Abstract
Background
Dental amalgams contain approximately 50% metallic mercury and emit small quantities of mercury vapor. Controversy surrounds whether fetal exposure to mercury vapor from maternal dental amalgams has neurodevelopmental consequences.
Methods
Maternal amalgam status during gestation (prenatal mercury vapor exposure) was determined retrospectively on 587 mother-child pairs enrolled in a prospective longitudinal cohort study of effects of prenatal and recent postnatal methylmercury exposure on neurodevelopment. Covariate-adjusted associations were examined between 6 age-appropriate neurodevelopmental tests administered at 66 months of age and prenatal maternal amalgam status. Models were fit without and with adjustment for prenatal and recent postnatal methylmercury exposure metrics.
Results
Mean maternal amalgams present during gestation were 5.1 surfaces (range 1-22) in the 42% of mothers with amalgams. No significant adverse associations were found between the number of prenatal amalgam surfaces and any of the 6 outcomes, with or without adjustment for prenatal and postnatal methylmercury exposure. Analyses using our secondary metric, prenatal amalgam occlusal point scores, showed an adverse association in males only on the Letter Word Recognition subtest of the Woodcock-Johnson Tests of Achievement, and several apparently beneficial associations for females only.
Conclusions
This study provides no support for the hypothesis that prenatal mercury vapor exposure from maternal dental amalgams results in neurobehavioral consequences in the child. These findings need confirmation from a prospective study of co-exposure to methyl mercury and mercury vapor.
Keywords: Mercury, Amalgam, Pregnancy, Outcomes
Introduction
The use of amalgams for dental restorations was introduced over 160 years ago. Favorable physical properties, superior durability, and economical cost made amalgam the preferred restorative material for billions of teeth. Recent studies indicate amalgam continues to be used frequently today. A dental practice-based research network (DPBRN) encompassing 229 dentists in Alabama, Mississippi, Florida, Georgia, Minnesota, Denmark, Norway, and Sweden, reported 38% of all recently placed restorations were amalgam, accounting for 45% of premolar restorations and 47% of molar restorations.1 Dentists participating in the Northwest PRECEDENT DPBRN (Oregon, Washington, Idaho, Montana, and Utah) reported using dental amalgam for 28% of restorations placed in children and adolescents, and for 22.7% of restorations placed in adults over the preceding 12 months.2 Placement of dental amalgam restorations remains significant in all age groups, including children and women of child-bearing age.
Dental amalgam is composed of approximately 50% metallic mercury, an inorganic form of mercury. Amalgams present in the oral cavity continuously expose an individual to small amounts of mercury vapor (Hg0) released from the surface over the lifetime of the restoration.3 Chronic exposure to higher levels of Hg0 is known to result in neurotoxicity consisting of various sensory, motor, cognitive and personality disturbances, but the lowest level of exposure where such associations occur is not presently known.3 Numerous reviews have suggested low level Hg0 exposure from dental amalgam restorations in adults is unlikely to result in adverse health effects.4-8 However, data for children is limited. Results from two randomized clinical trials in older children comparing postnatal exposure to Hg0 from dental amalgam to composite restorations found no significant differences in children with amalgam restorations on neurobehavioral assessments or nerve conduction velocity9, or in adverse neuropsychological and renal functions.10
Although Hg0 crosses the placenta, there are very limited scientific data to adequately assess whether there are health risks to the developing human fetus from maternal dental amalgams.8,11 Several animal studies suggest that adverse neurodevelopmental outcomes in offspring can be a consequence of prenatal exposure to Hg0 when exposure is at levels higher than those associated with dental restorations.12-15 Moreover, a recent study in rats found neurotoxic risk may be elevated in offspring co-exposed during gestation to Hg0 and methylmercury (MeHg), an organic form of mercury.16 Comparable human studies are lacking, as noted by an independent Scientific Committee of the European Union (2008) which stated, “with respect to populations at risk, there is a lack of information about effects in pregnant women”.6 More recently, the FDA (2009) reviewed the issue of dental amalgams and neurodevelopment and issued a report entitled ‘Final Regulation on Dental Amalgam’. In it the FDA similarly states “there is limited clinical information about the potential effects of dental amalgam fillings on pregnant women and their developing fetuses, and on children under six”.17
To address prenatal exposure to Hg0 from amalgam, we retrospectively reconstructed the maternal amalgam status during gestation for mothers of children enrolled in the Seychelles Child Development Study (SCDS) Main Cohort. This cohort of children was of interest because they were exposed to elevated levels of MeHg both prenatally and postnatally due to a diet high in fish and had already had extensive neurodevelopmental testing. We first examined the association between children's prenatal Hg0 exposure (using maternal amalgam status as a biological marker) and test results from their 66 month test battery. We then examined the association with adjustment for prenatal and recent postnatal MeHg exposures to determine whether co-exposure to the inorganic (Hg0) and organic (MeHg) forms of mercury influenced the analyses.
