Abstract
Background
Evidence-based guidelines recommend radical cystectomy for patients with muscle-invasive bladder cancer. However, many patients receive alternate therapies, such as chemotherapy or radiation. We examined factors that are associated with the use of radical cystectomy for invasive bladder cancer and compared the survival outcomes of patients with invasive bladder cancer by the treatment they received.
Methods
From linked Surveillance, Epidemiology, and End Results–Medicare data, we identified a cohort of 3262 Medicare beneficiaries aged 66 years or older at diagnosis with stage II muscle-invasive bladder cancer from January 1, 1992, through December 31, 2002. We examined the use of radical cystectomy with multilevel multivariable models and survival after diagnosis with the use of instrumental variable analyses. All statistical tests were two-sided.
Results
A total of 21% of the study subjects underwent radical cystectomy. Older age at diagnosis and higher comorbidity were associated with decreased odds of receiving cystectomy (for those ≥80 vs 66–69 years old, odds ratio [OR] = 0.10, 95% confidence interval [CI] = 0.07 to 0.14; for Charlson comorbidity index of 3 vs 0–1, OR = 0.25, 95% CI = 0.14 to 0.45). Long travel distance to an available surgeon was associated with decreased odds of receiving cystectomy (for >50 vs 0–4 miles travel distance to an available surgeon, OR = 0.60, 95% CI = 0.37 to 0.98). Overall survival was better for those who underwent cystectomy compared with those who underwent alternative treatments (for chemotherapy and/or radiation vs cystectomy, hazard ratio of death = 1.5, 95% CI = 1.3 to 1.8; for surveillance vs cystectomy, hazard ratio of death = 1.9, 95% CI = 1.6 to 2.3; 5-year adjusted survival: 42.2% [95% CI = 39.1% to 45.4%] for cystectomy; 20.7% [95% CI = 18.7% to 22.8%] for chemotherapy and/or radiation; 14.5% [95% CI = 13.0% to 16.2%] for surveillance).
Conclusions
Guideline-recommended care with radical cystectomy is underused for patients with muscle-invasive bladder cancer. Many bladder cancer patients whose survival outcomes might benefit with surgery are receiving alternative less salubrious treatments.
CONTEXT AND CAVEATS
Prior knowledge
Many patients who are diagnosed with muscle-invasive bladder cancer are treated with chemotherapy or radiation rather than with radical cystectomy and urinary diversion, as has been recommended by evidence-based guidelines.
Study design
Surveillance, Epidemiology, and End Results–Medicare data were used to examine factors that are associated with the use of radical cystectomy for invasive bladder cancer and to compare the survival outcomes of patients with invasive bladder cancer by the treatment they received.
Contribution
Only 21% of patients diagnosed with invasive bladder cancer underwent radical cystectomy. Patient characteristics that were associated with decreased odds of receiving cystectomy included older age at diagnosis, higher comorbidity, and long travel distance to an available surgeon. Overall survival was better for those who underwent cystectomy than for those who underwent alternative treatments.
Implications
Many bladder cancer patients whose survival outcomes might benefit from radical cystectomy with urinary diversion are not receiving this treatment. These patients need greater access to available surgeons and better information about the risks and benefits of their treatment options.
Limitations
The study sample was restricted to Medicare beneficiaries. Patients may have appropriately received treatments other than cystectomy based on their clinical characteristics and life expectancy. Chemotherapy use was underreported for the Surveillance, Epidemiology, and End Results–Medicare sample that was analyzed. Patients with stage III or IV cancers were excluded. Unmeasured confounders may have biased the results. Patient preference regarding the care received was not accounted for.
From the Editors
Bladder cancer is a heterogeneous disease that affects approximately 68 000 people in the United States annually (1). Patients who are diagnosed with noninvasive bladder cancers may have indolent, albeit recurrent, disease. Conversely, patients with more advanced muscle-invasive bladder cancers may have narrow windows of cure that require aggressive treatment to optimize their health outcomes.
National Comprehensive Cancer Network (NCCN) guidelines recommend radical cystectomy as the primary treatment for patients with muscle-invasive bladder cancer, whereas alternative treatments are reserved for patients with extensive comorbid conditions or poor performance status (2). Radical cystectomy involves removal of the urinary bladder and associated organs: the prostate in men, and the uterus, ovaries, and part of the vagina in women. The 5-year survival rate after radical cystectomy ranges from 62%–80% for those with stage II bladder cancers to 0%–36% for those with stage IV cancers (3). The aggressiveness of this malignancy mandates an aggressive approach to management.
Despite these recommendations, an ad hoc regionalization of radical cystectomy care that occurred in the 1990s may have created disparities in the use of this needed operation (4). For example, during the 1990s, fewer community surgeons performed this complex procedure, which was instead almost exclusively performed by surgeons at urban academic hospitals (4). This contraction of available surgeons may have restricted patient access to radical cystectomy, especially among underinsured patients and populations in underserved areas. We sought to characterize the utilization of guideline-recommended surgical care for muscle-invasive bladder cancer in the United States. We further sought to understand patient, provider, and health-care environment–specific factors that are associated with use of radical cystectomy, and we compared survival outcomes of patients with muscle-invasive bladder cancer by the treatment they received.
Methods
Patient Population
We used the linked database that merges the Surveillance, Epidemiology, and End Results (SEER) national cancer registry with Medicare claims to identify subjects with stage II transitional cell carcinoma of the urinary bladder (ie, muscle-invasive bladder cancer with no regional or distant metastases) who were diagnosed from January 1, 1992, through December 31, 2002, and for whom claims data were available through December 31, 2005. The combined SEER–Medicare dataset merges detailed cancer-specific information from SEER with Medicare claims, which permit an analysis of procedures performed, dates of any treatments received, and the determination of subjects’ comorbid conditions. We excluded subjects who were diagnosed with locally advanced cancers to restrict our sample to patients with unambiguous treatment options. According to the published guidelines (5) on the evaluation, treatment, and surveillance of bladder cancer, the majority of medically fit patients with stage II bladder transitional cell carcinoma should undergo radical cystectomy.
