Mycoplasma pneumoniae continues to be a significant cause of community-acquired pneumonia and can manifest as fulminant disease leading to mortality in healthy individuals.
Abstract
Background. Mycoplasma pneumoniae continues to be a significant cause of community-acquired pneumonia and, on rare occasions, manifests as fulminant disease that leads to mortality, even in healthy individuals.
Methods. We conducted a retrospective study on members of a family who were quarantined by the Centers for Disease Control and Prevention in 2002 for respiratory failure and death of a 15-year-old brother (sibling 1) and a 13-year-old sister (sibling 2). Collected airway, cerebrospinal fluid (CSF), and serum samples from both deceased siblings and serum samples from both parents and the remaining 3 ill siblings (sibling 3–5) were tested using a range of diagnostic assays. Autopsy lung tissue samples from sibling 2 were also assessed using immunohistochemical and immunoelectron microscopic methods.
Results. Autopsy evaluation of sibling 1 revealed cerebral edema consistent with hypoxic ischemic encepatholopathy and pulmonary findings of bronchiolitis obliterans with organizing pneumonia (BOOP). Postmortem lung examination of sibling 2 revealed lymphoplasmacytic bronchiolitis with intraluminal purulent exudate, BOOP, and pulmonary edema. Results of diagnostic assays implicated the household transmission of M. pneumoniae among all 5 siblings and both parents. Further analysis of lung tissue from sibling 2 demonstrated the presence of M. pneumoniae organisms and community-acquired respiratory distress syndrome toxin. M. pneumoniae was cultured directly from sibling 2 autopsy lung tissue.
Conclusion. Evidence is provided that M. pneumoniae was readily transmitted to all members of the household and that the resulting infections led to a spectrum of individual responses with variation in disease progression, including lymphoplasmacytic bronchiolitis, BOOP, and death.
Mycoplasma pneumoniae is an atypical bacterial pathogen without a cell wall that affects both the upper and lower respiratory tracts of children and adults. It is implicated in airway diseases including pneumonia, exacerbation of wheezing, tracheobronchitis, and pharyngitis, as well as extrapulmonary manifestations such as neurological, hematologic, gastrointestinal, and dermatological pathologies [1, 2]. Among M. pneumoniae–infected patients, it is estimated that 3%–10% develop clinical pneumonia and that <5% of cases of pneumonia are severe enough to require hospitalization. M. pneumoniae–mediated fulminant pneumonia is considered to be rare, regardless of the high prevalence of the infection in the general population [1, 2].
Although M. pneumoniae has been recognized as a significant clinical pathogen for decades, its virulence determinants have only been partially deciphered. Recently, we identified a unique M. pneumoniae adenosine diphosphate-ribosylating and vacuolating cytotoxin (designated community-acquired respiratory distress syndrome [CARDS] toxin) [3]. The severity of pulmonary disease caused by M. pneumoniae appears to be dependent on biological properties of individual mycoplasma strains and CARDS toxin concentration [4]. Interestingly, CARDS toxin transcription and translation are substantially up-regulated during infection, in contrast to in vitro growth [5]. Furthermore, CARDS toxin shares similarities to the toxin of Bordetella pertussis [3, 6].
The pulmonary presentations of M. pneumoniae pneumonia have included cellular bronchiolitis [7], bronchiolitis obliterans with organizing pneumonia (BOOP) or without organizing pneumonia [7–9], bronchiectasis, pleural effusion, adult respiratory distress syndrome [10], pulmonary embolism [11], chronic interstitial fibrosis [12], and lung abscess [13]. The current literature stresses the benign and often subclinical nature of many of these infections; hence, the term “walking pneumonia” is sometimes used to describe M. pneumoniae pneumonia. However, cases of fulminant pneumonia due to M. pneumoniae have been encountered with resultant respiratory failure or death [8].
In this study, we describe a range of clinical manifestations associated with M. pneumoniae infection in a previously healthy family. To our knowledge, for the first time, M. pneumoniae–mediated disease progression is described in terms of mycoplasma load, CARDS toxin detection, and individual subject immunological and clinical responsiveness to infection.
