Abstract
During development and disease, the exocytosis of signalling molecules, such as Wnt ligands, is essential to orchestrate cellular programs in multicellular organisms. However, it remains a largely unresolved question whether signalling molecules follow specialized transport routes through the exocytic pathway. Here we identify several Drosophila p24 proteins that are required for Wnt signalling. We demonstrate that one of these p24 proteins, namely Opossum, shuttles in the early secretory pathway, and that the Drosophila Wnt proteins are retained in the absence of p24 proteins. Our results indicate that Wnt secretion relies on a specialized anterograde secretion route with p24 proteins functioning as conserved cargo receptors.
Keywords: Wnt secretion, p24 proteins, protein transporting, signalling
Introduction
Wnt signalling has been implicated in many developmental processes, such as tissue homeostasis, axis patterning and the maintenance of stem cells, and is misregulated in several types of cancer (van Amerongen et al, 2008; Angers & Moon, 2009). Although much progress has been made in dissecting the intracellular Wnt signalling cascade, open questions remain whether and how Wnt ligands follow specialized exocytic pathways. Wnts are secreted as palmitoylated glycoproteins that require the multi-pass transmembrane protein Evenness interrupted (Evi/Wls) for post-Golgi transfer to the plasma membrane (Banziger et al, 2006; Bartscherer et al, 2006), and Retromer, a multiprotein sorting complex, for plasma membrane-to-Golgi recycling of Evi and the maintenance of Wnt secretion (Belenkaya et al, 2008; Franch-Marro et al, 2008; Port et al, 2008). Although past studies have mainly focused on this recycling pathway, it still remained to be resolved how Wnt proteins are transported through the early secretory pathway to the Golgi apparatus.
Here, we identify members of the p24 protein family, also known as EMP24/GP25L/Erp (endomembrane protein precursor of 24 kD) proteins, as being required for secretion of Wnt proteins, namely Wingless (Wg) and Wnt inhibitor of Dorsal (WntD). p24 proteins have been proposed to be cargo-adaptor proteins implicated in bidirectional transport processes at the endoplasmic reticulum (ER)–Golgi interface (Carney & Bowen, 2004; Strating & Martens, 2009). A specific association of p24 proteins with cargo molecules has also been shown for yeast.
We show that Opossum (Opm), a conserved member of the γ-subfamily of p24 proteins and two other Drosophila p24 proteins, CHOp24 and p24-1, are required in Wnt-producing cells for ER export of Wg. Our results indicate that p24 proteins might function as early cargo receptors upstream of Evi in a selective anterograde secretory route of Wnts.
Results
Opm is a conserved regulator of Wnt signalling
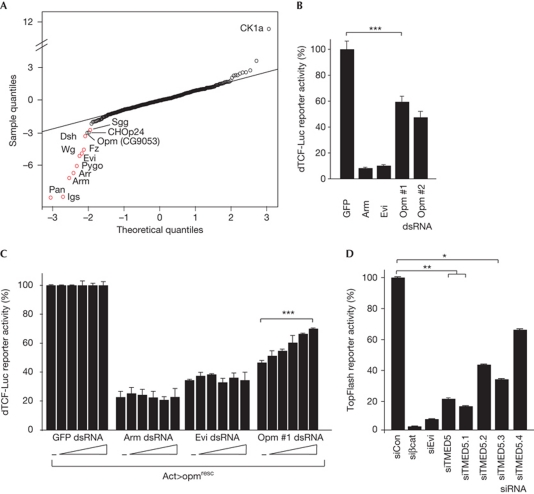
To dissect Wnt protein transporting, we analysed the effect of depleting 466 components of the cellular transporting machinery (supplementary Table S1 online) by RNA interference (RNAi) in Drosophila cells. Using a new Wg-specific luciferase reporter, we identified CG9053, named Opm, as being required for Wg signalling (Fig 1A). Depletion of Opm using independent double-stranded RNAs (dsRNAs) significantly affected Wg signalling in different cell lines and Wnt-reporter assays (Fig 1B; supplementary Fig S1A–C online). An RNAi-insensitive rescue construct (opmresc) significantly reverted the loss of Wg-reporter activity (Fig 1C; supplementary Fig S1D online).