Methods
Subjects
The SCDS Main Cohort is a well-described group of 779 mother-infant pairs residing in the Republic of Seychelles. They were enrolled in 1989-1990 in a prospective, double-blind, longitudinal study designed to test the hypothesis that prenatal MeHg exposure from a maternal diet high in fish is related to child neurodevelopmental outcomes. At enrollment the mothers consumed an average of 12 fish meals per week.18 The children were evaluated at multiple ages through age 19 years using batteries of neuropsychological tests to determine their cognitive and neurological development.19-23 Of the original 779 mother-child pairs enrolled in the SCDS Main Cohort, 711 were evaluated at 66 months of age and were eligible to participate in this dental study. We were able to recapitulate maternal dental status in 587 mothers. This study was reviewed and approved by the institutional review boards of the University of Rochester, Rochester, NY, and the Ministry of Health, Republic of Seychelles. Informed consent/assent was obtained from all study participants.
Determination of Maternal Dental Amalgam Status and Exposure
In the Republic of Seychelles dental care is free and most residents utilize the national dental facilities where comprehensive historic records are maintained. Approximately 10 years after the birth of the child, mothers were recalled and their dentition was clinically examined for the presence of amalgam restorations. Subsequently, dental personnel completed a retrospective abstraction of the mother's dental records. They specifically addressed placement and disposition of amalgam restorations.
The detailed strategy utilized to reconstruct the maternal dental amalgam status during gestation is shown in Table 1. In brief, amalgam restorations with documentation of placement prior to pregnancy and presence after the child's birth were considered as being present during gestation, as were amalgams known to have been placed during gestation. Amalgams documented as being placed prior to pregnancy, but with no further proof of retention, and also amalgams with no history of placement, but present at the time of examination, were considered as ‘possibly’ present during gestation. Amalgams known to have been initially placed after the birth of the child were excluded.
Table 1. Strategy for Determination of Maternal Dental Amalgam Status during Gestation.
| Record of Initial Amalgam Placement | Record of Amalgam Presence | ||||||
|---|---|---|---|---|---|---|---|
| Gestation | Dental Examination | Amalgam Present During Gestation? | Hg0 Exposure Level | ||||
| Pre-Pregnancy | (1989-1990) | Postnatal | Postnatal | (1999-2000) | |||
| YES | -- | -- | -- | YES | YES | Lower | |
| YES | -- | -- | YES | YES or NO | YES | Exposure | Upper |
| -- | YES | -- | YES or NO | YES or NO | YES | Limit | Exposure |
|
| |||||||
| YES | -- | -- | NO | NO | POSSIBLY | Limit | |
| NO | NO | NO | NO | YES | POSSIBLY | ||
|
| |||||||
| NO | NO | YES | YES or NO | YES or NO | NO | Excluded | |
Amalgam restorations with documentation of being placed in maternal permanent teeth prior to pregnancy and present after the child's birth were considered as being present during gestation, as were amalgam restorations placed during gestation. Amalgam restorations documented to be placed prior to pregnancy, but with no further proof of retention, and separately, amalgam restorations with no history of placement, but present at the time of examination, were considered as ‘possibly’ present during gestation. Restorations known to be initially placed after the birth of the child were excluded.