MATERIALS AND METHODS
Collection of Samples
Patients’ samples were obtained for testing from the family members after consent as approved by the institutional review board (IRB) of The University of Texas Southwestern Medical Center (IRB no. 0802-447). Informed consent was obtained from legal guardians and informed assent was obtained from patients ≥10 years of age. The University of Texas Health Science Center at San Antonio IRB determined that this study did not require IRB review.
Bacterial Strains and Recombinant Proteins
M. pneumoniae reference strain M129 and a clinical isolate designated SA1 were grown in SP4 broth. Recombinant P1 adhesin carboxyl immunodominant domain (rP1-C) and recombinant CARDS toxin (rCARDS toxin) were expressed and purified as reported elsewhere [3, 14].
Polymerase Chain Reaction (PCR) and Quantitative Real-time PCR (qPCR) Detection of M. pneumoniae Infection
Collected lung tissue, cerebrospinal fluid (CSF), and serum samples from both deceased siblings and serum samples alone from the parents and the remaining 3 ill siblings were subjected to DNA extraction using the QIAmp DNA Mini Kit (Qiagen, Valencia, CA). Detection of M. pneumoniae was by PCR and qPCR using P1 nucleotide sequences [15].
Isolation of M. pneumoniae Strain SA1 and Subtyping
Lung samples from the deceased 13-year-old subject were incubated in SP4 broth and subsequently plated on SP4 agar. Single mycoplasma colonies were picked and cloned in SP4 medium and compared with reference strain M129 by use of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of total mycoplasma proteins and immunoblotting against specific M. pneumoniae proteins. To further characterize the M. pneumoniae clinical isolate, we used P1 adhesin repetitive element-based PCR restriction fragment length polymorphism assays (P1-PCR-RFLP [16]). In addition, PCR-amplified full-length CARDS toxin gene was sequenced to determine nucleotide differences between reference and clinical strains (GenBank accession no. ABE27143).
Detection of Antibodies Against M. pneumoniae
Serum samples from each subject and CSF samples from siblings 1 and 2 were analyzed using enzyme-linked immunosorbent assay (ELISA) and immunoblotting against total cell lysates of both M. pneumoniae reference and SA1 strains and using ELISA against rCARDS toxin and rP1-C as indicator proteins [14].
Histological, Immunohistochemical, and Immunoelectron Microscopic Assessments
Lung autopsy samples were examined as described elsewhere [17]. Briefly, paraffin-embedded sections were cut at 4-μm thickness and stained with hematoxylin-eosin. Rabbit antiserum reactive against rCARDS toxin and mouse anti-P1 monoclonal antibody (US Biological, Swampscott, MA) at 1:1500 and 1:10 dilutions, respectively, were incubated with representative lung sections, which were then stained with diaminobenzidene chromagen (Vector Laboratories, Burlington, CA) [17]. Control sections were treated similarly but without primary antibodies. For immunogold electron microscopy, lung samples were processed and analyzed for the presence of M. pneumoniae using anti-P1 rabbit antiserum (1:100 dilution) as reported elsewhere [5].
RESULTS
Case Report
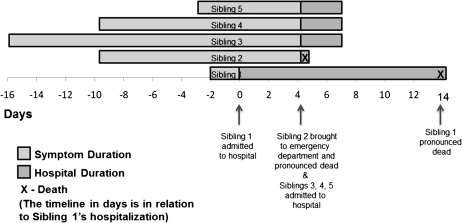
All 5 children of a family were admitted to a pediatric hospital in Dallas, Texas, over a period of 5 days; 2 of the children experienced a fatal outcome. All siblings were previously healthy without contributory past medical history and were homeschooled. Table 1 details the age, sex, clinical presentation, and diagnostic assessment of the subjects, and the timeline graph in Figure 1 indicates the onset of symptoms and time of diagnosis.