Figure 1.
Opm is a conserved positive regulator of Wg signalling. (A) Graphical representation of screening results as Q–Q plot of normally distributed quantiles against screening result quantiles. A perfect fit to a normal distribution is represented by a line. Values represent z-scores. Known positive regulators are highlighted in red. (B) dTCF-Luc reporter assay in S2R+ cells stimulated by overexpression of Wingless (Wg), in the presence of the indicated double-stranded RNAs (dsRNAs; ***P<0.0001, paired Student's t-test). (C) dTCF-Luc reporter system in Kc167 cells in the presence of increasing amounts of opmresc (***P<0.0001, analysis of variance test). (D) TOPFlash reporter assay in Hek293T cells activated by overexpression of mWnt1, in the presence of the indicated short interfering RNAs (siRNAs; **P<0.005, *P<0.05, paired Student's t-test). Data are shown as mean±s.d. of at least three independent experiments. Evi, Evenness interrupted; GFP, green fluorescent protein; opm, opossum.
Opm is a highly conserved type1a transmembrane protein of the conserved γ-subfamily of p24 proteins (supplementary Fig S1E online). Short interfering RNAs (siRNAs) targeting its human homologue, TMED5 (transmembrane emp24 protein transport containing domain 5), also known as CGI-100, significantly reduced Wnt-induced TOPFlash reporter activity in HEK293T cells that were activated by overexpression of mWnt1 (Fig 1D), confirming that Opm is a conserved regulator of canonical Wnt/Wg signalling.
Opm shuttles in the early secretory pathway
p24 proteins have been proposed to be recycling cargo adaptor proteins, as they are abundantly present in membranes of the early secretory pathway (Carney & Bowen, 2004; Strating and Martens, 2009). Therefore, we first examined the subcellular localization of an Opm–enhanced green fluorescent protein fusion protein, which colocalized with the ER marker KDEL in the Drosophila follicular epithelium. Similarly, TMED5–enhanced green fluorescent protein colocalized to the ER, the ER–Golgi intermediate compartment (ERGIC) and partially to the cis-Golgi network in HeLa cells. In accordance with a previous report (Koegler et al, 2010), TMED5 colocalized to ERGIC53-positive vesicles in cells treated with nocodazole and Brefeldin A, indicating that it shuttles in the early secretory pathway (supplementary Fig S2A,B online).
Loss of Opm does not affect general protein secretion
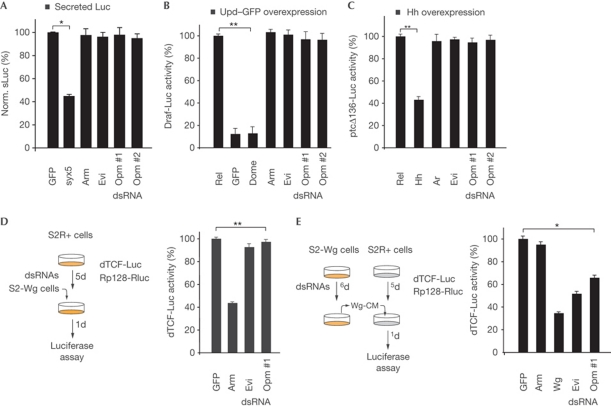
To test whether TMED5 is required for general protein transporting, we performed a vesicular stomatitis virus G-protein secretion assay. In accordance with a recent study (Koegler et al, 2010), vesicular stomatitis virus G-protein–GFP was detected at the plasma membrane in TMED5-depleted cells (supplementary Fig S2C online). Similar results were obtained with a secreted version of firefly luciferase (Fig 2A). In addition, both a JAK/signal transducers and activators of transcription (STAT)- and Hedgehog (Hh)-specific reporter assay were unaffected by opm depletion (Fig 2B,C), indicating that Opm is not required for global protein secretion.
