SOURCES OF FUNDING This study was supported by research grants from the American Heart Association (SDG grant BRW, pre-doctoral fellowship to MYL), the National Institutes of Health (HL 081682 to BRW), and APS post-doctoral fellowship to SMG.
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47:168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A, Rao GN. A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation. Biochem J. 2002;368:183–190. doi: 10.1042/BJ20020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Dronadula N, Rao GN. A novel role for nuclear factor of activated T cells in receptor tyrosine kinase and G protein-coupled receptor agonist-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:41218–41226. doi: 10.1074/jbc.M406917200. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of Nuclear Factor of Activated T Cells Activation Signaling Suppresses Balloon Injury-induced Neointima Formation in a Rat Carotid Artery Model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz AC, Rauch BH, Freidel K, Schror K. Regulation of protease-activated receptor-1 by vasodilatory prostaglandins via NFAT. Cardiovasc Res. 2009;83:778–784. doi: 10.1093/cvr/cvp163. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, Wide-Swensson D, Hellstrand P, Lydrup ML, Gomez MF. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C1167–C1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, Sigvardsson M, Bosc LV Gonzalez, Smith ML, Salehi A, Agardh E, Fredrikson GN, Agardh CD, Nilsson J, Wamhoff BR, Hultgardh-Nilsson A, Gomez MF. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol. 2010;30:218–224. doi: 10.1161/ATVBAHA.109.199299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpurapu M, Wang D, Van QD, Kim TK, Kundumani-Sridharan V, Pulusani S, Rao GN. Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem. 2010;285:3510–3523. doi: 10.1074/jbc.M109.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karpurapu M, Wang D, Singh NK, Li Q, Rao GN. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. J Biol Chem. 2008;283:26577–26590. doi: 10.1074/jbc.M800423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robida AM, Xu K, Ellington ML, Murphy TJ. Cyclosporin A selectively inhibits mitogen-induced cyclooxygenase-2 gene transcription in vascular smooth muscle cells. Mol Pharmacol. 2000;58:701–708. doi: 10.1124/mol.58.4.701. [DOI] [PubMed] [Google Scholar]
- 12.Orr AW, Lee MY, Lemmon JA, Yurdagul A, Jr., Gomez MF, Bortz PD, Wamhoff BR. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225–231. doi: 10.1161/ATVBAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MY, Garvey SM, Baras AS, Lemmon JA, Gomez MF, Bortz PD Schoppee, Daum G, LeBoeuf RC, Wamhoff BR. Integrative genomics identifies DSCR1 (RCAN1) as a novel NFAT-dependent mediator of phenotypic modulation in vascular smooth muscle cells. Hum Mol Genet. 2010;19:468–479. doi: 10.1093/hmg/ddp511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djuric SW, BaMaung NY, Basha A, Liu H, Luly JR, Madar DJ, Sciotti RJ, Tu NP, Wagenaar FL, Wiedeman PE, Zhou X, Ballaron S, Bauch J, Chen YW, Chiou XG, Fey T, Gauvin D, Gubbins E, Hsieh GC, Marsh KC, Mollison KW, Pong M, Shaughnessy TK, Sheets MP, Smith M, Trevillyan JM, Warrior U, Wegner CD, Carter GW. 3,5-Bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J Med Chem. 2000;43:2975–2981. doi: 10.1021/jm990615a. [DOI] [PubMed] [Google Scholar]
- 15.Bretschneider E, Uzonyi B, Weber AA, Fischer JW, Pape R, Lotzer K, Schror K. Human vascular smooth muscle cells express functionally active endothelial cell protein C receptor. Circ Res. 2007;100:255–262. doi: 10.1161/01.RES.0000255685.06922.c7. [DOI] [PubMed] [Google Scholar]
- 16.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type Voltage-Gated Ca2+ Channels Modulate Expression of Smooth Muscle Differentiation Marker Genes via a Rho Kinase/Myocardin/SRF-Dependent Mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 17.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garvey SM, Sinden DS, Bortz PD Schoppee, Wamhoff BR. Cyclosporine upregulates KLF4 in vascular smooth muscle cells and drives phenotypic modulation in vivo. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.109.163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corjay MH, Blank RS, Owens GK. Platelet-derived growth factor-induced destabilization of smooth muscle alpha-actin mRNA. J Cell Physiol. 1990;145:391–397. doi: 10.1002/jcp.1041450302. [DOI] [PubMed] [Google Scholar]
- 20.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- 21.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 22.Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cozar FJ, Okamura H, Aramburu JF, Shaw KT, Pelletier L, Showalter R, Villafranca E, Rao A. Two-site interaction of nuclear factor of activated T cells with activated calcineurin. J Biol Chem. 1998;273:23877–23883. doi: 10.1074/jbc.273.37.23877. [DOI] [PubMed] [Google Scholar]
