Abstract
Activating mutations in the KATP-channel cause neonatal diabetes mellitus (NDM), and patients have been safely transitioned from insulin to sulfonylureas. We report a male infant with permanent NDM (PNDM), born to a PNDM mother. Blood glucose began to rise on day of life (DOL) 2, and sulfonylurea (glyburide) therapy was initiated on DOL 5. Glucose was subsequently well controlled and normal at 3 months. A KATP mutation (R201H; KCNJ11) was detected in the infant, the mother, and 6-yr-old sister with PNDM; both were also subsequently transitioned off insulin onto glyburide. To our knowledge, this is the youngest NDM patient to receive oral glyburide and, importantly, the only one deliberately initiated on sulfonylureas. Strikingly, the current dose (0.017 mg/kg/d) is below the reported therapeutic range and approximately 75-fold lower than doses required by the affected sister and mother. Pancreatic insulin disappears in an animal model of KATP-induced NDM, unless glycemia is well controlled, thus, a dramatically lower glyburide requirement in the infant may reflect preserved insulin content because of early sulfonylurea intervention. Safe and effective initiation of glyburide in an insulin-naïve neonatal patient with KATP-dependent PNDM argues for early detection and sulfonylurea intervention.
Keywords: KATP, KCNJ11, Kir6.2, neonatal diabetes, sulfonylurea
The KATP-channel regulates insulin secretion by coupling cellular metabolism to electrical activity (1), and it is now recognized that activating mutations in KCNJ11 and ABCC8, which encode the pore-forming (Kir6.2) and regulatory (SUR1) subunits of the channel, respectively, represent common genetic causes of neonatal diabetes mellitus (NDM). NDM is rare and characterized by persistent hyperglycemia presenting in the first 6 months of life and lasting more than 2 wk. Activating mutations decrease ATP-sensitivity of the channel, thereby preventing channel closure and subsequent release of insulin (4). Patients with NDM secondary to KATP mutations have been safely transitioned from insulin to sulfonylureas (5–7), with doses typically higher than in type 2 diabetes, although the sulfonylurea requirements of NDM patients can typically be reduced over time (5). Moreover, phenotypic heterogeneity has been reported among unrelated NDM patients with the same activating KATP mutation, implicating varying degrees of β-cell dysfunction (8). In this report, we discuss a family with permanent NDM (PNDM) because of one such mutation.
Results
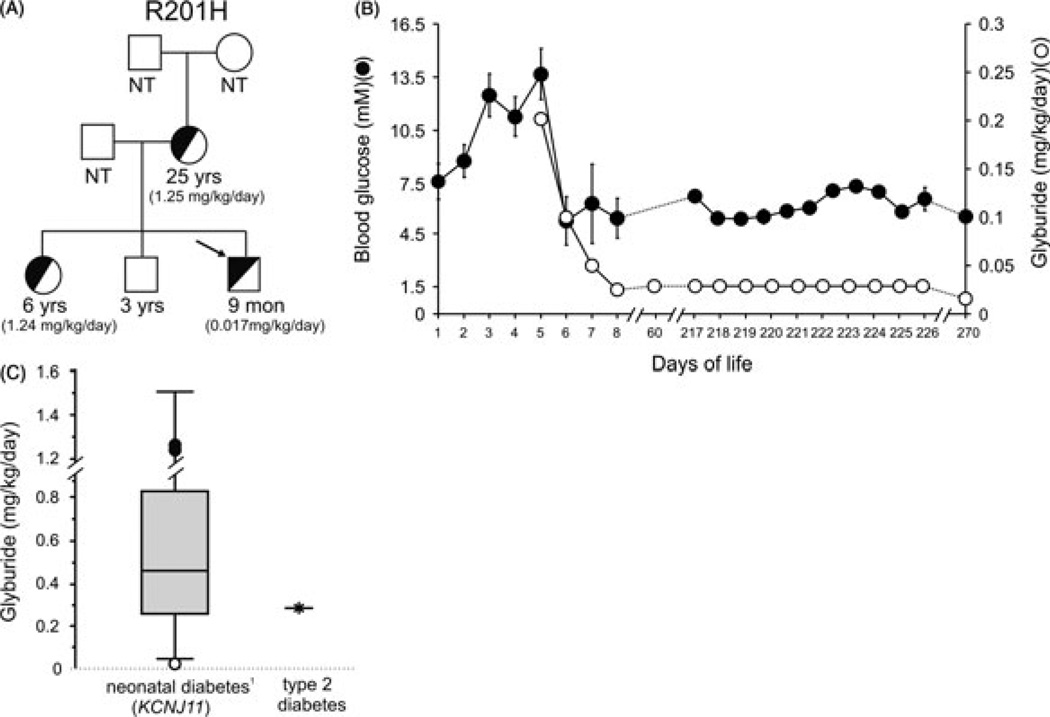
We report a term male, born to a 24-yr-old mother diagnosed with PNDM at 10 wk of age. The mother had a previous spontaneous abortion, a daughter diagnosed with PNDM at 7 wk of age, and a healthy son. The father is not diabetic. The family provided informed consent for testing and reporting. A heterozygous mutation R201H (c.601G > A) was detected in the KCNJ11 gene of the proband [day of life (DOL) 7]. The same R201H mutation, known to underlie PNDM (4), had previously been identified in the mother and sister, and not in the healthy brother (Fig. 1A). Given his family history, blood glucoses (BGs) were monitored in our infant. Initial glucose was 5.6 mM and ranged from 5.6 to 8.4 mM on DOL 1. BGs were 7.6–10.1 mM on DOL 2 and 10.8–13.8 mM on DOL 3. BGs remained elevated (11–16.5 mM) on DOL 4–5. Urine remained negative for ketones. Birth weight was 3057 g (50th percentile) and length and head circumference [orbital frontal cortex (OFC)] 50th percentile. The physical examination was unremarkable.