Table 1.
Characteristics of Mycoplasma pneumoniae Infection Within a Family By Use of Serum Samples
| Parameters | Sibling 1a | Sibling 2a | Sibling 3 | Sibling 4 | Sibling 5 | Father | Mother |
| Age in years | 15 | 13 | 6 | 10 | 17 | NA | NA |
| Sex | Male | Female | Male | Female | Male | Male | Female |
| Diagnosis | Respiratory failure/arrest | Respiratory failure/arrest | Respiratory infection | Respiratory infection | Pneumonia | NCE | NCE |
| Test results | |||||||
| PCR | + | + | + | + | + | − | − |
| ELISA IgM P1b | ++ | − | ++ | + | ++++ | − | − |
| CARDS toxin | ++++ | − | ++ | ++ | ++++ | − | − |
| ELISA IgG P1b | ++++ | − | + | ++ | +++ | ++ | ++ |
| CARDS toxin | − | + | + | + | ++++ | ++ | ++ |
| Immunoblot P1c | +++ | − | + | ++ | ++ | + | + |
| CARDS toxinb | + | ± | − | +++ | + | + | + |
| M. pneumoniae WCLd | ++ | ± | + | ++ | +++ | +++ | ++ |
Abbreviations: CARDS, community-acquired respiratory distress syndrome; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobin; NA, not available; NCE, not clinically evaluated; PCR, polymerase chain reaction; WCL, whole cell lysate.
Death.
ELISA positivity was determined based on optical density (405 nm) readings. OD405 values of <0.2 were considered to be negative. ELISA positivity: −, negative; +, 0.2–0.4; ++, 0.41–0.6; +++, 0.6–0.8; ++++, >0.8.
Immunoblot positivity: −, negative; +/−, threshold level; +, low intensity; ++, medium intensity; +++, strong intensity.
Immunoblot profiles using M. pneumoniae strains SA1 and M129 were similar.
Figure 1.
Timeline graph showing the onset of symptoms and time of diagnosis of the siblings. Further details of the siblings are included in Table 1.
15-Year-Old Deceased Male Sibling (Sibling 1)
A 15-year-old white male was admitted in August 2000 to the pediatric intensive care unit for sudden onset of respiratory failure. He was in his usual state of health until 2 days prior to admission when he developed sore throat, cough, and rhinorrhea with no noted fever, headache, or altered mental status. On the morning of admission, his family was not able to arouse him from sleep but confirmed him to be breathing and with heartbeat. The family then noted cessation of breathing and called paramedics, who found the patient to be pulseless with cardiac electrical activity and initiated cardiopulmonary resuscitation (CPR) including endotracheal intubation. On admission to the hospital, physical examination was only notable for a febrile (39°C), sedated, and mechanically ventilated male patient. Initial chest X-ray radiograph revealed multifocal parenchymal opacities involving the perihilar regions and lower lobes. The patient continued with intermittent fevers throughout his hospital course, during which a maculopapular rash developed. General laboratory studies (complete blood cell count, liver function tests, electrolyte levels, and creatinine levels) were remarkable for an initial white blood cell count of 13.2 k/μL, initial aspartate aminotransferase level of 247 U/L, and initial alanine aminotransferase level of 506 U/L; these values normalized over the course of hospitalization. Toxicology testing was negative. CSF indices were within reference limits, except for a white blood cell count of 22 cells/μL.