The most recent release of the linked SEER–Medicare dataset contains information on persons with newly diagnosed cancers in 13 US regions that are generalizable to the US population (ie, metropolitan Atlanta, Detroit, and Seattle/Puget Sound; rural Georgia; and the entire states of California, Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah) (6). Linkage of the SEER registry to the Medicare Patient Entitlement and Diagnosis Summary File provides demographic information and detailed cancer-specific information, including stage, grade, histology, and treatments that were received within 4 months of diagnosis. The Medicare Provider Analysis and Review file contains diagnosis and procedure codes for each hospital admission, provider billing records, and a hospital file derived from the Healthcare Cost Report and Provider-of-Service survey. We restricted the study sample to subjects who had Medicare Fee-for-Service coverage and for whom Medicare Part A and Part B claims data were available.
Study subjects with stage II bladder cancer, based on American Joint Committee on Cancer and International Union on Cancer TNM Classification and Stage groupings (7), were identified from the primary malignancy diagnosis codes and extent-of-disease codes in SEER. Cancer stage in SEER conforms to the standards of the North American Association of Central Cancer Registries, and case ascertainment in SEER is 98% complete (8). We specified the treatment group based on treatment codes in SEER and International Classification of Diseases, Ninth Revision (ICD-9) (9) or Current Procedural Terminology Coding System, Fourth Edition (CPT-4) (10) codes in the Medicare claims. Radical cystectomy subjects were identified based on procedure codes that are indicative of radical cystectomy (ICD-9 code 57.71; CPT-4 code 51570 or 51575). Patients who received radiation were classified on the basis of diagnosis and procedure codes in Medicare claims that are consistent with radiotherapeutic procedures in the absence of a concomitant code for radical cystectomy (ie, ICD-9 codes 92, V580, V661, or V771; CPT codes 77261, 77399, 77400, 77490, 77750, or 77797). Because bladder-sparing therapeutic protocols for invasive bladder cancer typically combine radiation and chemotherapy (3), we combined subjects who received chemotherapy alone (n = 402), radiation alone (n = 271), or combination chemotherapy and radiation (n = 249) into one treatment group. We identified subjects who received chemotherapy based on ICD-9 and CPT-4 codes that are consistent with chemotherapeutic agents commonly used to manage bladder cancer in the absence of a concomitant code for radical cystectomy (ie, J6360, J9000, J9001, J9060, J9062, J9201, J9250, or J9260). Subjects who underwent surgery in combination with radiation or chemotherapy were included with the radical cystectomy group. Subjects who did not have SEER–Medicare variable specifications consistent with radical cystectomy, chemotherapy, or radiation were deemed to have received no further aggressive cancer-directed care and thus were categorized as having received surveillance.
Covariates
To examine factors that are associated with undergoing radical surgery among patients who are newly diagnosed with stage II bladder cancer, we created multilevel multivariable models that incorporated covariates that are representative of predisposing characteristics (ie, immutable factors such as age and race), enabling characteristics (ie, factors that facilitate care, such as health-care insurance and household income), and measures of need (eg, disease severity). Characteristics of the environment in which patients receive care (eg, physician density, health maintenance organization penetration) have also been shown to affect health-care utilization and were included in the models (11–13). In this analysis, these contextual variables combined with subject predisposing, enabling, and need characteristics as factors associated with the use of radical cystectomy for muscle-invasive bladder cancer.
Subject age, sex, race, and marital status were derived from SEER demographic variables. Although most beneficiaries enter Medicare at age 65 years, we restricted the study sample to subjects who were aged 66 years or older at diagnosis to ensure that all subjects had at least 1 year of claims data from which to derive the burden of comorbidity. Given that our study sample contained few nonwhite subjects, we dichotomized race into white and nonwhite. We dichotomized marital status into partnered (classified as married in SEER) and unpartnered (single, separated, divorced, or widowed) individuals. We assessed subject socioeconomic status by ascribing to them the socioeconomic characteristics of their neighborhood of residence (14,15). We evaluated the median household income in the subject’s zip code and the proportion of residents within that zip code who did not have a high school education, both from the 2000 US Census, and categorized these variables into quartiles. We quantified subject comorbidity with the Klabunde modification of the Charlson comorbidity index (16,17), which gives a weighted score based on diagnosis claims in which a higher score indicates a subject with a greater burden of comorbid conditions. We used the SEER registry classification of bladder cancer grade (ie, grades 1 through 4), in which a higher grade indicates more undifferentiated cancers. We categorized grades 3 and 4 cancers as high-grade malignancies. We identified the surgeon who diagnosed the muscle-invasive cancer (hereafter referred to as the primary surgeon) using encrypted Unique Physician Identification Numbers that are included with the Medicare claims.
To account for the environmental context in which the study subjects received care, we linked the subject's zip code to the Area Resource File (18). We examined a priori the density of primary care physicians and urologists in subjects’ zip codes (by quartiles), health maintenance organization penetration (quartiles), and the density of the local population as a continuum from large urban areas to rural locales as categorized in the Area Resource File for each study subject. We incorporated contextual covariates that were associated with use of radical cystectomy in univariate models at an alpha level of .15 or lower into our multivariable models. We hypothesized that patient travel distance to an available surgeon would be associated with the use of radical surgery. We used ArcGIS software (ESRI, Redlands, CA) to compute the linear distance (also known as “as the crow flies”) from the center of the residence zip code of each study subject to the center of the zip code of the nearest cystectomy provider identified in the Medicare claims data. Linear distance has been shown to be highly correlated with patient-reported travel times (19). Categories of travel distance were based on tertiles and included a category that corresponds to published travel lengths at which patients abandon needed surgical care (at least 50 miles of travel to a cystectomy provider) (20,21).
Statistical Analysis
We compared study subjects by the treatment they received by using χ2 analysis for categorical variables and analysis of variance for continuous variables. We selected covariates for inclusion in the multivariable models that were associated with use of radical cystectomy in univariate models at an alpha level of .15 or lower. Despite the known importance of race with respect to access to health-care services, we excluded the non-statistically significant race variable from our multivariable models because of the paucity of nonwhite subjects in the study sample. The choice of treatment after a diagnosis of muscle-invasive bladder cancer is likely to depend on the practice patterns of the surgeon who diagnoses the cancer. To account for the effect of clustering of patients within surgical providers on utilization of surgery, we created a multilevel model with random effects to examine factors associated with receipt of radical cystectomy. Characteristics of the individual subjects represented the level 1 fixed effects of our model, and the surgeon who performed the bladder tumor biopsy diagnostic of muscle invasion (represented by the Unique Physician Identification Number) represented level 2. Because the outcome, receipt of cystectomy, was common, we calculated predictive margins or the percent difference in likelihood of the outcome for the covariate compared with the reference category. The predictive margins were estimated from solutions for the best linear unbiased predictors accounting for random effects in the multivariable model. We bootstrapped with 1000 repetitions to obtain 95% confidence intervals (CI) for the predictive margins. The multilevel model also permitted estimation of the partitioned variance in use of radical cystectomy that was attributable to the primary surgeon, which was calculated from the residual intraclass correlation coefficient (22–24).
