Voltage-gated ion channels clearly are involved in the pathogenesis of epilepsy, with evidence implicating derangement of Na+, K+, and Ca2+ voltage-gated channels, in both inherited and acquired forms of epilepsy (1). A newcomer to this list of ion channels involved in epilepsy is the hyperpolarization-activated cation channel or h-channel (otherwise known as Ih or the pacemaker channel). This voltage-gated channel now is known to play a significant role in regulating neuronal excitability and recently has been shown to be modulated by seizures. Unlike other channels implicated in epilepsy whose function in normal neurons can clearly be labeled “excitatory” (Na+ and Ca2+) or “inhibitory” (K+), the unique physiologic behavior of the h-channel allows it to both augment and decrease the excitability of neurons. Thus the role of Ih in epilepsy, at present, is controversial and is a growing area of intense investigation (2, 3).
H-Channels Are Both Inhibitory and Excitatory
The h-channel or Ih is widely distributed in the cortex, hippocampus, and thalamus, as well as in peripheral nerve and in the heart, where it was first described as a regulator of cardiac pacemaking (4). Ih possesses unusual biophysical properties that allow it to play a chameleon-like role in neuronal excitability. Its structure represents an evolutionary marriage between the voltage-gated K+ channel (which it most strongly resembles) and the cyclic nucleotide–gated, non–voltage-gated K+ channel. Thus Ih possesses a high permeability to K+ ions, is voltage gated, but also is modulated by intracellular cyclic adenosine monophosphate (cAMP) levels, allowing activity-dependent regulation. More important, the channel has substantial permeability to Na+, such that on opening at typical neuronal resting potential, it generates an inward current, causing the cell to depolarize; yet the channel is activated not by depolarization (as with virtually all voltage-gated channels) but by hyperpolarization. Because hyperpolarization produces activation, which in turn leads to depolarization (resulting in channel deactivation), the h-channel possesses an inherent negative-feedback property.
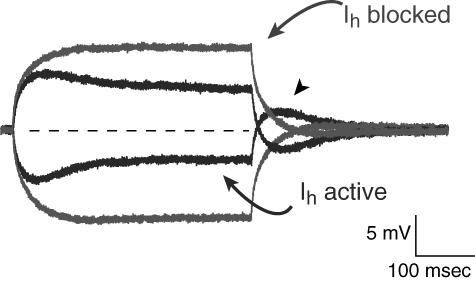
This negative-feedback principle is evident in the contribution of Ih to neuronal excitability, as illustrated in Fig. 1. In a neuron recorded at rest with Ih inactive, a small depolarizing or hyperpolarizing input rapidly produces a steady-state change in voltage. With Ih active, however, a hyperpolarizing input causes slow Ih activation, producing a depolarizing current that returns the membrane potential toward rest. Conversely, a depolarizing input causes deactivation of the Ih that was active at rest; the loss of a tonic depolarizing current causes a hyperpolarization, again returning membrane potential toward rest. Thus Ih tends to stabilize membrane potential toward the resting potential against either depolarizing or hyperpolarizing inputs. More precisely, Ih diminishes input resistance, the voltage change produced by a given synaptic current.
FIGURE 1 .
Actions of hyperpolarization-activated cation channel (Ih) on neuronal excitability. Current-clamp recording from CA1 hippocampal pyramidal neuron dendrite with superimposed responses to depolarizing and hyperpolarizing current injections (lighter traces) when Ih is blocked (with ZD-7288) show that the voltage response quickly reaches a steady-state plateau. When Ih is active (darker traces), a hyperpolarizing input elicits a slow, depolarizing “sag” in membrane potential, reflecting Ih activation. Note the rebound afterdepolarization (arrowhead) at the end of the hyperpolarizing input. Similarly, a depolarizing input yields a hyperpolarizing sag in membrane potential, representing Ih deactivation. Thus Ih tends to stabilize membrane potential toward resting potential (dotted line) against both hyperpolarizing and depolarizing inputs.