An infectious diseases evaluation was performed. Endotracheal aspirates revealed many white blood cells with no bacteria seen on Gram stain and no growth of bacteria on routine culture. Viral direct fluorescence assays on nasopharyngeal specimens for respiratory syncytial virus, adenovirus, influenza virus, and parainfluenza virus and viral culture were negative. Blood, urine, and cerebral spinal fluid bacterial cultures were without growth. Commercially available PCR assays for enterovirus, herpes simplex virus, and M. pneumoniae on CSF were negative. Serum immunofluorescence assays for St Louis encephalitis virus immunoglobulin M (IgM) and immunoglobulin G (IgG), West Nile virus IgM and IgG, California encephalitis virus IgM and IgG, and Legionella species were negative. Serum Chlamydia species IgG and human immunodeficiency virus (HIV) ELISAs were negative. However, serum M. pneumoniae IgM and IgG ELISAs were positive at 1:80 and 1:160, respecively (ARUP Laboratories, UT); serum M. pneumoniae complement fixation was positive at >1:256. Total serum IgM and IgG levels were normal. Over the hospital course, the subject received broad-spectrum antibiotics, including gatifloxacin, clindamycin, ceftriaxone, azithromycin, and doxycycline. His neurological status never improved beyond minimal response to painful stimuli, even when all sedation was stopped, and computed tomography of the brain was consistent with severe global hypoxic ischemic injury. Support was withdrawn 14 days after admission. The findings recorded in the autopsy report included cerebral edema consistent with hypoxic ischemic encepatholopathy, with no evidence of encephalitis or meningitis, and acute and organizing pneumonia with BOOP. Because the complement fixation test to detect M. pneumoniae infection is based on crude lipid antigen that contains glycolipids that occur commonly in plants and bacteria [18] and even in human brain tissue [19], we further confirmed the positive M. pneumoniae serologic results by measuring IgM and IgG serum responses to M. pneumoniae unique rP1-C and rCARDS toxin proteins. As shown in Table 1, the subject exhibited strong immunoresponsiveness against these M. pneumoniae proteins. In addition, PCR analysis for M. pneumoniae was positive.
13-Year-Old Deceased Female Sibling (Sibling 2)
The 13-year-old sister was noted to exhibit 2 weeks of respiratory symptoms similar to those preceding sibling 1’s sudden onset of respiratory failure. Four days after sibling 1 was admitted to the hospital, sibling 2 developed fever and complained of dyspnea. She was found unresponsive in her bed the next morning by her family; paramedics confirmed her to be asystolic and initiated CPR. She was pronounced dead after advanced cardiac life support failed to revive her in the emergency center. Premortem bacterial blood and sputum cultures and nasopharyngeal viral culture were unrevealing. Total serum immunoglobulin levels were low (IgG level, 322 mg/dL; IgM level, 24 mg/dL; IgA level, 32 mg/dL). HIV test was negative.
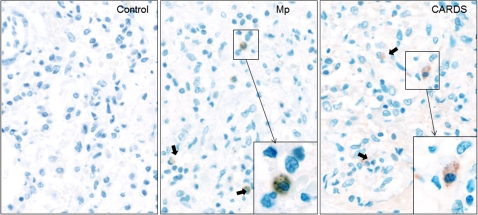
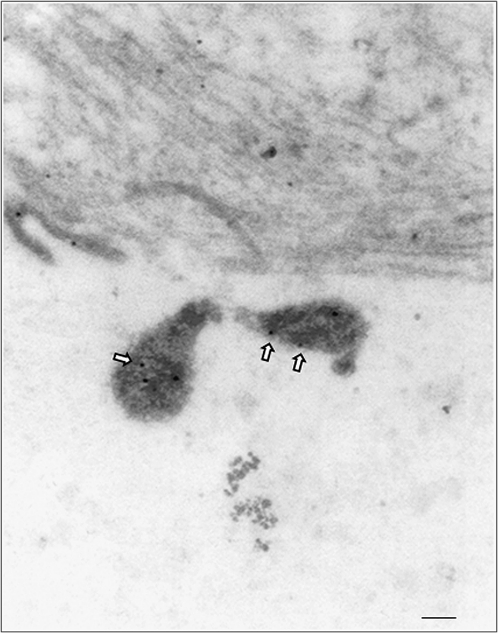
Detection of serum M. pneumoniae IgM and IgG levels was negative by commercial laboratory testing, in contrast to sibling 1. Lymphoplasmatic bronchiolitis and focal sites of BOOP and alveolar edema and intra-alveolar hemorrhages were histopathological findings at autopsy. Some of the bronchioles also contained an intraluminal exudate of neutrophils, admixed with monocytes and epithelial debris that extended into subjacent alveoli, (ie, focal bronchopneumonia). Postmortem lung stains and/or cultures were negative for routine bacteria, acid-fast bacilli, fungi, Legionella species, and viruses. However, M. pneumoniae PCR (ARUP Laboratories, UT) was positive on lung tissue. Furthermore, our studies indicated positive M. pneumoniae serum PCR (Table 1) and lung tissue qPCR; in the latter case, 8 × 107 M. pneumoniae genome copies were detected per gram of lung tissue. Immunostaining using mouse monoclonal antibodies reactive against P1 adhesin