- 24.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 25.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LV. Endothelial cell protein C receptor cellular localization and trafficking: potential functional implications. Blood. 2009;114:1974–1986. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle alpha-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418–427. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 28.Minami T, Miura M, Aird WC, Kodama T. Thrombin-induced Autoinhibitory Factor, Down Syndrome Critical Region-1, Attenuates NFAT-dependent Vascular Cell Adhesion Molecule-1 Expression and Inflammation in the Endothelium. J Biol Chem. 2006;281:20503–20520. doi: 10.1074/jbc.M513112200. [DOI] [PubMed] [Google Scholar]
- 29.Rance JB, Follows GA, Cockerill PN, Bonifer C, Lane DA, Simmonds RE. Regulation of the human endothelial cell protein C receptor gene promoter by multiple Sp1 binding sites. Blood. 2003;101:4393–4401. doi: 10.1182/blood-2002-05-1570. [DOI] [PubMed] [Google Scholar]
- 30.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C Element Mediates Repression of the SM22{alpha} Promoter Within Phenotypically Modulated Smooth Muscle Cells in Experimental Atherosclerosis. Circ Res. 2004;95:981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 31.Andres V, Urena J, Poch E, Chen D, Goukassian D. Role of Sp1 in the induction of p27 gene expression in vascular smooth muscle cells in vitro and after balloon angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:342–347. doi: 10.1161/01.atv.21.3.342. [DOI] [PubMed] [Google Scholar]
- 32.Madsen CS, Hershey JC, Hautmann MB, White SL, Owens GK. Expression of the smooth muscle myosin heavy chain gene is regulated by a negative-acting GC-rich element located between two positive-acting serum response factor-binding elements. J Biol Chem. 1997;272:6332–6340. doi: 10.1074/jbc.272.10.6332. [DOI] [PubMed] [Google Scholar]
- 33.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao S, Matsui K, Fine A, Zhu B, Marshak-Rothstein A, Widom RL, Ju ST. FasL promoter activation by IL-2 through SP1 and NFAT but not Egr-2 and Egr-3. Eur J Immunol. 1999;29:3456–3465. doi: 10.1002/(SICI)1521-4141(199911)29:11<3456::AID-IMMU3456>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, Van Noorden CJ, Spek CA. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–1973. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loubele ST, Spek CA, Leenders P, van OR, Aberson HL, Hamulyak K, Ferrell G, Esmon CT, Spronk HM, ten CH. Activated protein C protects against myocardial ischemia/ reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2009;29:1087–1092. doi: 10.1161/ATVBAHA.109.188656. [DOI] [PubMed] [Google Scholar]
- 37.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 38.Taylor FB, Jr., Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 39.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 40.Schuepbach RA, Feistritzer C, Fernandez JA, Griffin JH, Riewald M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemost. 2009;101:724–733. doi: 10.1160/th08-10-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia JG, Patterson C, Bahler C, Aschner J, Hart CM, English D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis, and platelet-derived growth factor mRNA expression in cultured endothelium. J Cell Physiol. 1993;156:541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- 42.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 43.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 44.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 45.ndrade-Gordon P, Derian CK, Maryanoff BE, Zhang HC, Addo MF, Cheung W, Damiano BP, D’Andrea MR, Darrow AL, de GL, Eckardt AJ, Giardino EC, Haertlein BJ, McComsey DF. Administration of a potent antagonist of protease-activated receptor-1 (PAR-1) attenuates vascular restenosis following balloon angioplasty in rats. J Pharmacol Exp Ther. 2001;298:34–42. [PubMed] [Google Scholar]
- 46.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 47.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang JM, Sica A, Peri G, Walter S, Padura IM, Libby P, Ceska M, Lindley I, Colotta F, Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1166–1174. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- 49.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 50.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 51.Kurosawa S, Stearns-Kurosawa DJ, Hidari N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J Clin Invest. 1997;100:411–418. doi: 10.1172/JCI119548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697–4703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]
- 53.von Zur MC, von ED, Bassler N, Neudorfer I, Steitz B, Petri-Fink A, Hofmann H, Bode C, Peter K. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): implications on imaging of atherosclerotic plaques. Atherosclerosis. 2007;193:102–111. doi: 10.1016/j.atherosclerosis.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 54.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.