Our primary metric of prenatal Hg0 exposure was the total number of amalgam surfaces present in the mother during gestation. This metric takes into account all surfaces of amalgam available for Hg0 release and has been extensively used as a measure of exposure.10,24-30 We also used a secondary exposure metric modified from the “amalgam points” scoring of Olstad et al.31 For this metric we considered only the occlusal surfaces of amalgams on premolars and molars, and assigned a score of 1 for small size occlusal amalgams such as pits, 2 for medium size occlusal amalgams on premolars, and 3 for large size occlusal amalgams on molars. Significant release of Hg0 from amalgams during chewing has been well documented and likely occurs primarily from the occlusal amalgam surfaces.32 Maserejian and coworkers studied various amalgam exposure measures in children and found the metric that included posterior occlusal surfaces to be the best predictor of cumulative urinary Hg excretion, the common biomarker of Hg0 exposure.30 The summed scores constitute our “occlusal points” score and are more representative of the surface area available to actively release Hg0 from amalgams during chewing.
To account for the uncertainty regarding the true maternal gestational amalgam status in this retrospective reconstruction, we determined two levels of exposure to the amalgam metrics in our statistical models (Table 1). The Lower Exposure Limit (LEL) is the total of all amalgam surfaces or occlusal points with a high likelihood of having been present during gestation, while the Upper Exposure Limit (UEL) includes all the LEL points or surfaces, plus those amalgams possibly present during gestation.
We also created indicator variables for whether any amalgam surfaces or any occlusal points were placed during pregnancy. These variables were considered in secondary models.
Metrics of Other Exposures
Prenatal MeHg exposure was previously determined by assessing the concentration of total mercury (THg) in a segment of maternal hair growing during gestation.19 Recent postnatal MeHg was previously determined by measuring THg in the 1 cm segment of hair closest to the child's scalp at the time the test battery was administered.21 Greater than 80% of THg in hair samples from a fish-eating population is in the form of MeHg, and THg is a commonly used marker of MeHg exposure.33,34,35 Mercury concentrations in hair correlate with consumption of fish, but not with Hg0 or the number of amalgam restorations.26,36,37,38 Maternal amalgam status and maternal THg therefore represent separate and discreet exposure metrics for prenatal Hg0 and prenatal MeHg, respectively. Exposure levels of other toxicants in Seychelles are low. Lead levels in whole blood from Seychellois children and mothers were determined previously to be less than 0.48 μmol/L (10 μg/dL).33 Levels of polychlorinated biphenyls in blood from a subset of 49 cohort children at age 66 months were below the limit of detection (0.2 ng/mL).21
Outcomes and Other Measures
At age 66 months a comprehensive test battery was administered to the children.21 The test battery assessed overall intelligence [General Cognitive Index (GCI) of the McCarthy Scales of Children's Abilities (MSCA)]39, expressive and receptive language ability [Preschool Language Scale (PLS) Total Score]40, reading and arithmetic achievement [Letter Word Recognition and Applied Problems subtests of the Woodcock-Johnson (W-J) Tests of Achievement]41, drawing and copying to measure visual-spatial ability (Bender Gestalt test using the Koppitz scoring protocol)42, and the child's social and adaptive behavior [Child Behavior Checklist (CBCL)]43. For the GCI, PLS, and both W-J Tests of Achievement a higher score indicates better performance. For the Bender Gestalt and CBCL a lower score indicates improved performance
Covariates measured during the evaluation included sex, birth weight, maternal age, the preschool version of the Home Observation for Measurement of the Environment (HOME: an in home observation that rates quality of the home environment and interactions between parent and child)44, Hollingshead Four- Factor SES45 (an index combining educational level and job classification of both parents), pure tone hearing thresholds on the children, caregiver IQ (Raven Standard Progressive Matrices, a culture-free test which uses visual geometric drawings of increasing complexity)46.