protein revealed M. pneumoniae organisms localized to the surface of bronchiolar epithelium (Figure 2, Mp). Importantly, immunostaining of lung sections also revealed the presence of CARDS toxin (Figure 2, CARDS) associated with alveolar macrophages. Furthermore, immunoelectron microscopy clearly demonstrated M. pneumoniae organisms in the lungs (Figure 3). We also were able to successfully culture M. pneumoniae organisms from sibling 2’s lung specimens at 2 independent research laboratories (The University of Texas Southwestern Medical Center at Dallas and The University of Texas Health Science Center at San Antonio). In an attempt to understand the unusual severity of these M. pneumoniae infections, we further characterized the M. pneumoniae clinical isolate designated as SA1, which was isolated in San Antonio. This strain was confirmed as M. pneumoniae by sequencing of the CARDS toxin gene, by comparing SA1 protein profiles with reference strain M129 using SDS-PAGE and immunoblotting against major M. pneumoniae proteins (P1, P30, ClpB, CARDS toxin, pyruvate dehydrogenase subunits A and B, and elongation factor Tu), and by subtyping, which determined the clinical isolate to be categorized as type II-b using P1-PCR-RFLP. Interestingly, sibling 2 did not demonstrate a notable humoral immune response to M. pneumoniae proteins (Table 1).
Figure 2.
Immunolocalization of Mycoplasma pneumoniae and community-acquired respiratory distress syndrome (CARDS) toxin in lung specimen of sibling 2. In focal peribronchiolar sites, intra-alveolar collections of alveolar macrophages and monocytes were present, some of which showed positively-immunostained intracytoplasmic mycoplasma aggregates and CARDS toxin. Bold arrows indicate immunostained aggregates of mycoplasma organisms (Mp panel) and CARDS toxin (CARDS panel) that are similar in size and distribution (original magnification ×40). Insets indicate the higher magnification of same (original magnification ×60).
Figure 3.
Immunogold electron microscopic detection of Mycoplasma pneumoniae in lung tissue of sibling 2. M. pneumoniae cells were gold-particle-labeled with anti–M. pneumoniae antibodies (arrows) as described in the Materials and Methods. Mycoplasma cells were visualized with a JEOL 1230 transmission electron microscope. Gold labeling (arrows) reveals M. pneumoniae with its characteristic tip organelle (scale bar, 0.1 μm).
Siblings 3, 4, and 5, and Parents
The 6-year-old brother, 10-year-old sister, and 17-year-old brother were admitted to the hospital on the same day that sibling 2 died. These children had been experiencing respiratory symptoms, mainly consisting of cough and rhinorrhea without fever, for 1–3 weeks. Two of the children had experienced a nonspecific erythematous rash, and one had experienced myalgias and arthralgias prior to admission. On admission, general laboratory studies (complete blood cell count, liver function tests, electrolyte levels, and creatinine levels) and physical examination were unremarkable, except that one child had scattered rales on chest auscultation. In addition, total IgG, IgM, and IgA levels were normal for all siblings. Chest X-ray radiographs revealed focal infiltrates consistent with pneumonia and bronchial wall involvement among the surviving 3 siblings. The 3 siblings were tested for infectious agents in a similar manner as for sibling 1, except CSF samples were not obtained, and the results were similarly unrevealing. However, serum ELISA for M. pneumoniae total IgG and IgM were positive in siblings 3, 4, and 5. All 3 children plus both parents (Table 1) were immunoblot positive against M. pneumoniae whole cell lysates, CARDS toxin, and/or P1 (Table 1). Both parents exhibited detectable serum M. pneumoniae IgG levels. Furthermore, PCR analysis revealed the presence of M. pneumoniae in serum samples of the 6-, 10-, and 17-year-olds but not in those of the mother or father. Azithromycin therapy was prescribed for these siblings, and recovery was uneventful. The parents were not clinically evaluated (Table 1).