Unadjusted survival estimates by treatment received for muscle-invasive bladder cancer were assessed by the Kaplan–Meier method. We used instrumental variable methods to account for the substantial differences in clinical and demographic characteristics between subjects who underwent radical cystectomy for bladder cancer and those who received chemotherapy, radiation, or surveillance. Instrumental variable methods balance measured and unmeasured characteristics that differ between treatment groups; unbalanced characteristics are a major limitation of observational analyses with administrative claims databases (25,26). We chose as the instrumental variable the travel distance to the nearest cystectomy provider because it was statistically significantly associated with treatment and was not independently associated with survival in Cox proportional hazards models, for which we verified that the proportional hazards assumption was not violated by visual inspection of the log-minus-log plots. We further validated travel distance as a candidate instrumental variable by examining the covariate balance by minimum cystectomy travel distance categories and found no association between subject-level characteristics (age [P = .66], sex [P = .21], marital status [P = .13], Charlson comorbidity index [P = .16], and cancer grade [P = .06]) and travel distance.
For survival analyses, conventional two-stage instrumental variable methods may produce biased estimates in these inherently nonlinear situations (27), and two-stage least squares regression fails to account for time to death and disregards censoring. Thus, for adjusted survival analyses, we used two-stage residual inclusion estimation (Supplementary Methods, available online). Terza et al. (28) showed that two-stage residual inclusion estimation is consistent across a variety of nonlinear models, including parametric survival models with a Weibull distribution. The first stage of the model predicts treatment modality and outputs raw residuals for the endogenous variables. In this study, the first stage of the model follows from the covariate selection for the multilevel model of use of radical cystectomy described above. However, to examine survival outcomes across three treatment groups (rather than comparing patients who received cystectomy with those who did not), we used a multinomial model in the first stage. The raw residuals were calculated by subtracting the predicted likelihood of receiving chemotherapy and/or radiation or surveillance vs cystectomy from the actual value of the treatment received. The second stage of the model incorporates these residuals as additional covariates along with the endogenous treatment variables and other relevant covariates. Cox proportional hazards models were used to examine associations between covariates and overall survival. Covariates that were statistically significantly associated with survival at P < .05 were incorporated into the second-stage Weibull models for the two-stage residual inclusion estimation of treatment effects on survival. For this nonstandard method, we bootstrapped 1000 samples of the original cohort with replacement to obtain the 95% confidence intervals for the hazard ratio (HR) estimates for overall mortality as well as for the adjusted 2- and 5-year survival estimates for each treatment group (29). For all Cox proportional hazards models, we verified nonviolation of the proportional hazards assumption with log-minus-log plots.
All statistical tests were two-sided, and all analyses were performed with the use of SAS software (version 9.2; SAS Institute, Cary, NC). Variables that were associated with use of cystectomy or survival at an alpha level less than .05 were considered statistically significant.
Results
Overall, 678 (21%) of the 3262 study subjects underwent radical cystectomy for muscle-invasive bladder cancer (Table 1). Subjects who underwent cystectomy were younger and had fewer comorbid conditions compared with subjects who underwent chemotherapy, radiation, or surveillance (P < .001 for both). Subjects who received cystectomy were more likely to have a high-grade cancer compared with subjects who received alternative treatments (P < .001). Those who underwent cystectomy had shorter travel distances to an available cystectomy provider compared with those who did not (P = .003). We identified statistically significant regional variation in the use of radical cystectomy (P < .001): Cystectomy was more common in SEER regions with higher populations or more urban locales, such as Detroit, Seattle, Connecticut, and California, than in SEER regions with lower populations or more rural locales, such as Iowa.
Table 1.
Characteristics of the entire study sample by treatment received (n = 3262)*
| Characteristic | Radical cystectomy | Chemotherapy or radiation | Surveillance | P† |
| Overall, No. (%) | 678 (21) | 922 (28) | 1662 (51) | |
| Subject demographic characteristics | ||||
| Age at diagnosis, y | ||||
| Mean (SD) | 74.5 (5.5) | 78.8 (6.6) | 81.3 (7.4) | <.001 |
| 66–69, No. (%) | 132 (20) | 91 (10) | 125 (8) | <.001 |
| 70–74, No. (%) | 229 (34) | 148 (16) | 205 (12) | |
| 75–79, No. (%) | 192 (28) | 256 (28) | 329 (20) | |
| ≥80, No. (%) | 125 (18) | 427 (46) | 1003 (60) | |
| Sex, No. (%) | ||||
| Male | 480 (71) | 656 (71) | 1118 (67) | .07 |
| Female | 198 (29) | 266 (29) | 544 (33) | |
| Race, No. (%) | ||||
| White | 605 (89) | 832 (90) | 1483 (89) | .70 |
| Nonwhite | 73 (11) | 90 (10) | 179 (11) | |
| Partnered, No. (%) | 627 (93) | 873 (95) | 1546 (93) | .15 |
| Subject socioeconomic status | ||||
| Percentage of residents in subject's zip code with less than a high school education, No. (%) | ||||
| <10 | 199 (29) | 250 (27) | 440 (27) | .67 |
| 10–20 | 293 (43) | 401 (44) | 722 (43) | |
| 20–30 | 107 (16) | 157 (17) | 270 (16) | |