Statistical Analysis
We examined the covariate-adjusted associations between each of the six outcomes and prenatal Hg0 exposure, using four different estimators of prenatal Hg0 exposure: the number of amalgam surfaces using the LEL, amalgam surfaces using the UEL, occlusal point score using the LEL, and occlusal point score using the UEL. Models for each outcome and Hg0 exposure metric were fit without and then with adjustment for prenatal and recent postnatal MeHg exposure metrics. Each model was first examined for an interaction between the prenatal Hg0 metric and sex. If the interaction was significant, then the model was reported with the interaction. Otherwise, the model was rerun and reported without this interaction. The Hg0 metric by sex interaction allows the slope relating the Hg0 metric to the outcomes to differ for males and females. Because there was no biological reason to expect non-additive effects of prenatal Hg0 and MeHg exposures, we did not examine an interaction between MeHg exposure and the prenatal Hg0 metric.
All regression models adjusted for the same covariates as in the primary analysis for this cohort, which were chosen based on their potential to impact the association between Hg and outcomes.21 The child-related covariates included sex, birth weight, child's medical history, and child's hearing status. The maternal and family-related covariates included maternal age, HOME score, Hollingshead SES, and caregiver intelligence (Raven score). All covariates were treated as continuous variables except for sex, child medical history, and the child's hearing level. The latter was modeled in three discrete levels (0-25, 26-35, and >35 dB). We included the postnatal MeHg by sex interaction only for the Bender Gestalt Errors outcome, to be consistent with previously reported results.21 Models for each outcome were fit using subjects with complete covariate and dental data, and non-missing values for the outcome.
Only results from significant models (p≤ 0.05) are reported. Models that are significant may have significant and/or non-significant predictors within them. Thus prenatal Hg0 may or may not be a significant predictor in any given model. Model assumptions were checked using standard methods, and if violated, transformations were considered.47 Model results include outlying values, when they are present.
Additional, secondary models were fit that adjusted for whether the mother had any amalgams or occlusal points placed during pregnancy.
Results
Complete dental and covariate data were available on 587 women. Some children were unable to complete all the tests. Summary statistics for all continuous variables, both overall and by sex, are presented in Table 2. In the LEL group, 249 mothers (42.4%) had at least one amalgam. Three of these had only non-occlusal amalgams and thus no occlusal points, while the remaining 246 had at least one occlusal amalgam. For both the LEL and UEL, the occlusal point score and number of amalgam surface metrics were highly correlated (r= 0.928 for the LEL and r= 0.916 for the UEL, data not shown).
Table 2. Summary Statistics for Continuous Variables, Overall and by Sex.
| All Groups | Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Mean | SD | Minimum | Maximum | Mean | SD | Mean | SD |