DISCUSSION
Since the first report of fatal M. pneumoniae infection [20], severe cases have been reported sporadically. In the previous reviews of fatal M. pneumoniae infection, death was associated with diffuse pneumonia, adult respiratory distress syndrome, vascular thrombosis, and disseminated intravascular coagulation [20–23]. Histopathological analyses of tissues from fatal cases of M. pneumoniae pneumonia [7–9, 24] and from M. pneumoniae–infected animal models [17, 25] have shown ulceration and destruction of ciliated epithelium of bronchi and bronchioles, edema of bronchial and bronchiolar walls, bronchiolar and alveolar infiltrates of macrophages and lymphocytes, and bronchiolitis obliterans. Chan and Welsh [8] reviewed cases of M. pneumoniae pneumonia that resulted in respiratory failure and death and reported that fulminant cases were more common in young healthy adults. Rollins et al [7] observed BOOP based on open lung biopsy and emphasized that M. pneumoniae causation is often omitted from differential diagnosis. In the present study, both deceased siblings had experienced respiratory symptoms for about 2 weeks before their acute cardiopulmonary failure episodes, so the findings of a reparative lung response, such as BOOP, could be expected. However, the primary lung finding in sibling 2 was lymphoplasmacytic bronchiolitis with intraluminal exudate, a pattern reported in M. pneumoniae–infected humans and experimentally infected animals.
In earlier animal studies centered on localizing M. pneumoniae in tissue sites, mycoplasmas were identified primarily on the surface of airway epithelium whereas evidence of tissue inflammation and tissue injury throughout the bronchial walls suggested diffusion of unknown mycoplasma products from the surface epithelium into deeper tissues. Since our discovery of CARDS toxin, we showed that CARDS toxin, along with M. pneumoniae organisms, can be detected on respiratory epithelium surfaces of infected lungs and in peribronchiolar alveolar spaces that contained inflammatory exudates of edema, neutrophils, and alveolar macrophages and monocytes [17]. Furthermore, rCARDS toxin alone elicits airway vacuolization and cytotoxicity and extensive peribronchial and perivascular cellular inflammation in baboons and mice after inoculation into lungs [26]. We also reported that CARDS toxin concentrations in bronchiolar lavage fluids of infected mice are directly related to M. pneumoniae organism load and disease severity [4]. In the lung tissue of sibling 2, we observed the localization and distribution of M. pneumoniae and CARDS toxin by immunohistochemical staining. We also detected M. pneumoniae organisms by immunogold electron microscopy. The substantial load of M. pneumoniae genomes within the lung of sibling 2, the detection of CARDS toxin, and the extensive lung pathology clearly reinforce the contributory roles of M. pneumoniae to disease progression. Most likely, the heavily infected lung tissue of sibling 2 permitted isolation of M. pneumoniae strain SA1 subtype II-b from lung specimen. Interestingly, in our prior investigation of various M. pneumoniae strains and associated elicited pulmonary disease severity, it was the type II strain that was most adept at colonizing, replicating, and persisting, thus resulting in high concentrations of CARDS toxin in bronchiolar lavage fluids and extensive lung disease [4]. Why there was such a discrepancy in the M. pneumoniae IgM and IgG responses between siblings 1 and 2 is unresolved.