Figure 2.
Opm functions in the Wg-secreting cell. (A) sLuc secretion assay in the presence of the indicated double-stranded RNAs (dsRNAs). Syntaxin 5 (syx5) serves as control. (B,C) JAK/signal transducers and activators of transcription- and Hedgehog (Hh)-specific reporter assays in the presence of the indicated dsRNAs. (D) S2R+ cells treated with the indicated dsRNAs (highlighted in yellow) and transfected with the dTCF-Luc reporter system were mixed with S2-Wg cells. Time of treatment in days (d) is indicated. (E) S2R+ cells activated with Wingless (Wg)-conditioned medium collected from S2-Wg cells (Wg-CM) pretreated with the indicated dsRNAs (highlighted in yellow). Data are shown as mean±s.d. of at least three independent experiments (*P<0.001, **P<0.0001, paired Student's t-test). Evi, Evenness interrupted; GFP, green fluorescent protein; opm, opossum; sLuc, secreted version of firefly luciferase; Upd, unpaired.
Epistatically, Opm function was mapped to the Wnt/Wg-secreting cells as cell-mixing experiments revealed that Opm function is not required for signal transduction in the receiving cell (Fig 2D–E).
Opm is required for Wg function in vivo
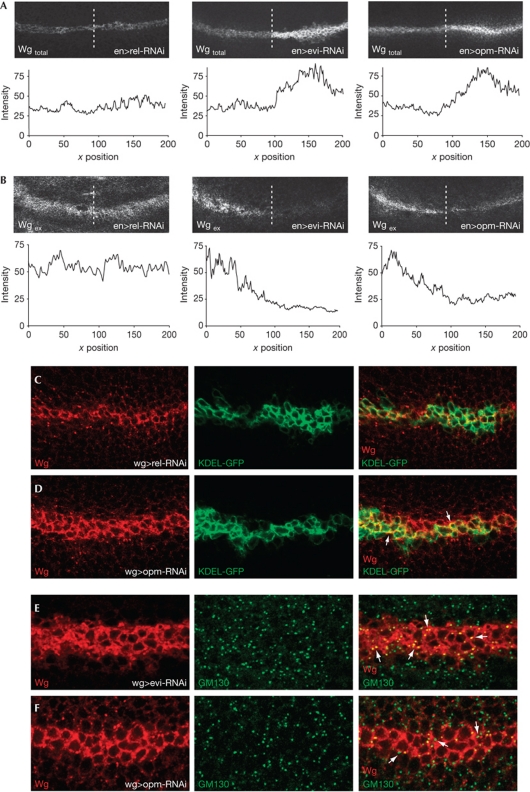
To determine the requirement of Opm for Wg signalling in vivo, we depleted opm by tissue-specific RNAi in the developing Drosophila wing disc. Total Wg protein accumulated in Wg-expressing cells of the opm-depleted tissue, whereas the extracellular Wg gradient was significantly reduced (Fig 3A,B). Furthermore, Wg colocalized to a higher extent with the ER marker KDEL, but not the Golgi marker GM130, as observed for evi-deficient cells (Fig 3C–F; supplementary Fig S3A online). These experiments suggest that Wg does not reach the Golgi apparatus in the absence of Opm.
Figure 3.
Opm is required for Wg secretion in vivo. (A,B) Total and extracellular Wg staining of larval wing discs of the indicated genotypes (n>5 for all genotypes). (C,D) Total Wg protein staining of larval wing discs colabelled with KDEL-GFP. Quantification of the degree of colocalization for n=3 was done through the JACoP plugin in ImageJ, resulting in an average Pearson's coefficient of P=0.56 and P=0.68 for rel-RNAi and opm-RNAi, respectively, which supports an enhanced colocalization in the ER on opm-RNAi. To complement the analysis, Mander's correlation coefficients were calculated (M=0.47 for rel-RNAi versus M=0.59 for opm-RNAi). (E,F) Total Wg protein staining of third-instar larval wing discs co-stained with GM130 (n=5 for all genotypes). ER, endoplasmic reticulum; Evi, Evenness interrupted; GFP, green fluorescent protein; opm, opossum; RNAi, RNA interference; Wg, Wingless.