| McCarthy GCI | 587 | 94.48 | 12.44 | 49.00 | 126.00 | 95.63 | 12.74 | 93.35 | 12.04 |
| PLS Total Score | 547 | 70.10 | 6.55 | 49.00 | 90.00 | 70.80 | 6.77 | 69.43 | 6.27 |
| W-J Applied Problems | 584 | 87.33 | 17.23 | 8.00 | 138.00 | 88.70 | 17.79 | 85.98 | 16.57 |
| W-J Letter Word | 583 | 77.01 | 10.57 | 47.00 | 116.00 | 77.76 | 10.57 | 76.27 | 10.53 |
| Bender Gestalt Errors Raw Scores | 573 | 10.18 | 3.79 | 0.00 | 21.00 | 10.54 | 3.73 | 9.82 | 3.81 |
| CBCL Total T Score | 586 | 59.64 | 9.90 | 24.00 | 92.00 | 59.40 | 10.06 | 59.88 | 9.75 |
| Prenatal MeHg | 587 | 6.71 | 4.43 | 0.54 | 22.74 | 6.84 | 4.48 | 6.59 | 4.38 |
| Postnatal MeHg | 587 | 6.44 | 3.30 | 0.88 | 25.81 | 6.68 | 3.28 | 6.21 | 3.30 |
| Prenatal Hg0 (LEL Surfaces) | 249 | 5.12 | 4.11 | 1.00 | 22.00 | 4.82 | 4.07 | 5.37 | 4.13 |
| Prenatal Hg0 (UEL Surfaces) | 433 | 6.75 | 5.29 | 1.00 | 31.00 | 6.16 | 4.85 | 7.28 | 5.60 |
| Prenatal Hg0 (LEL Occlusal Points) | 246 | 8.43 | 5.77 | 2.00 | 28.00 | 8.14 | 5.63 | 8.66 | 5.89 |
| Prenatal Hg0 (UEL Occlusal Points) | 431 | 11.05 | 7.17 | 2.00 | 45.00 | 10.59 | 6.77 | 11.46 | 7.50 |
| Birth Weight (kg) | 587 | 3.21 | 0.49 | 1.50 | 5.32 | 3.15 | 0.47 | 3.27 | 0.51 |
| Maternal Age | 587 | 25.96 | 5.78 | 14.33 | 44.79 | 26.06 | 5.64 | 25.86 | 5.92 |
| HOME Score | 587 | 33.30 | 5.32 | 13.00 | 47.00 | 33.58 | 4.96 | 33.03 | 5.65 |
| Hollingshead SES | 587 | 26.03 | 10.27 | 5.00 | 61.00 | 26.15 | 10.48 | 25.91 | 10.08 |
| Caregiver Intelligence (Raven) | 587 | 23.30 | 10.44 | 3.00 | 56.00 | 23.55 | 10.18 | 23.06 | 10.71 |
Summary statistics for all data by group and by sex [ Females (n = 291), Males (n = 296)] are shown. Values for maternal amalgam metrics include only the subset of mothers with amalgams. Abbreviations: GCI (General Cognitive Index); PLS (Preschool Language Score); W-J (Woodcock-Johnson); CBCL (Child Behavior Checklist; MeHg (Methylmercury); LEL (Lower Exposure Limit); UEL (Upper Exposure Limit); HOME (Home Observation for Measurement of the Environment); SES (Socioeconomic Status).
Prenatal and recent postnatal MeHg exposure levels for this subset of the original cohort were comparable with the original full cohort.21 The mean maternal hair THg level was 6.7 (±4.4) ppm (range 0.5-22.8 ppm) for the 587 subset mothers with full covariate data. The hair THg level in this subset of children at 66 months (n = 587) was 6.4 (±3.3) ppm (range 0.9-25.8 ppm). There was no association between maternal LEL surfaces and prenatal (r = 0.01) or postnatal (r = -0.07) MeHg (hair THg) exposure. The means of the outcomes and covariates by prenatal MeHg and Hg0 exposure categories are shown in Table 3.
Table 3. Means of Outcomes and Covariates by Prenatal Mercury Exposure Categories and by Amalgam Status.
| Prenatal Hg0 Exposure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Surfaces | Number of Occlusal Points | |||||||||||
| Prenatal MeHg Exposure (ppm) | LEL | UEL | LEL | UEL | ||||||||
| 0-3 | 3 - 6 | 6 - 9 | 9+ | 0 | 1+ | 0 | 1+ | 0 | 1+ | 0 | 1+ | |
| Sample Size (n) | 135 | 170 | 127 | 155 | 338 | 249 | 154 | 433 | 341 | 246 | 156 | 431 |
| McCarthy GCI | 95.00 | 94.17 | 95.24 | 93.75 | 93.84 | 95.35 | 92.01 | 95.36 | 93.91 | 95.28 | 92.07 | 95.35 |