In physiological terms, Ih can be either excitatory or inhibitory with respect to its influence on action potential firing. As described earlier, Ih diminishes the effect of excitatory inputs and the likelihood they will produce action potential firing. Conversely, Ih helps set the level of resting potential, depolarizing it from the K+ reversal potential and toward action potential firing threshold, a potentially excitatory influence. Furthermore, in cells with a tonically active inward current, addition of the negative-feedback behavior of Ih contributes to oscillatory behavior, such as is seen in sinoatrial node cells and thalamic relay neurons (5). Thus like the ancient Chinese principle of yin–yang, the h-channel embodies two opposing influences on neuronal excitability, preventing simple characterization as either inhibitory or excitatory.
Although all h-channels possess the fundamental properties described earlier, Ih represents a family of currents with differing kinetics and tissue distributions. Ih is encoded by the HCN family of genes, of which four subtypes have been identified (6). The predominance of various h-channel subtypes varies by location, with HCN1 and HCN2 most prevalent in cortex and hippocampus, and HCN2 and HCN4 predominating in the thalamus (7). HCN3 is only modestly expressed in brain. Because the biophysical properties of HCN subtypes vary significantly (with HCN1 having faster kinetics but less cAMP modulation compared with HCN2 and HCN4), the contribution of Ih to neuronal behavior also varies by both location and neuron type in each region, with individual neurons expressing varying amounts of different HCN isoforms.
H-Channels Affect Neuronal Function
Because Ih has the potential to affect excitability in a number of ways, modulation of Ih can significantly affect neuronal behavior. Some of the first evidence in this regard involved a study of Ih in thalamocortical neurons (8). The work demonstrated that the frequency of slow oscillations underlying “spindle waves,” mediated in part by Ih, was slowed when Ih was upregulated by a Ca2+-dependent increase in intracellular cAMP concentration. The resulting persistent activation of Ih caused a sustained depolarization, thus breaking the oscillatory cycle. Other evidence showing that modulation of Ih can set the firing rates of rhythmically active neurons appears in studies involving pain transduction in dorsal root ganglion neurons. In a rat model of neuropathic pain, Ih was upregulated in response to neuronal injury, with the resulting depolarization of rhythmically active sensory neurons increasing pathologic action potential firing—possibly underlying pain sensation (9).
H-channels also exert powerful effects on the pyramidal neurons of the hippocampus and neocortex that are independent of their effects on rhythmicity. Understanding of their action in these neurons began with the startling observation that h-channels were distributed in pyramidal neurons in density gradients, with the apical dendrites possessing up to 10-fold the channel density seen at the cell body (10). This Ih gradient reduces the temporal summation of synaptic inputs in the dendrites compared with the soma, minimizing the influence of dendritic cable properties on synaptic inputs localized in the distal apical dendrites and “normalizing” their effect on action potential firing at the soma (11).
The gradient of h-channels in pyramidal dendrites may explain, in part, the action of a commonly used antiepileptic drug (AED), lamotrigine (LTG). A recent study demonstrated that LTG caused an upregulation of Ih by altering its voltage-dependent activation (12). In the dendrites, where Ih is at high density, synaptic inputs were attenuated in their ability to drive action potential firing at the soma. Conversely, at the soma, where Ih density is lowest, synaptic inputs and action potential firing were only minimally affected. Thus by virtue of a nonuniform cellular distribution, modulation of Ih by LTG can have differential effects on neuronal excitability, selectively inhibiting excitatory synaptic inputs, which are localized predominantly to the apical dendrites. LTG also may act on h-channels located on thalamic neurons, which may explain the drug's action on primarily generalized seizures. Other evidence suggests that the AED gabapentin (GBP) also may act in part via upregulation of Ih (13).
Are H-Channels Involved in Epilepsy?
Evidence is accumulating that suggests a role in for Ih in epileptogenesis. The first studies to connect h-channels and seizures demonstrated that in an animal model of provoked seizures (the hyperthermia model of febrile seizures), both Ih and γ-aminobutyric acid (GABA)-mediated inhibition were enhanced as measured at the soma of hippocampal pyramidal neurons (14, 15). Although the increase in inhibition might be a compensatory response to seizure activity, its conjunction with increased Ih appeared to produce hyperexcitability by facilitating rebound action potential firing after the hyperpolarization induced by a GABAergic inhibitory postsynaptic potential (Ih supplying the transient rebound potential, as shown in Fig. 1). The increase in Ih was mediated by a small depolarizing shift in its half-maximal activation voltage but with a slowing in its activation and deactivation kinetics—a result that cannot be explained solely by greater cAMP modulation.