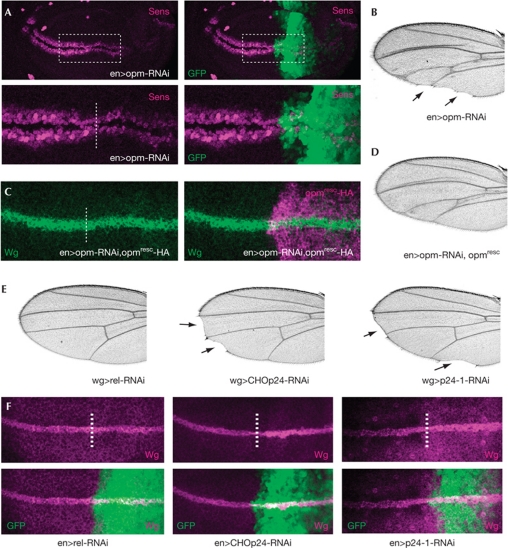
We next examined the Wg short-range target Senseless (Sens), which was reduced in opm-depleted cells leading to adult wing margin defects that phenocopy a loss of Wg function (Fig 4A,B). However, opm depletion seems not to block Wg secretion completely as the observed phenotypes are milder compared with evi knockdown, indicating that opm might function in part redundantly with other factors.
Figure 4.
p24 proteins are required for Wg secretion. (A) Sens staining of wing discs of the genotype enGal4, UAS-GFP/UAS-opm-RNAi (n=5 analysed). GFP marks the RNAi expression domain. The dashed line indicates the anterior/posterior (A/P) boundary. (B) Adult wing of the genotype enGal4/UAS-opm-RNAi (n>50 analysed). Note the characteristic wing margin defects. (C) Total Wg staining of third-instar larvae of the genotype: enGal4/UAS-opm-RNAi;UAS-opmresc-HA/+. The dashed line indicates the A/P boundary. (D) Adult wing of the genotype enGal4/UAS-opm-RNAi;UAS-opmresc/+ (n>50 analysed). (E) Adult wings of the genotypes wgGal4/UAS-relRNAi, wgGal4/UAS-CHOp24-RNAi and wgGal4/+; UAS-p24-1-RNAi/+ (n>100 analysed). Note the wing margin defects and the loss of sensory bristles (arrows). (F) Total Wingless (Wg) staining of wing discs of the indicated genotypes, anterior to the left: enGal4,UAS-GFP/UAS-relRNAi, enGal4,UAS-GFP/UAS-CHOp24-RNAi, enGal4,UAS-GFP/UAS-p24-1-RNAi (n=4 analysed). GFP marks the RNAi expression domain; the dashed line indicates the A/P boundary. GFP, green fluorescent protein; HA, haemagglutinin; opm, opossum; RNAi, RNA interference; Sens, Senseless.
To test for the specificity of the phenotype, we first examined the Hh target gene Patched in opm-depleted wing discs, which was unaffected, confirming that Opm is not required for Hh signalling (supplementary Fig S3B online). Second, we rescued the effect on Wg protein by expression of an RNAi-insensitive opmresc construct in the opm-RNAi background, which restores total Wg protein to almost wild-type amounts and partially rescues the RNAi-induced wing margin defects (Fig 4C,D; supplementary Fig S3C,D online).