| ≥30 | 79 (12) | 114 (12) | 230 (14) | |
| Quartiles of median household income in subject's zip code‡, No. (%) | ||||
| 1 (lowest) | 139 (21) | 227 (25) | 449 (27) | .002 |
| 2 | 178 (26) | 247 (26) | 389 (23) | |
| 3 | 158 (23) | 227 (25) | 431 (26) | |
| 4 (highest) | 203 (30) | 221 (24) | 393 (24) | |
| Subject clinical characteristics | ||||
| Charlson comorbidity index, No. (%) | ||||
| 0 | 526 (78) | 575 (62) | 974 (58) | <.001 |
| 1 | 102 (15) | 218 (24) | 365 (22) | |
| 2 | 33 (5) | 80 (9) | 179 (11) | |
| ≥3 | 17 (2) | 49 (5) | 144 (9) | |
| High-grade cancer, No. (%) | 601 (89) | 825 (90) | 1400 (84) | <.001 |
| Year of diagnosis, No. (%) | ||||
| 1992–1995 | 257 (38) | 307 (33) | 541 (32) | .09 |
| 1996–1999 | 202 (30) | 270 (29) | 508 (31) | |
| 2000–2002 | 219 (32) | 345 (38) | 613 (37) | |
| Subject environment | ||||
| Travel distance to cystectomy provider, No. (%), miles | ||||
| 0–4 | 329 (49) | 399 (43) | 702 (42) | .003 |
| 5–19 | 231 (34) | 293 (32) | 551 (33) | |
| 20–49 | 66 (10) | 123 (13) | 221 (13) | |
| ≥50 | 45 (7) | 99 (11) | 177 (11) | |
| Missing | 7 (1) | 8 (1) | 11 (1) | |
| Local population of subject residence§, No. (%) | ||||
| Metropolitan, >1 000 000 residents | 407 (60) | 520 (56) | 927 (56) | .11 |
| Metropolitan, 250 000–1 000 000 residents | 136 (20) | 172 (19) | 311 (19) | |
| Metropolitan, <250 000 residents | 106 (16) | 166 (18) | 319 (19) | |
| Rural | 29 (4) | 64 (7) | 105 (6) | |
| No. of urologists per 100 000 residents§, mean (SD) | 68.9 (106.3) | 61.7 (101.2) | 63.5 (106.5) | .38 |
| Health professional shortage area§,║, No. (%) | 600 (89) | 778 (84) | 1446 (87) | .04 |
| SEER registry, No. (%) | ||||
| Connecticut | 102 (15) | 118 (13) | 174 (10) | <.001 |
| Detroit | 112 (17) | 140 (15) | 210 (13) | |
| Hawaii | 6 (1) | 10 (1) | 22 (1) | |
| Iowa | 57 (8) | 152 (16) | 231 (14) | |
| New Mexico | 27 (4) | 24 (3) | 74 (4) | |
| Seattle | 74 (11) | 89 (10) | 128 (8) | |
| Utah | 22 (3) | 33 (4) | 73 (4) | |
| Atlanta | 23 (3) | 37 (4) | 63 (4) | |
| Rural Georgia | 0 (0) | 3 (0) | 9 (1) | |
| Kentucky | 15 (2) | 21 (2) | 44 (3) | |
| Louisiana | 12 (2) | 24 (3) | 40 (2) | |
| New Jersey | 30 (5) | 41 (4) | 103 (6) | |
| California | 198 (29) | 230 (25) | 491 (30) | |
SEER = Surveillance, Epidemiology, and End Results.
Estimated from χ2 analysis for categorical variables and analysis of variance for continuous variables.
Quartile 1: ≤$36 998; quartile 2: $36 999–$46 475; quartile 3: $46 476–$58 016; quartile 4: >$58 016.
Derived from linkage of subject zip code of residence to the Area Resource File.
Health professional shortage areas are areas designated by the US Health Resources and Services Administration as being critically understaffed in primary care practitioners, dentists, or mental health providers.
We used a multilevel multivariable model to identify factors that were associated with receipt of radical cystectomy (Table 2). There was a stepwise decrease in the odds ratio (OR) and in the predictive margin of undergoing radical cystectomy with increasing age, independent of other covariates. Subjects who were aged 80 years or older at diagnosis had 90% lower odds (OR = 0.10, 95% CI = 0.07 to 0.14) and 38% lower predicted rate (95% CI = −45% to −32%) of radical cystectomy compared with those who were aged 66–69 years at diagnosis. Increasing comorbidity was associated with decreasing odds and predictive margin of undergoing radical cystectomy. The sickest subjects (ie, Charlson comorbidity index of 3 or higher) had 75% lower odds (OR = 0.25, 95% CI = 0.14 to 0.45) and 18% lower predicted rate (95% CI = −23% to −13%) of radical cystectomy compared with those without substantial comorbid conditions (ie, Charlson comorbidity index of 0 or 1). Those with high-grade cancers had a greater likelihood of receiving surgery than those with low-grade cancers, all other covariates held constant (OR = 1.41, 95% CI = 1.01 to 1.97; predictive margin = 4.9%, 95% CI = 0.6% to 9.1%).
Table 2.
Multilevel multivariable model of factors associated with receipt of radical cystectomy*
| Variable | OR (95% CI) | Predictive margin† (95% CI) |
| Age (vs 66–69), y | ||
| 70–74 | 0.83 (0.59 to 1.18) | −0.040 (−0.12 to 0.036) |
| 75–79 | 0.40 (0.29 to 0.57) | −0.19 (−0.26 to −0.12) |
| ≥80 | 0.10 (0.07 to 0.14) | −0.38 (−0.45 to −0.32) |
| Female (vs male) | 1.01 (0.79 to 1.28) | 0.001 (−0.033 to 0.034) |
| Partnered (vs unpartnered) | 0.94 (0.62 to 1.44) | −0.010 (−0.073 to 0.053) |
| Subject zip code income quartile (vs 4 [highest]) | ||
| 1 (lowest) | 0.72 (0.50 to 1.03) | −0.049 (−0.099 to 0.001) |
| 2 | 0.93 (0.67 to 1.29 | −0.010 (−0.058 to 0.037) |
| 3 | 0.75 (0.55 to 1.04) | −0.052 (−0.095 to −0.010) |
| Charlson comorbidity index (vs 0–1) | ||
| 2 | 0.48 (0.37 to 0.62) | −0.11 (−0.14 to −0.076) |
| ≥3 | 0.25 (0.14 to 0.45) | −0.18 (−0.23 to −0.13) |
| High-grade cancer | 1.41 (1.01 to 1.97) | 0.049 (0.006 to 0.091) |
| Travel distance to cystectomy provider (vs 0–4), miles | ||
| 5–19 | 0.90 (0.69 to 1.17) | −0.015 (−0.053 to 0.022) |
| 20–49 | 0.73 (0.48 to 1.12) | −0.046 (−0.099 to 0.008) |
| ≥50 | 0.60 (0.37 to 0.98) | −0.074 (−0.14 to −0.013) |
| Local population of subject residence (vs metropolitan, >1 000 000 residents)‡ | ||
| Metropolitan, 250 000–1 000 000 residents | 0.91 (0.65 to 1.28) | −0.016 (−0.059 to 0.027) |
| Metropolitan, <250 000 residents | 1.10 (0.67 to 1.81) | 0.012 (−0.055 to 0.078) |
| Rural | 1.07 (0.51 to 2.24) | 0.008 (−0.092 to 0.11) |
| Urologist density quartile (vs 4 [highest])‡,§ | ||
| 1 (lowest) | 0.89 (0.50 to 1.57) | −0.015 (−0.090 to 0.061) |
| 2 | 0.90 (0.62 to 1.30) | −0.014 (−0.062 to 0.034) |
| 3 | 1.15 (0.82 to 1.61) | 0.021 (−0.022 to 0.065) |
| Health professional shortage area‡ | 1.02 (0.71 to 1.47) | 0.003 (−0.045 to 0.051) |
CI = confidence interval; OR = odds ratio.