Due to the high prevalence of M. pneumoniae infections worldwide, severe cases of pneumonia are not uncommon, but they are probably underdiagnosed. However, new methods have been developed to identify M. pneumoniae infections [27, 28]. Furthermore, we reported that the CARDS toxin gene-based qPCR assay and the CARDS toxin antigen-based capture assay are highly specific and sensitive in the detection of active infection of M. pneumoniae among mechanically ventilated subjects [29] and in a high proportion of adult subjects with refractory asthma [30]. As previously noted, the increase in the severity of M. pneumoniae pneumonia in younger patients may be biased, possibly due to a greater prevalence of infection or increased susceptibility in younger patients. Possibly the siblings (1 and 2) with a fatal outcome had an immunodeficiency, whereas the other siblings who did not have a fatal outcome did not share this immunodeficiency.
In summary, this report highlights the potentially serious nature, as well as the diversity of individual immune response and outcome, of M. pneumoniae infection in a previously healthy family. The relatively young age of patients with fatal M. pneumoniae infection is of unclear significance. Furthermore, this study is the first, to our knowledge, to link M. pneumoniae infection, mycoplasma genome number, and CARDS-toxin-mediated events in human disease, emphasizing the need for improved diagnostic and treatment modalities.
Notes
Acknowledgments.
We thank Mark Blaylock for his help with electron microscope images and Rose Garza for her assistance in assembling the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support.
This study was funded by grants from the National Institutes of Health (U19AI070412) and The Kleberg Foundation, to J. B. B.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–73. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 2.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103:6724–9. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Techasaensiri C, Tagliabue C, Cagle M, et al. Variation in colonization, ADP-ribosylating and vacuolating cytotoxin, and pulmonary disease severity among Mycoplasma pneumoniae strains. Am J Respir Crit Care Med. 2010;182:797–804. doi: 10.1164/rccm.201001-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannan TR, Musatovova O, Balasubramanian S, et al. Mycoplasma pneumoniae community acquired respiratory distress syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol Microbiol. 2010;76:1127–41. doi: 10.1111/j.1365-2958.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein A binding protein of Mycoplasma pneumoniae. Infect Immun. 2005;73:2828–34. doi: 10.1128/IAI.73.5.2828-2834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins S, Colby T, Clayton F. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch Pathol Lab Med. 1986;110:34–41. [PubMed] [Google Scholar]
- 8.Chan ED, Welsh CH. Fulminant Mycoplasma pneumoniae pneumonia. West J Med. 1995;162:133–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Llibre JM, Urban A, Garcia E, Carrasco MA, Murcia C. Bronchiolitis obliterans organizing pneumonia associated with acute Mycoplasma pneumoniae infection. Clin Infect Dis. 1997;25:1340–2. doi: 10.1086/516124. [DOI] [PubMed] [Google Scholar]
- 10.Fischman RA, Marschall KE, Kislak JW, Greenbaum DM. Adult respiratory distress syndrome caused by Mycoplasma pneumoniae. Chest. 1978;74:471–3. doi: 10.1378/chest.74.4.471. [DOI] [PubMed] [Google Scholar]
- 11.Simmons BP, Aber RC. Mycoplasma pneumoniae pneumonia: symptoms mimicking pulmonary embolism with infarction. JAMA. 1979;241:1268–9. doi: 10.1001/jama.241.12.1268. [DOI] [PubMed] [Google Scholar]
- 12.Tablan OC, Reyes MP. Chronic interstitial pulmonary fibrosis following Mycoplasma pneumoniae pneumonia. Am J Med. 1985;79:268–70. doi: 10.1016/0002-9343(85)90021-x. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi S, del Giudice MM, Spicuzza L, Saporito M, Nipitella G, La Rosa M. Lung abscess in a child with Mycoplasma pneumoniae infection. Eur J Pediatr. 2010;169:1413–5. doi: 10.1007/s00431-010-1223-6. [DOI] [PubMed] [Google Scholar]