As Opm is a member of the p24 protein family consisting of nine predicted members in Drosophila, we systematically analysed RNAi-induced phenotypes of other p24 proteins in vivo (supplementary Table S2 online). Knockdown of CHOp24 (CG3564 or p24/p24β1) and p24-1 (CG1967 or p26/p24γ4) caused wing margin defects mimicking opm depletion and an accumulation of total Wg protein (Fig 4E,F). However, as we were not able to completely rescue the observed wing margin defects of opm-RNAi animals, we analysed the expression of other p24 transcripts. Indeed, we observed both reduction and upregulation of several p24 transcripts on knockdown of a single p24 protein (supplementary Fig S3E online), indicating that several p24 proteins might be affected and suggesting a cross-regulatory compensation by several p24 proteins on knockdown of a single family member. In addition, we cannot exclude changes in protein expression of p24s causing a combined RNAi phenotype. Taken together, our results strongly indicate that several p24 proteins contribute to Wnt secretion and might have partially redundant functions.
Opm is required for embryonic development
To further analyse the role of opm during development, we generated a loss-of-function allele by homologous recombination (opm9.3; 13A on the X chromosome). Despite the fact that offspring of homozygous opm9.3 mutant females die early during embryogenesis due to severe patterning defects (supplementary Fig S4A,B online), opm9.3 homozygous mutants are viable, suggesting a strong maternal contribution, which has indeed been reported for most of the p24 proteins (Boltz et al, 2007). Consistently, we found that one wild-type allele of opm is sufficient to rescue the embryonic lethality of progeny of opm9.3/opm9.3 females. However, the phenotype of opm9.3 seems to be more complex and does not resemble the classical Wg loss-of-function phenotype, indicating that its function might affect more processes and is possibly redundant with other p24 proteins.
We next tested whether the secretion of Wg is affected in mitotic opm mutant clones in wing imaginal discs. Total Wg protein was increased in large clones of opm mutant tissues compared with adjacent wild-type tissues, and Sens was reduced, although residual Sens remained detectable (supplementary Fig S4C,D online), confirming that Wg is retained in the absence of opm. However, the block of Wg secretion is incomplete compared with the strong phenotype of evi mutants (Banziger et al, 2006; Bartscherer et al, 2006). Furthermore, we did not observe a significant change in the expression of Sens in small or medium-sized opm9.3 mutant clones, indicating a potential rescue effect from wild-type tissue or a partial redundancy with other p24 proteins.
Opm is required for Wg secretion
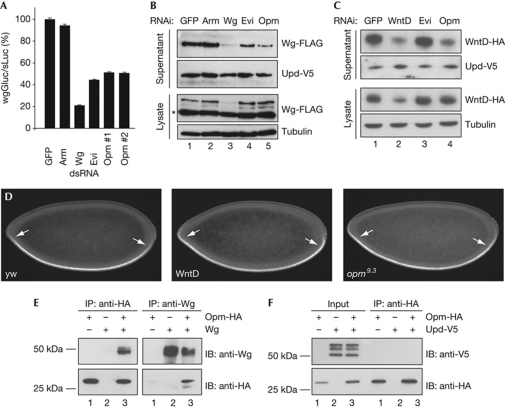
To further confirm a role of Opm in Wg secretion, we analysed the effect of opm depletion on a Wg–Gaussia luciferase (Wg–Gluc) fusion protein and secreted Wg protein. Opm-RNAi reduced both secretion of Wg–Gluc and FLAG-tagged Wg, whereas the Drosophila JAK/STAT ligand Unpaired (Upd) was secreted normally (Fig 5A,B; supplementary Fig S5A online), suggesting a specific requirement for Opm in Wg, but not Upd, secretion.
Figure 5.