| PLS Total Score | 69.86 | 69.58 | 70.06 | 70.91 | 69.59 | 70.79 | 68.66 | 70.60 | 69.57 | 70.83 | 68.68 | 70.60 |
| W-J Applied Problems | 85.56 | 87.84 | 87.55 | 88.15 | 86.54 | 88.42 | 83.09 | 88.85 | 86.59 | 88.38 | 83.28 | 88.81 |
| W-J Letter Word | 76.55 | 77.61 | 77.12 | 76.67 | 77.08 | 76.92 | 75.77 | 77.45 | 77.09 | 76.90 | 75.83 | 77.44 |
| Bender Gestalt Errors | 10.06 | 10.32 | 9.98 | 10.29 | 10.36 | 9.94 | 11.09 | 9.86 | 10.34 | 9.95 | 11.03 | 9.87 |
| CBCL Total T Score | 60.19 | 59.68 | 58.97 | 59.68 | 59.26 | 60.16 | 59.62 | 59.65 | 59.33 | 60.07 | 59.62 | 59.65 |
| Sex | 0.55 | 0.47 | 0.47 | 0.53 | 0.47 | 0.55 | 0.44 | 0.53 | 0.47 | 0.55 | 0.44 | 0.53 |
| Birth weight (kg) | 3.16 | 3.17 | 3.20 | 3.30 | 3.19 | 3.23 | 3.16 | 3.22 | 3.19 | 3.23 | 3.17 | 3.22 |
| Maternal age | 25.12 | 26.52 | 25.76 | 26.25 | 26.52 | 25.20 | 27.05 | 25.57 | 26.55 | 25.15 | 26.99 | 25.59 |
| HOME score | 32.76 | 33.35 | 33.64 | 33.46 | 32.72 | 34.10 | 31.12 | 34.08 | 32.76 | 34.06 | 31.21 | 34.06 |
| Hollingshead SES | 26.33 | 26.03 | 27.02 | 24.97 | 24.64 | 27.92 | 22.31 | 27.35 | 24.69 | 27.89 | 22.48 | 27.32 |
| Caregiver Intelligence | 23.44 | 23.29 | 23.61 | 22.95 | 22.79 | 24.01 | 20.96 | 24.14 | 22.76 | 24.06 | 21.24 | 24.05 |
Abbreviations: GCI (General Cognitive Index); PLS (Preschool Language Score); W-J (Woodcock-Johnson); CBCL (Child Behavior Checklist; LEL (Lower Exposure Limit); UEL (Upper Exposure Limit); HOME (Home Observation for Measurement of the Environment); SES (Socioeconomic Status). Higher GCI, PLS, and W-J Applied Problems/Letter Word scores indicate improved performance. Lower Bender Gestalt Errors and CBCL Total Scores indicate improved performance.
Primary analyses
All models were significant for both amalgam surfaces and points. We first used the number of amalgam surfaces to estimate regression coefficients for the six outcome variables for covariate-adjusted models using the LEL without (model 1) and with (model 2) adjustment for prenatal and postnatal MeHg. These results are presented in Table 4.
Table 4.
Linear regression coefficients (p-values) for covariate-adjusted models using LEL amalgam SURFACES to estimate Hg0 exposure. Models are without (Model 1) and with (Model 2) adjustment for prenatal and recent postnatal MeHg exposure. Any model for which there is a significant sex by exposure interaction (p<0.05) shows separate exposure slopes for males and females. If this interaction is not significant, the overall exposure effect is shown.
| Parameter Estimates | Regression Coefficients (p-values) | |||||
|---|---|---|---|---|---|---|
| Woodcock-Johnson Tests of Achievement | ||||||
| McCarthy GCI | PLS Total Score | Applied Problems | Letter and Word Recognition | Bender Gestalt Errors | CBCL Total T Score | |
| Sample Size (n) | 587 | 547 | 584 | 583 | 573 | 586 |
|
| ||||||
| Prenatal Hg0 Without Adjustment for MeHg (Model 1) | ||||||
|
| ||||||
| LEL Prenatal Hg0 Amalgam surfaces | N/A | 0.05 (0.50) | -0.16 (0.40) | -0.12(0.12) | -0.05 (0.28) | 0.06 (0.61) |
|
| ||||||
| LEL Prenatal Hg0Amalgam Surfaces:Females | 0.29 (0.17) | N/A | N/A | N/A | N/A | N/A |
|
| ||||||
| LEL Prenatal Hg0 Amalgam Surfaces:Males | -0.25(0.17) | N/A | N/A | N/A | N/A | N/A |