Predictive margin is the difference between the predicted probability of cystectomy for the covariate compared with the reference category estimated from the solutions for random effects in the multilevel multivariable model.
Derived from linkage of subject zip code of residence to the Area Resource File.
Quartile 1: five or less urologists per 100 000 persons; quartile 2: six to 26 urologists per 100 000 persons; quartile 3: 27 to 53 urologists per 100 000 persons; quartile 4: 54 or more urologists per 100 000 persons.
Longer travel distances to an available cystectomy provider were associated with a statistically significant reduction in the odds and predicted likelihood of undergoing radical cystectomy compared with short distances. Subjects with requisite travel distances to an available surgeon of at least 50 miles had 40% lower odds (OR = 0.60, 95% CI = 0.37 to 0.98) and 7.4% lower predicted likelihood (95% CI = −14% to −1.3%) of cystectomy compared with those who had to travel 0–4 miles.
Of the variance in this study of receipt of radical cystectomy that could be explained by our model, 31% was attributable to the diagnosing surgeon. Subject-level socioeconomic variables explained 22% of the variance in receipt of cystectomy.
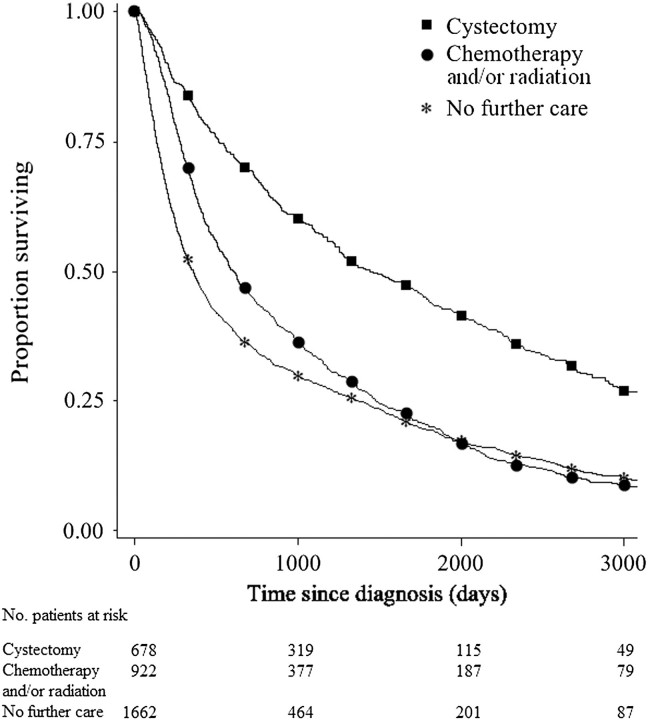
Figure 1 displays the unadjusted survival curves for the three treatment groups. We used conventional Cox proportional hazards models and instrumental variable analyses to compare overall and disease-specific survival by treatment group (Table 3). Median follow-up in SEER for the study sample was 39.0 months for subjects who received cystectomy, 20.3 months for subjects who received chemotherapy and/or radiation, and 12.1 months for subjects who received surveillance. After adjusting for measured and unmeasured differences between cystectomy subjects and subjects who underwent chemotherapy, radiation, or surveillance, the subjects who received cystectomy had better overall and bladder cancer–specific survival (for chemotherapy and/or radiation vs cystectomy, HR of death = 1.5, 95% CI = 1.3 to 1.8; for surveillance vs cystectomy, HR of death = 1.9, 95% CI = 1.6 to 2.3; 5-year adjusted survival: 42.2%, 95% CI = 39.1% to 45.4%, for cystectomy; 20.7%, 95% CI = 18.7% to 22.8%, for chemotherapy and/or radiation; 14.5%, 95% CI = 13.0% to 16.2%, for surveillance). Stratified survival analyses demonstrated no statistically significant interaction between age or sex and treatment group (data not shown).
Figure 1.
Kaplan–Meier unadjusted overall survival curves stratified by treatment received for muscle-invasive bladder cancer (for chemotherapy and/or radiation vs cystectomy, unadjusted hazard ratio of death = 1.8, 95% confidence interval = 1.7 to 1.9; for surveillance vs cystectomy, unadjusted hazard ratio of death = 2.2, 95% confidence interval = 2.0 to 2.3).
Table 3.