Opm is required for Wnt secretion. (A) Wingless (Wg)–Gluc secretion assay normalized to sLuc (Wg–Gluc/sLuc). Data are shown as mean±s.d. (*P<0.0001, Student's t-test, n=4). (B) Western blot of Wg-FLAG in supernatants and lysates of S2 wg-FLAG cells in the presence of the indicated double-stranded RNAs (dsRNAs); Unpaired (Upd)-V5 serves as loading control. The asterisk indicates a non-specific band observed in all lysates. (C) Wnt inhibitor of Dorsal (WntD) secretion assay in S2 cells transfected with WntD-HA, in the presence of the indicated dsRNAs. (D) Lateral view of stage 4 embryos of the indicated genotypes stained for anti-Dorsal, anterior to the left (n>30 for each genotype). Note the extension of nuclear Dorsal at the termini for WntD (94% of embryos, P<0.001) and opm9.3 (77% of embryos, P<0.001, χ2-test). (E,F) Co-IPs of Opm-HA and Wg or Upd-V5 from lysates of transfected S2 cells. Evi, Evenness interrupted; GFP, green fluorescent protein; Gluc, Gaussia luciferase; HA, haemagglutinin; IB, immunoblot; IP, immunoprecipitation; Luc, secreted version of firefly luciferase; opm, opossum; RNAi, RNA interference; Upd, unpaired; yw, yellow white control embryos.
We next analysed the secretion of WntD, a distant member of the Drosophila Wnt family, that is not palmitoylated as other Wnts and is secreted in an Evi-independent manner (Gordon et al, 2005; Ching et al, 2008). In contrast to Evi-depleted cells, WntD secretion was significantly impaired in opm-deficient cells (Fig 5C; supplementary Fig S5B,C online), indicating that opm is required for Wg and WntD secretion. We confirmed this by comparing opm9.3 and WntD early embryonic phenotypes. opm9.3 mutants phenocopy the extended gradient of nuclear Dorsal of WntD mutants (Gordon et al, 2005) and the altered expression of the terminal gap genes tailless and huckebein (Fig 5D; supplementary Fig S5D online). We speculate that these defects in WntD signalling might also mask a Wg loss-of-function phenotype, resulting in a complex, partially overlapping phenotype in opm mutants.
As Opm mainly localized to the ER and ERGIC functioning in the ligand-secreting cell, we proposed that it might interact with Wnt proteins. Co-immunoprecipitation experiments in transfected cells supported our model that Opm interacts with Wg, although this interaction might be indirect, leading to an altered Wg localization and significantly smaller amounts of plasma membrane-bound Wg in opm-deficient cells (Fig 5E; supplementary Fig S5E online). We were not able to demonstrate a biochemical interaction of Opm with WntD, Por or Evi. Opm did not interact with the secreted JAK/STAT ligand Upd or with a control ER-resident transmembrane protein (Fig 5F; supplementary Fig S5F online). However, we cannot exclude a requirement of p24 proteins in transporting other signalling components, which remains to be investigated.
Discussion
Different models have been proposed for cargo transport along the exocytic pathway. The bulk flow model proposes a passive transport of proteins, that is, unless retention mechanisms interfere. In contrast, other models require receptor-mediated export from the ER, where retention is the default unless cargo is associated with specific receptors that mediate sorting into transport vesicles.
The data presented here favour the latter view that Wnt proteins require receptors from the p24 protein family, a class of highly conserved transmembrane proteins, to exit from the ER. We identified several p24 proteins involved in Wnt transporting and show that Opm is required for secretion of the Drosophila Wnt proteins Wg and WntD. We also show that the human homologue of Opm is required for Wnt signalling. Similarly, at least two other p24 proteins, CHOp24 and p24-1, are required for Wg secretion, as knockdown of these p24 proteins phenocopies previously reported Evi loss-of-function phenotypes, such as cell-autonomous retention of Wg in secreting cells. Our results indicate that Wnt secretion relies on a specialized anterograde route with several p24 proteins functioning as highly conserved cargo receptors. The requirement of several Drosophila p24 proteins for Wnt secretion was also demonstrated in a recent report by Port et al (2011). p24 proteins are one of the first examples of Wnt growth factor-specific cargo receptor of the early secretory pathway. However, it remains to be investigated whether these p24 proteins function as complexes as previously proposed (Carney & Bowen, 2004).