A closer inspection of the molecular determinants of Ih seen in animals after febrile seizures revealed the reason for this paradoxical shift in Ih properties: HCN1, the predominant isoform in pyramidal neurons, was persistently downregulated after seizures, whereas HCN2, an isoform with slower kinetics, was upregulated (although not as persistently as HCN1) (16). This switch to a predominance of the HCN2 isoform explained the slowed kinetics of Ih seen after hyperthermia-induced seizures. Notably, these changes were most pronounced in area CA1 and less so in CA3.
A similar result was obtained in animal studies in which the entorhinal cortex was lesioned. These animals exhibited spontaneous “limbic” seizures and demonstrated an early diffuse downregulation of HCN expression throughout the hippocampal formation, which later recovered after reinnervation by afferents from the contralateral cortex (17). This reduction in Ih could be interpreted as compensatory with regard to decreased levels of afferent input from the entorhinal cortex; however, it is similarly possible that decreased HCN expression contributed to the acute seizures.
Whereas the animal model studies have demonstrated a convincing link between epileptiform activity and modulation of h-channels, they do not address whether such changes occur in the setting of human epilepsy. However, in a recent study conducted with human tissue resected from patients with temporal lobe epilepsy, dentate granule cells, which normally express low levels of Ih, showed an upregulation of HCN1 messenger RNA (mRNA) with little change in HCN2 (18). The change in HCN expression was evident only in cases of end-stage hippocampal sclerosis, long after the onset of epilepsy, suggesting that it represented a “compensatory” change in granule cell excitability in an attempt to reduce excitatory inputs into the hippocampal formation. Interestingly, in these patients, ∼80% of CA1 neurons had degenerated, but HCN1 mRNA did not appear to be reduced in surviving neurons; rather, significant HCN1 mRNA expression was seen in CA1 interneurons.
H-channels affect the behavior of interneurons as well as principal neurons and have been shown to be present in at least several classes of hippocampal interneurons (19, 20, 21). So far, limited investigation has occurred of Ih in interneurons, but several studies suggest that the presence of Ih supports spontaneous and rhythmic firing of hippocampal interneurons either by depolarizing resting potential or by mediating pacemaker activity, thus increasing the tonic inhibition of pyramidal neurons (20, 21). These finding suggest that increases in Ih in interneurons tend to oppose hyperexcitability.
Pharmacologic evidence for the role of h-channels in epilepsy remains contradictory. As mentioned, upregulation of Ih by AEDs would suggest that Ih exerts a fundamentally anticonvulsant effect (12, 13), yet blockade of h-channels with the organic blocker ZD-7288 raised afterdischarge threshold in an in vivo model of stimulus-evoked seizures, and blockade of Ih in vitro was not observed to produce epileptiform discharges (22, 23). Of course, the specificity of pharmacologic agents acting on Ih is far from absolute: AEDs likely act on multiple ion channel targets, and doubt has been cast on the specificity of ZD-7288, suggesting it may block excitatory synaptic transmission in an Ih-independent fashion as well (24).
Perhaps the strongest evidence linking h-channels with epilepsy relates to the role of Ih in the thalamocortical discharges underlying primarily generalized epilepsy. HCN2 is strongly expressed in thalamus and is clearly involved in spontaneous firing of thalamocortical neurons, whether in spindle oscillations or in the 3-Hz spike-and-wave pattern characteristic of absence epilepsy. Previous in vitro studies suggested that Ih played a critical role in determining the frequency of these oscillations and that either up- or downregulation of Ih could abolish rhythmic firing (25, 26). However, a recent study using genetically modified mice, in which HCN2 was deleted, demonstrated that these animals had spontaneous absence seizures as well as a cardiac sinus arrhythmia, suggesting that the absence of HCN2 at least is strongly proconvulsive. Similar knockouts of the HCN1 gene have yet to be fully characterized (27).
H-Channels and Epilepsy: Causation or Compensation?