Analysis of overall and disease-specific survival following a diagnosis of muscle-invasive bladder cancer*
| Treatment group | Overall survival |
Disease-specific survival |
||||||
| Cox PH | 2SRI |
Cox PH | 2SRI |
|||||
| HR of death (95% CI) | HR of death (95% CI) | 2-y survival, % (95% CI) | 5-y survival, % (95% CI) | HR of death (95% CI) | HR of death (95% CI) | 2-y survival, % (95% CI) | 5-y survival, % (95% CI) | |
| Radical cystectomy | 1.00 (referent) | 1.00 (referent) | 67.4 (65.0 to 69.8) | 42.2 (39.1 to 45.4) | 1.00 (referent) | 1.00 (referent) | 81.1 (78.8 to 83.5) | 66.6 (62.9 to 70.3) |
| Chemotherapy or radiation | 1.43 (1.27 to 1.62) | 1.53 (1.28 to 1.83) | 47.5 (45.2 to 49.8) | 20.7 (18.7 to 22.8) | 1.33 (1.11 to 1.59) | 1.37 (1.01 to 1.77) | 68.3 (65.7 to 71.0) | 48.0 (44.5 to 51.9) |
| Surveillance | 1.68 (1.50 to 1.88) | 1.87 (1.59 to 2.26) | 38.9 (36.9 to 41.1) | 14.5 (13.0 to 16.2) | 1.46 (1.23 to 1.73) | 1.52 (1.16 to 1.97) | 64.2 (61.8 to 66.8) | 43.1 (40.0 to 46.3) |
Overall and disease-specific survival adjusted for subject age, sex, marital status, Charlson comorbidity index, and cancer grade. 2SRI = two-stage residual inclusion estimation instrumental variable analysis; CI = confidence interval; Cox PH = conventional Cox proportional hazards models; HR = hazard ratio.
Discussion
In the context of a health-care system that is burdened by the overuse of costly and possibly unnecessary tests, medications, and procedures, we found marked underuse of guideline-recommended care for invasive bladder cancer. According to NCCN guidelines, the majority of patients with muscle-invasive bladder cancer should undergo radical cystectomy and urinary diversion. However, only 21% of the subjects in the study cohort underwent radical surgery. This pronounced underuse of recommended care may harm patients who receive alternative treatment regimens. Subjects who underwent chemotherapy, radiation, or surveillance for their muscle-invasive bladder cancer had a higher hazard of death over time than those who underwent radical cystectomy.
Unlike many contemporary surgical procedures for which guidelines may lag behind evidence for their preferred use (22), national guidelines for the management of muscle-invasive bladder cancer have recommended radical cystectomy for years (30). Nevertheless, our data indicate that much of the variance in the receipt of radical cystectomy is attributable to the diagnosing surgeon. Some of this variance may reflect the evidence base behind the NCCN guidelines: Its recommendation for cystectomy is based on expert opinion rather than on data from randomized clinical trials. Because the evidence base for the cystectomy recommendation is limited, providers may have substantial uncertainty about the best clinical management of muscle-invasive bladder cancer patients. Other barriers may also preclude widespread adoption of cystectomy at the provider level. For example, the primary urologist may be unwilling to offer radical cystectomy and urinary diversion. It is a complex procedure with an operative time ranging from 4 to 8 or more hours depending on the cancer severity and the amount of urinary reconstruction involved. Medicare reimbursement rates for this procedure, adjusted for inflation, declined by 32% from 1995 to 2004 (31). Because most physicians operate essentially as small business owners, the economic incentive to do less risky but better remunerated work in the clinic or in an ambulatory surgery center may compel them to avoid providing cystectomy. Beyond financial concerns, radical cystectomy confers high rates of morbidity and mortality (32) and requires intensive perioperative nursing care as patients learn to manage their new urinary reconstructions. High-volume providers may have the infrastructure to manage this care efficiently and effectively, whereas low-volume providers may be dissuaded by these concerns. If, for any reason, the primary urologist is not comfortable performing cystectomy, the patient must be referred, typically to a tertiary academic center, for appropriate treatment.
To a large extent, such regionalization of cystectomy care has already occurred (33) and may represent a positive change: Volume–outcome studies for radical cystectomy suggest lower rates of mortality for patients who undergo surgery by high-volume providers (34–36). Conversely, regionalization of radical cystectomy may explain the low rates of cystectomy documented in this study. The reduction of available surgeons is likely to differentially affect patients who live in urban vs rural underserved areas, which could exacerbate existing disparities in bladder cancer care. Rural patients who receive care at a tertiary referral center may have to return to their primary urologist for follow-up care, which could create a burden that many community urologists may be unwilling to accept. This transition of care may harm patients; readmissions to secondary hospitals for other complex procedures were found to be associated with worse morbidity and mortality outcomes compared with readmission to the hospital where the surgery was performed (37). Although regionalization of cystectomy care may benefit those who undergo surgery, the larger impact of this process may be to compromise invasive bladder cancer care.
We found that longer travel distance was associated with lower odds of radical cystectomy for invasive bladder cancer. Regionalization of cystectomy care evokes concern about urban–rural disparities in health-care delivery, a concern that is validated by our findings. Patients who had to travel more than 50 miles to an available surgeon had a lower likelihood of undergoing cystectomy compared with patients who had shorter travel distances. Rural patients are known to have reduced access to cancer screening services and surgical treatment after diagnosis (38–40). Long travel times to providers further limit their treatment options (41). Rural cancer patients also tend to be older than urban cancer patients, which may further restrict their access to transportation and affect their medical candidacy for needed complex surgical services (42). Our findings confirm those of a previous study (20) that reported that patients sacrifice survival benefits—in our study, those conferred by surgery for invasive bladder cancer—when confronted with an extensive travel burden.
Patient factors were also associated with the treatment that was received. Younger healthier patients had higher odds, independent of the other covariates in our model, of undergoing radical cystectomy for their invasive bladder cancers. It is understandable that surgery is more common in patients who are better able to withstand the medical stress of a long surgery and sufficiently functionally capable of adapting to their new urinary tract reconstructions. Furthermore, older age and increased comorbidity are often intertwined (43). However, prognosis for patients with invasive bladder cancer is more highly correlated with cancer severity than with patient age (44,45). Moreover, radical cystectomy has previously demonstrated survival benefits even among the elderly (46). Thus, although age and comorbidity should determine medical candidacy for this surgery, the majority of invasive bladder cancer patients without contraindication to prolonged anesthesia should undergo potentially curative radical surgery.