Until now, the biological function of p24 proteins has been elusive, and only few studies have characterized p24 mutant phenotypes. Whereas in yeast and Caenorhabditis elegans p24 proteins have no effect on viability and morphology, two Drosophila p24 proteins have been shown to be maternal-effect lethal and are required for the activity of maternally expressed thickvein (Bartoszewski et al, 2004). Our data indicate that several p24 proteins might be involved in transporting Wnt proteins or might function synergistically in accordance with a proposed oligomeric behaviour (Carney & Bowen, 2004). Recent studies in yeast have implicated p24 proteins in the export of glycosyl phosphatidylinositol-anchored proteins, linking the cargo protein to the COPII coat (Castillon et al, 2011). Whether a similar or divergent mechanism underlies the export of Wnt proteins remains to be investigated. Although we could show a biochemical interaction of Opm and Wg, we have so far failed to detect a similar interaction with WntD. Therefore, we cannot at present exclude the possibility that the effect on the secretion of WntD might be indirect.
Although several p24 proteins seem to be affected by RNAi, only a single p24 protein seems to be altered in opm9.3 mutants, possibly explaining the weak Wg loss-of-function phenotype. In addition, the observed phenotypes are milder than evi loss of function, indicative of an incomplete block of Wg secretion. We speculate that p24 proteins function partially redundantly, and that more p24 proteins contribute to Wg secretion in a tissue-specific manner, which would be consistent with the report by Port et al (2011). It is also likely that a regulatory compensation mechanism balances p24 levels as suggested previously (Boltz & Carney, 2008; Takida et al, 2008).
In summary, our data support a model in which Wnt proteins follow a specific route of secretion and require specialized transport components upstream of the Golgi network. Our data also indicate that temporally and spatially precisely coordinated Wnt activity can only be achieved by receptor-mediated recruitment of Wnt proteins into the secretory pathway. p24 proteins seem to have a crucial role by functioning as cargo receptors for Wnt proteins, and might serve as a model for specific receptor-mediated secretory routes of other growth factors.
Methods
Cell culture. Drosophila S2R+, Kc167, S2 hs-wg and S2 wg-FLAG cells were maintained in Schneider's medium supplemented with 10% FCS and 50 μg−1μl−1 penicillin/streptomycin. S2 hs-wg and S2 wg-FLAG cells were selected with 125 μg−1μl−1 hygromycin. Cells were transfected using Effectene (Qiagen). HEK293T cells were maintained in DMEM (Gibco) supplemented with 10% FCS and 50 μg−1μl−1 penicillin/streptomycin (Invitrogen). Cells were transfected with 50 ng DNA and 50 nM siRNA in 384-well plates using FuGene (Roche) and Dharmafect1 (Dharmacon), respectively.
RNAi screening. For the initial screen, 104 S2R+ cells were seeded on 250 ng dsRNA in 384-well plates. After 24 h, dTCF-Luc, pRP128-RL and pAc-wg were transfected. Cells were grown at 25 °C for 4 days to allow protein depletion. The screen was performed in duplicates. Data analysis was performed using Bioconductor/R and cellHTS.
RNAi experiments. dsRNAs were designed using GenomeRNAi (Horn et al, 2007). Each RNAi experiment was conducted at least three times in quadruplicate for each dsRNA (for primer sequences see supplementary Table S3 online). For the paracrine signalling assay, S2R+ cells treated with dsRNAs for 5 days and transfected with the reporter plasmids were mixed in a 1:3 ratio with S2 hs-wg cells that were activated by heat shock at 37 °C for 45 min 24 h before readout.
RNAi experiments in human cells were conducted as described in Bartscherer et al (2006), using the following siRNAs: β-catenin (M-003482-00), hEvi (M-018728-01), TMED5 (M-007854-01) and non-targeting siRNA (siCon, D-001206-13-05; Dharmacon).