Fig. 1.
(A) Pedigree of family with Kir6.2 (KCNJ11) mutation. Arrow indicates the R201H proband; half-filled and empty symbols indicate clinically and genetically affected and unaffected individuals, respectively. The current ages of the family members and the glyburide dosing regimen (given in parentheses) are indicated. NT, not tested. (B) Glycemic control and sulfonylurea dosing in R201H proband. Blood glucose values (solid circles) and glyburide dose (open circles), vs. day of life. For days 1–8, multiple glucose readings (mean ± SEM) were measured in the hospital. Subsequent days are self-reported, random glucose measures (three times daily; mean ± SEM) from the patient’s mother. (C) Sulfonylurea dosing of the R201H proband (open circle) and the affected mother and sister (closed circles) relative to 44 NDM patients who successfully switched from insulin to sulfonylurea therapy (Box and Whisker plot adapted from Pearson et al. (5); bars represent the dosing range, box represents the lower and upper quartiles, and solid line represents median). For comparison, the maximal recommended glyburide dose to treat type 2 diabetes is shown (based on a 75 kg individual).
Given the family history of KATP-induced PNDM, and prior to the results of genetic testing, the sulfonylurea glyburide was initiated [0.2 mg/kg/d divided twice a day (BID)] on DOL 5 (Fig. 1B). BGs decreased to less than 5.5 mM and the dose was decreased to 0.1 mg/kg/d divided BID and subsequently to 0.05 mg/kg/d once daily. Because there is no widely available, stable suspension of glyburide, the dose was prepared by crushing a 5 mg tablet and suspending in 5 mL water. Our infant tolerated glyburide well without any reported side effects, and he was discharged on DOL 8. BGs were 3.3–7.2 mM and glyburide was decreased to 0.025 mg/kg/d divided BID. At 2 months, BGs were 5.1–11.6 mM and growth was normal. Glyburide was increased to 0.03 mg/kg/d divided three times a day (TID). At 9 months, BGs were 3.4–11.4 mM (95% 3.9–8.3) on glyburide 0.017 mg/kg/d divided TID. Weight 8.5 kg (25%), length and OFC was normal. Hemoglobin A1C was 5.9% (normal 4.0–6.2%).
The 6-yr-old sister has PNDM and was treated with insulin since 7 wk. She was transitioned off insulin to glyburide (current dose 1.24 mg/kg/d); BGs are 2.42–12.7 mM (67% 3.9–8.3) and hemoglobin A1C is 5.8%. Mother was also transitioned off insulin to glyburide (current dose 1.25 mg/kg/d) with BGs 4.4–8.8 mM, and hemoglobin A1C 1 month after transition was 9.3%. Notably, both the mother and the 6-yr-old are on glyburide doses approximately 75-fold higher than the infant brother.
Discussion
We report an infant with familial PNDM due to heterozygous KCNJ11 mutation (R201H), who was successfully treated with oral sulfonylurea at DOL 5 without ever receiving insulin. To our knowledge, this is the youngest patient to commence oral glyburide therapy for treatment of NDM because of a Kir6.2 mutation, and the only one deliberately not treated first with insulin. Significantly, the glyburide dose for this infant (currently 0.017 mg/kg/d) is below the reported therapeutic range (0.05–1.50 mg/kg/d) (Fig. 1C). Moreover, it is approximately 75-fold lower than the glyburide doses required by his sister and mother, who had received insulin for 6 and 24 yr, respectively. Other studies have demonstrated a trend toward reduced effectiveness of sulfonylureas the later patients are transitioned to the drug (9). The underlying basis for this observation is unclear. However, we recently reported that in an animal model of KCNJ11-induced NDM, pancreatic β-cells and insulin content disappear quite rapidly, unless glycemia is well controlled (2). It is also apparent from several studies that glycemic control of NDM patients is frequently not very good with insulin treatment alone (e.g., see reference (10)). We speculate that the dramatically lower glyburide requirement in our infant reflects preservation of insulin content because of early sulfonylurea intervention and consequent avoidance of repeated hyperglycemic episodes.
In summary, this case dramatically illustrates safe and effective initiation of glyburide in insulin-naïve neonatal patients with PNDM due to mutations in KATP channels. The data critically argue for early detection and early use of sulfonylureas in cases of KATP-dependent NDM, as well other cases of NDM in which sulfonylurea sensitivity is unaffected.
Acknowledgements
This work was supported in part by American Diabetes Association Grant-In-Aid (1-08-RA-141) and NIH DK69445 (to CGN).
Footnotes
Conflict of interest
We declare that we have no conflicts of interest.
References
- 1.Girard CA, Wunderlich FT, Shimomura K, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remedi MS, Kurata HT, Scott A, et al. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 4.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 5.Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations.[see comment] N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 6.Massa O, Iafusco D, D’Amato E, et al. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Human Mutation. 2005;25:22–27. doi: 10.1002/humu.20124. [DOI] [PubMed] [Google Scholar]
- 7.Sagen JV, Raeder H, Hathout E, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- 8.Klupa T, Edghill EL, Nazim J, et al. The identification of a R201H mutation in KCNJ11, which encodes Kir6.2, and successful transfer to sustained-release sulphonylurea therapy in a subject with neonatal diabetes: evidence for heterogeneity of beta cell function among carriers of the R201H mutation. Diabetologia. 2005;48:1029–1031. doi: 10.1007/s00125-005-1731-5. [DOI] [PubMed] [Google Scholar]
- 9.Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 10.Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab. 2008;93:1054–1061. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]