Our results beg the question that underlies all studies that identify disparate or inappropriate care: What next? How do we ensure broader use of radical surgery for invasive bladder cancer? Guidelines confirm that radical cystectomy is the gold standard treatment for invasive bladder cancer. Our results support that designation: patients who received cystectomy had substantially better survival outcomes compared with patients who received alternative treatments. So how should we address the dual reluctance of providers to commit to managing this complex surgery and of patients to submitting to its life-altering consequences? Although a regionalization of cystectomy care resulting from established physician referral patterns and patient self-referrals may have contributed to the underuse we document, some form of organized regionalization of cystectomy may be an answer. For example, invasive bladder cancer care could revolve around a prescribed network of cystectomy providers to which all patients would have access rather than being dependent on the primary urologist and his or her clinical biases, tendencies, and local referral practices. Patients themselves must also become more empowered in the decision-making process. It seems unlikely that a majority of patients would willingly select a treatment (ie, a treatment other than cystectomy) with up to an 80% increase in the risk of death over time. Increasing the health literacy of bladder cancer patients may increase the proportion that chooses to undergo radical cystectomy.
This study has a number of limitations that may have biased the results. First, restriction of the study sample to Medicare beneficiaries may compromise the generalizability of the results. However, the majority of incident bladder cancers occur in Medicare-aged men and women (47). Second, patients may have appropriately received treatments other than cystectomy on the basis of patient clinical characteristics and life expectancy. Although we attempt to capture patient comorbidity through Medicare claims data, this approach provides an imperfect estimation. Patients who did not receive cystectomy may have had more severe comorbidity than could be identified by using a claims-based methodology. Third, chemotherapy use was underreported in 2004 and 2005 in the SEER–Medicare sample that we analyzed. However, because our cohort included cancers diagnosed through 2002, this underreporting is likely to have had a very small effect on the distribution of patients to the chemotherapy and/or radiation and surveillance treatment groups. Fourth, we excluded patients with stage III or IV cancers (ie, cancers that extend into the perivesical fat or invade local structures and metastatic cancers) to minimize the number of patients who received appropriate alternative treatment regimens, such as chemotherapy. However, cancer stage in SEER is classified at diagnosis and may not capture clinical understaging. Many patients in our sample may have been found to have stage III or IV cancers at the time of cystectomy. Fifth, any comparison of treatment groups derived from nonrandomized cohorts as in this study is prone to bias, especially from unmeasured confounders. We attempted to adjust for both the measured differences among treatment groups, such as age and comorbidity, and the unmeasured group differences by using an instrumental variable analysis. We contend that the two-stage residual inclusion estimation that we applied to the data, a technique that has been shown to be reliable for addressing selection bias in observational data (28), permits a valid interpretation of our survival outcomes.
Sixth, we could not account for patient preferences in care. Radical cystectomy is a life-altering intervention because it requires urinary tract reconstruction. Although most cancer surgeries require convalescence, few require such a profound alteration to daily living as changing how one empties the bladder. Patients who receive an incontinent intestinal conduit reconstruction have a urostomy that is covered with a bag that collects the voided urine. Those who undergo continent urinary diversion with a neobladder void either by performing the Valsalva maneuver to increase abdominal pressure or through intermittent self-catheterization. Some radical cystectomy patients may also incur some degree of bowel dysfunction depending on the length of intestine that was used in their reconstruction (48). Sexual dysfunction is nearly universal among patients who have undergone radical cystectomy (48). It can be difficult to convince a patient to sacrifice these aspects of quality of life, despite the proven benefit of cystectomy to the quantity of life. That balance, between quantity and quality of life, may drive many of the decisions that underlie the underuse of cystectomy that we have documented. Even with radical surgery or radiation therapy, survival after an invasive bladder cancer diagnosis is limited. Quality of life may be paramount in patients’ minds as they opt for less beneficial therapies that offer bladder preservation.
Finally, our results conflict with those of population-based studies from other countries that demonstrated similar survival outcomes between cystectomy and radiation therapy treatment groups. For example, population-based cancer registries in the United Kingdom and Canada have shown equivalent survival outcomes for surgery and radiation on comparative retrospective analyses (49–52). Yet the majority of patients in these countries receive radiation therapy as the initial treatment with curative intent. The patients who underwent cystectomy had higher rates of lymph node involvement (52) or locally advanced stage T4 cancers compared with patients who received radiation, thereby predisposing cystectomy patients to worse survival outcomes (49,51). Furthermore, these analyses did not address known and unmeasured imbalances between these treatment groups nor did they stratify survival outcomes by clinical stage at presentation.
Despite these limitations, we demonstrated substantial underuse of radical cystectomy for muscle-invasive bladder cancer. This underuse of guideline-recommended care may have condemned patients who received alternative treatments to premature mortality. Maximizing the number of patients who receive appropriate care for muscle-invasive bladder cancer requires overcoming the formidable obstacles of patient and provider resistance to this procedure. To increase the use of radical cystectomy and urinary diversion for invasive bladder cancer, we must ensure that patients have access to available surgeons and promote patient knowledge so they may fully understand the risks and benefits of their treatment options.
Funding
Collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. This work was supported by the UCLA Robert Wood Johnson Clinical Scholars Program and the National Institute of Diabetes and Digestive and Kidney Diseases N01-DK-7-0003 (Principal investigator: M.S.L.).