- 14.Berg CP, Kannan TR, Klein R, et al. Mycoplasma antigens as a possible trigger for the induction of antimitochondrial antibodies in primary biliary cirrhosis. Liver Int. 2009;29:797–809. doi: 10.1111/j.1478-3231.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 15.Ursi D, Dirven K, Loens K, Ieven M, Goossens H. Detection of Mycoplasma pneumoniae in respiratory samples by real-time PCR using an inhibition control. J Microbiol Methods. 2003;55:149–53. doi: 10.1016/s0167-7012(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T, Kenri T, Okazaki N, et al. Epidemiological study of Mycoplasma pneumoniae infections in Japan based on PCR-restriction fragment length polymorphism of the P1 cytadhesin gene. J Clin Microbiol. 1996;34:447–9. doi: 10.1128/jcm.34.2.447-449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan TR, Coalson JJ, Cagle M, Musatovova O, Hardy RD, Baseman JB. Synthesis and distribution of CARDS toxin during Mycoplasma pneumoniae infection in a murine model. J Infect Dis. 2011;204:1596–1604. doi: 10.1093/infdis/jir557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny GE, Newton RM. Close serological relationship between glycolipids of Mycoplasma pneumoniae and glycolipids of spinach. Ann New York Acad Sci. 1973;225:54–61. [Google Scholar]
- 19.Biberfeld G. Antibodies to brain and other tissues in cases of Mycoplasma pneumoniae infection. Clin Exp Immunol. 1971;8:319–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Maisel JC, Babbitt LH, John TJ. Fatal Mycoplasma pneumoniae infection with isolation of organisms from lung. JAMA. 1967;202:287–90. [PubMed] [Google Scholar]
- 21.Koletsky RJ, Weinstein AJ. Fulminant Mycoplasma pneumoniae infection: report of a fatal case, and a review of the literature. Am Rev Respir Dis. 1980;122:491–6. doi: 10.1164/arrd.1980.122.3.491. [DOI] [PubMed] [Google Scholar]
- 22.Williamson J, Marmion BP, Kok T, Antic R, Harris RJ. Confirmation of fatal Mycoplasma pneumoniae infection by polymerase chain reaction detection of the adhesin gene in fixed lung tissue. J Infect Dis. 1994;170:1052–3. doi: 10.1093/infdis/170.4.1052. [DOI] [PubMed] [Google Scholar]
- 23.Carrascosa MF, Lucena MI, Andrade RJ, et al. Fatal acute hepatitis after sequential treatment with levofloxacin, doxycycline, and naproxen in a patient presenting with acute Mycoplasma pneumoniae infection. Clin Ther. 2009;31:1014–9. doi: 10.1016/j.clinthera.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Wachowski O, Demirakca S, Muller KM, Scheurlen W. Mycoplasma pneumoniae associated organising pneumonia in a 10 year old boy. Arch Dis Child. 2003;88:270–2. doi: 10.1136/adc.88.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36:465–78. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 26.Hardy RD, Coalson JJ, Peters J, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4:e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degrange S, Cazanave C, Charron A, Renaudin H, Bebear C, Bebear CM. Development of multiple-locus variable-number tandem-repeat analysis for molecular typing of Mycoplasma pneumoniae. J Clin Microbiol. 2009;47:914–23. doi: 10.1128/JCM.01935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loens K, Ursi D, Goossens H, Ieven M. Molecular diagnosis of Mycoplasma pneumoniae respiratory tract infections. J Clin Microbiol. 2003;41:4915–23. doi: 10.1128/JCM.41.11.4915-4923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muir MT, Cohn SM, Louden C, Kannan TR, Baseman JB. Novel toxin assays implicate Mycoplasma pneumoniae in prolonged ventilator course and hypoxemia. Chest. 2011;139:305–10. doi: 10.1378/chest.10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters J, Singh H, Brooks EG, et al. Persistence of community-acquired respiratory distress syndrome toxin-producing mycoplasma pneumoniae in refractory asthma. Chest. 2011;140:401–7. doi: 10.1378/chest.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]