The findings from an outpouring of research on h-channels incontrovertibly show that these ion channels contribute to the excitability of neurons and are modulated by neuronal activity. The question is, “What does this mean for epilepsy?” Given the multitude of actions that this current may exert—varying across brain regions, HCN channel subtypes, even within the same neuron—it is impossible to make generalizations about Ih activity as exclusively either excitatory or inhibitory. With regard to epileptogenesis, the question is further complicated by whether an observed change in Ih represents causation (producing the state of hyperexcitability) or compensation (amelioration of the hyperexcitable state). So far, the evidence is not conclusive in either direction.
Further experiments may clarify the role for Ih in epileptogenesis. Such research would ideally include studies in animal models that probe changes in Ih during the development of the epileptic state; analysis of HCN mutant animals, with conditional and brain region–specific knockouts of HCN subtypes; the identification of human HCN mutants and their correlation with disease; and the development of new pharmaceutic agents with channel-subtype specificity. Even with these data, controversy is likely to persist, but given the ubiquity of h-channels in the central nervous system (CNS) and their multiple actions on neuronal excitability, a better understanding of Ih is clearly imperative in the aim of curing epilepsy and other neurologic diseases.
References
- 1.Steinlein OK, Noebels JL. Ion channels and epilepsy in man and mouse. Curr Opin Genet Dev 2000;10: 286–291. [DOI] [PubMed] [Google Scholar]
- 2.Santoro B, Baram TZ. The multiple personalities of the h-channels. Trends Neurosci 2003;26: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K, Aradi I, Santhakumar V, Soltesz I. H-channels in epilepsy: New targets for seizure control?Trends Pharmacol Sci 2002;23: 552–557. [DOI] [PubMed] [Google Scholar]
- 4.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol 2003;65: 453–480. [DOI] [PubMed] [Google Scholar]
- 5.Luthi A, McCormick DA. H-current: Properties of a neuronal and network pacemaker. Neuron 1998;21: 9–12. [DOI] [PubMed] [Google Scholar]
- 6.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 1998;93: 717–729. [DOI] [PubMed] [Google Scholar]
- 7.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 2000;20: 5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luthi A, McCormick DA. Modulation of a pacemaker current through Ca2+-induced stimulation of cAMP production. Nat Neurosci 1999;2: 634–641. [DOI] [PubMed] [Google Scholar]
- 9.Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 2003;23: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 1998;18: 7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 1999;2: 508–514. [DOI] [PubMed] [Google Scholar]
- 12.Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci 2002;5: 767–774. [DOI] [PubMed] [Google Scholar]
- 13.Surges R, Freiman TM, Feuerstein TJ. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia 2003;44: 150–156. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med 1999;5: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med 2001;7: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci 2002;22: 4591–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brauer AU, Savaskan NE, Kole MH, Plaschke M, Monteggia LM, Nestler EJ, Simburger E, Deisz RA, Ninnemann O, Nitsch R. Molecular and functional analysis of hyperpolarization-activated pacemaker channels in the hippocampus after entorhinal cortex lesion. FASEB J 2001;15: 2689–2701. [DOI] [PubMed] [Google Scholar]
- 18.Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci 2003;23: 6826–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1–4 suggests evolving roles in the developing rat hippocampus. Neuroscience 2001;106: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupica CR, Bell JA, Hoffman AF, Watson PL. Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol 2001;86: 261–268. [DOI] [PubMed] [Google Scholar]
- 21.Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 1996;497(Pt 1):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitayama M, Miyata H, Yano M, Saito N, Matsuda Y, Yamauchi T, Kogure S. Ih blockers have a potential of antiepileptic effects. Epilepsia 2003;44: 20–24. [DOI] [PubMed] [Google Scholar]
- 23.Xiong ZQ, Stringer JL. Cesium induces spontaneous epileptiform activity without changing extracellular potassium regulation in rat hippocampus. J Neurophysiol 1999;82: 3339–3346. [DOI] [PubMed] [Google Scholar]
- 24.Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci U S A 2002;99: 9538–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue BW, Huguenard JR. The role of H-current in regulating strength and frequency of thalamic network oscillations. Thalamus Rel Syst 2001;1: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luthi A, Bal T, McCormick DA. Periodicity of thalamic spindle waves in abolished by ZD7288, a blocker of Ih. J Neurophysiol 1998;79: 3284–3289. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 2003;22: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]