Footnotes
The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health the National Cancer Institute (NCI), and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services (IMS), Inc; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
All authors had access to the data. No authors endorse conflicts of interest or financial relationships that affect publication of this manuscript. The sponsor had no role in the data analysis or construction of the manuscript. This work was previously presented at the 2009 American Urological Association Annual Meeting.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Montie JE, Clark PE, Eisenberger MA, et al. Bladder cancer. J Natl Compr Canc Netw. 2009;7(1):8–39. doi: 10.6004/jnccn.2009.0002. [DOI] [PubMed] [Google Scholar]
- 3.Schoenberg MP, Gonzalgo ML. Management of invasive and metastatic bladder cancer. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelphia, PA: Saunders; 2006. pp. 2468–2478. [Google Scholar]
- 4.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. The regionalization of radical cystectomy to specific medical centers. J Urol. 2005;174(4, pt 1):1385–1389. doi: 10.1097/01.ju.0000173632.58991.a7. discussion 1389. [DOI] [PubMed] [Google Scholar]
- 5.Montie JE, Abrahams NA, Bahnson RR, et al. Bladder cancer. Clinical guidelines in oncology. J Natl Compr Canc Netw. 2006;4(10):984–1014. doi: 10.6004/jnccn.2006.0083. [DOI] [PubMed] [Google Scholar]
- 6.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–748. [PubMed] [Google Scholar]
- 7.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 8.Hofferkamp JE. Standards for Cancer Registries Volume III: Standards for Completeness, Quality, Analysis, Management, Security, and Confidentiality of Data. Springfield, IL: North American Association of Central Cancer Registries; 2008. [Google Scholar]
- 9.ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification, Sixth Edition [computer program]. Version Official. Washington, DC: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Health Care Financing Administration; 1997. [Google Scholar]
- 10.American Medical Association. Current Procedural Terminology: CPTStandard Edition. Chicago, IL: American Medical Association; 1998. [Google Scholar]
- 11.Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351–1362. [PMC free article] [PubMed] [Google Scholar]
- 12.Kravet SJ, Shore AD, Miller R, Green GB, Kolodner K, Wright SM. Health care utilization and the proportion of primary care physicians. Am J Med. 2008;121(2):142–148. doi: 10.1016/j.amjmed.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(117):1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 14.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Best AE. Secondary data bases and their use in outcomes research: a review of the area resource file and the Healthcare Cost and Utilization Project. J Med Syst. 1999;23(3):175–181. doi: 10.1023/a:1020515419714. [DOI] [PubMed] [Google Scholar]
- 19.Haynes R, Jones AP, Sauerzapf V, Zhao H. Validation of travel times to hospital estimated by GIS. Int J Health Geogr. 2006;5:40. doi: 10.1186/1476-072X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: implications for regionalization. Med Care. 1999;37(2):204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 22.Miller DC, Saigal CS, Banerjee M, Hanley J, Litwin MS. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112(8):1708–1717. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London, UK: Sage Publications; 1999. [Google Scholar]
- 24.Subramanian S, Jones K, Duncan C. Multilevel methods for public health research. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. Oxford, UK: Oxford University Press; 2003. pp. 65–111. [Google Scholar]
- 25.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 26.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health Serv Res. 2008;43(3):1102–1120. doi: 10.1111/j.1475-6773.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wooldridge JM. Introductory Econometrics: A Modern Approach. 3rd ed. Mason, OH: Thomson/South-Western; 2006. [Google Scholar]
- 30.Scher H, Bahnson R, Cohen S, et al. NCCN urothelial cancer practice guidelines. National Comprehensive Cancer Network. Oncology (Williston Park) 1998;12(7A):225–271. [PubMed] [Google Scholar]
- 31.Lotan Y, Cadeddu JA, Roehrborn CG, Stage KH. The value of your time: evaluation of effects of changes in Medicare reimbursement rates on the practice of urology. J Urol. 2004;172(5, pt 1):1958–1962. doi: 10.1097/01.ju.0000142016.51680.fa. [DOI] [PubMed] [Google Scholar]
- 32.Dahl DM, McDougal WS. Use of intestinal segments in urinary diversion. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelphia, PA: Saunders; 2006. pp. 2534–2578. [Google Scholar]
- 33.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. Regionalization of radical cystectomy. J Urol. 2004;171(4 suppl) doi: 10.1097/01.ju.0000173632.58991.a7. 36. Abstract 137. [DOI] [PubMed] [Google Scholar]
- 34.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 35.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69(5):871–875. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005;173(5):1695–1700. doi: 10.1097/01.ju.0000154638.61621.03. [DOI] [PubMed] [Google Scholar]
- 37.Ryoo JJ, Kunitake H, Frencher SK, et al. Continuity of care: readmission to the same hospital following gastric cancer resection. J Am Coll Surg. 2009;209(3):S16–17. [Google Scholar]
- 38.Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390–399. doi: 10.1111/j.1748-0361.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroen AT, Brenin DR, Kelly MD, Knaus WA, Slingluff CL., Jr Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol. 2005;23(28):7074–7080. doi: 10.1200/JCO.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Tropman SE, Ricketts TC, Paskett E, Hatzell TA, Cooper MR, Aldrich T. Rural breast cancer treatment: evidence from the Reaching Communities for Cancer Care (REACH) project. Breast Cancer Res Treat. 1999;56(1):59–66. doi: 10.1023/a:1006279117650. [DOI] [PubMed] [Google Scholar]
- 41.Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer. 2008;44(7):992–999. doi: 10.1016/j.ejca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Virnig BA, Moscovice IS, Durham SB, Casey MM. Do rural elders have limited access to Medicare hospice services? J Am Geriatr Soc. 2004;52(5):731–735. doi: 10.1111/j.1532-5415.2004.52213.x. [DOI] [PubMed] [Google Scholar]
- 43.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol. 2003;170(5):1765–1771. doi: 10.1097/01.ju.0000091620.86778.2e. [DOI] [PubMed] [Google Scholar]
- 45.Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: comparison of survival between younger and older patients. Cancer. 2005;103(3):546–552. doi: 10.1002/cncr.20805. [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck BK, Miller DC, Taub D, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64(2):292–297. doi: 10.1016/j.urology.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert SM, Wood DP, Dunn RL, et al. Measuring health-related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI) Cancer. 2007;109(9):1756–1762. doi: 10.1002/cncr.22556. [DOI] [PubMed] [Google Scholar]
- 49.Chahal R, Sundaram SK, Iddenden R, Forman DF, Weston PM, Harrison SC. A study of the morbidity, mortality and long-term survival following radical cystectomy and radical radiotherapy in the treatment of invasive bladder cancer in Yorkshire. Eur Urol. 2003;43(3):246–257. doi: 10.1016/s0302-2838(02)00581-x. [DOI] [PubMed] [Google Scholar]
- 50.Hayter CR, Paszat LF, Groome PA, Schulze K, Mackillop WJ. The management and outcome of bladder carcinoma in Ontario, 19821994. Cancer. 2000;89(1):142–151. [PubMed] [Google Scholar]
- 51.Munro NP, Sundaram SK, Weston PM, et al. A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in Yorkshire, UK. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 52.Scrimger RA, Murtha AD, Parliament MB, et al. Muscle-invasive transitional cell carcinoma of the urinary bladder: a population-based study of patterns of care and prognostic factors. Int J Radiat Oncol Biol Phys. 2001;51(1):23–30. doi: 10.1016/s0360-3016(01)01591-7. [DOI] [PubMed] [Google Scholar]