Secretion assays and immunoprecipitation. The Wg and WntD secretion assays were performed as described in Bartscherer et al (2006). Antibodies used were mouse-α-FLAG-HRP (Sigma), rabbit-α-V5 (Rockland), mouse-α-HA (Cell Signaling), α-Wg (DSHB), mouse-α-tubulin (Sigma), α-rabbit-HRP and α-mouse-HRP (TrueBlot ULTRA, eBiosciences). For immunoprecipitation, cells were processed as described in Belenkaya et al (2002).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank T. Horn, C. Kalla, A. Ragab and K. Bartscherer for support and critical comments on the manuscript. We are particularly grateful to Z. Paroush and R. Helman for critical discussions and support with the analysis of the embryonic WntD phenotype. We thank A. Teleman, B. Thompson, B. Dixon, K. Basler, N. Perrimon, H. Bellen, R. Nusse and M. Mlodzik for providing reagents. Support by the DKFZ Light Microscopy Facility is gratefully acknowledged. This work was supported by a Marie-Curie Excellence grant from the European Commission and by the Deutsche Forschungsgemeinschaft (FOR1036).
Footnotes
The authors declare that they have no conflict of interest.
References
- Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477 [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522 [DOI] [PubMed] [Google Scholar]
- Bartoszewski S, Luschnig S, Desjeux I, Grosshans J, Nusslein-Volhard C (2004) Drosophila p24 homologues eclair and baiser are necessary for the activity of the maternally expressed Tkv receptor during early embryogenesis. Mech Dev 121: 1259–1273 [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533 [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J (2002) pygopus encodes a nuclear protein essential for Wingless/Wnt signaling. Development 129: 4089–4101 [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X (2008) The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 14: 120–131 [DOI] [PubMed] [Google Scholar]
- Boltz KA, Carney GE (2008) Loss of p24 function in Drosophila melanogaster causes a stress response and increased levels of NF-kappaB-regulated gene products. BMC Genomics 9: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz KA, Ellis LL, Carney GE (2007) Drosophila melanogaster p24 genes have developmental, tissue-specific, and sex-specific expression patterns and functions. Dev Dyn 236: 544–555 [DOI] [PubMed] [Google Scholar]
- Carney GE, Bowen NJ (2004) p24 proteins, intracellular trafficking, and behavior: Drosophila melanogaster provides insights and opportunities. Biol Cell 96: 271–278 [DOI] [PubMed] [Google Scholar]
- Castillon GA, Aguilera-Romero A, Manzano-Lopez J, Epstein S, Kajiwara K, Funato K, Watanabe R, Riezman H, Muniz M (2011) The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Mol Biol Cell 22: 2924–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R (2008) Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem 283: 17092–17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP (2008) Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Dionne MS, Schneider DS, Nusse R (2005) WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature 437: 746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn T, Arziman Z, Berger J, Boutros M (2007) GenomeRNAi: a database for cell-based RNAi phenotypes. Nucleic Acids Res 35: D492–D497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegler E, Bonnon C, Waldmeier L, Mitrovic S, Halbeisen R, Hauri HP (2010) p28, a novel ERGIC/cis Golgi protein, required for Golgi ribbon formation. Traffic 11: 70–89 [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K (2008) Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10: 178–185 [DOI] [PubMed] [Google Scholar]
- Port F, Hausmann G, Basler K (2011) A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO Rep 12: 1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating JR, Martens GJ (2009) The p24 family and selective transport processes at the ER-Golgi interface. Biol Cell 101: 495–509 [DOI] [PubMed] [Google Scholar]
- Takida S, Maeda Y, Kinoshita T (2008) Mammalian GPI-anchored proteins require p24 proteins for their efficient transport from the ER to the plasma membrane. Biochem J 409: 555–562 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R (2008) Alternative wnt signaling is initiated by distinct receptors. Sci Signal 1: re9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.