|
|
||||||
| Prenatal Hg0 With Adjustment for MeHg (Model 2) | ||||||
|
| ||||||
| LEL Prenatal Hg0 Amalgam Surfaces | N/A | 0.06 (0.39) | -0.14 (0.48) | -0.17 (0.15) | -0.05 (0.22) | 0.06 (0.61) |
|
| ||||||
| LEL Prenatal Hg0 Amalgam Surfaces:Females | 0.31 (0.13) | N/A | N/A | N/A | N/A | N/A |
|
| ||||||
| LEL Prenatal Hg0 Amalgam Surfaces:Males | -0.24 (0.19) | N/A | N/A | N/A | N/A | N/A |
|
| ||||||
| Prenatal MeHg | -0.08 (0.48) | 0.12 (0.06) | 0.11 (0.50) | -0.01 (0.88) | 0.01 (0.70) | -0.02 (0.80) |
|
| ||||||
| Recent Postnatal MeHg | 0.35 (0.03) | 0.22 (0.01) | 0.54 (0.01) | 0.19 (0.15) | N/A | 0.01 (0.92) |
|
| ||||||
| Recent Postnatal MeHg:Females | N/A | N/A | N/A | N/A | 0.02 (0.74) | N/A |
|
| ||||||
| Recent Postnatal MeHg:Males | N/A | N/A | N/A | N/A | -0.20 (0.01) | N/A |
|
| ||||||
| Birth weight | 1.97 (0.06) | 1.02 (0.07) | 3.68 (0.01) | 0.17 (0.85) | -0.33 (0.30) | -0.21 (0.81) |
|
| ||||||
| Child's medical history | -1.49 (0.59) | 1.01 (0.48) | -0.59 (0.88) | -1.89 (0.43) | 1.24 (0.14) | 1.77 (0.43) |
|
| ||||||
| Maternal age | -0.05 (0.55) | -0.01 (0.91) | -0.02 (0.86) | 0.02 (0.80) | -0.01 (0.79) | -0.26 (≪0.01) |
|
| ||||||
| HOME | 0.54 (≪ 0.01) | 0.28 (≪0.01) | 0.68 (≪0.01) | 0.36 (≪0.01) | -0.18 (≪0.01) | -0.26 (≪0.01) |
|
| ||||||
| Hollingshead SES | 0.07 (0.25) | 0.04 (0.26) | 0.07 (0.39) | 0.16 (≪0.01) | 0.00 (0.95) | -0.10 (0.03) |
|
| ||||||
| Hearing group one | 0.42 (0.85) | 0.41 (0.74) | 3.16 (0.30) | -1.38 (0.47) | -0.85 (0.20) | 0.27 (0.88) |
|
| ||||||
| Hearing group two | 3.75 (0.35) | 1.62 (0.52) | 7.27 (0.22) | 0.76 (0.84) | -1.33 (0.28) | -1.42 (0.66) |
|
| ||||||
| Caregiver Intelligence (Raven) | 0.09 (0.09) | 0.06 (0.03) | 0.17 (0.02) | 0.01 (0.79) | -0.03 (0.07) | -0.03 (0.56) |
Abbreviations: Hg0 (Mercury Vapor); MeHg (Methylmercury); LEL (Lower Exposure Limit); HOME (Home Observation for Measurement of the Environment); SES (Socioeconomic Status); N/A (Not Applicable). Higher GCI, PLS, and W-J Applied Problems/Letter Word scores indicate improved performance. Lower Bender Gestalt Errors and CBCL Total Scores indicate improved performance.
Amalgam surfaces were not significantly associated with any outcome, either without or with adjustment for prenatal and recent postnatal MeHg. However, there was a significant interaction of sex by LEL amalgam surfaces for the GCI endpoint (p = 0.04) after adjusting for prenatal and recent postnatal MeHg and covariates. For the GCI, the amalgam slope was positive for females (0.31, 95% CI: -0.09 to 0.72) and negative (-0.24, 95% CI: -0.59 to 0.12) for males, but neither slope was significantly different from zero (Table 4). The relationship between LEL amalgam surfaces and GCI is illustrated in Figure 1. The plot suggests that for a small number of amalgam surfaces, the GCI outcomes are similar for both sexes. However, as the number of maternal amalgam surfaces increases, males are predicted to perform less well, while females are predicted to have better performance. As expected, a higher HOME score was a very significant predictor of improved scores in all models and higher SES and caregiver IQ were also significant predictors of improved scores in some models.
Figure 1.
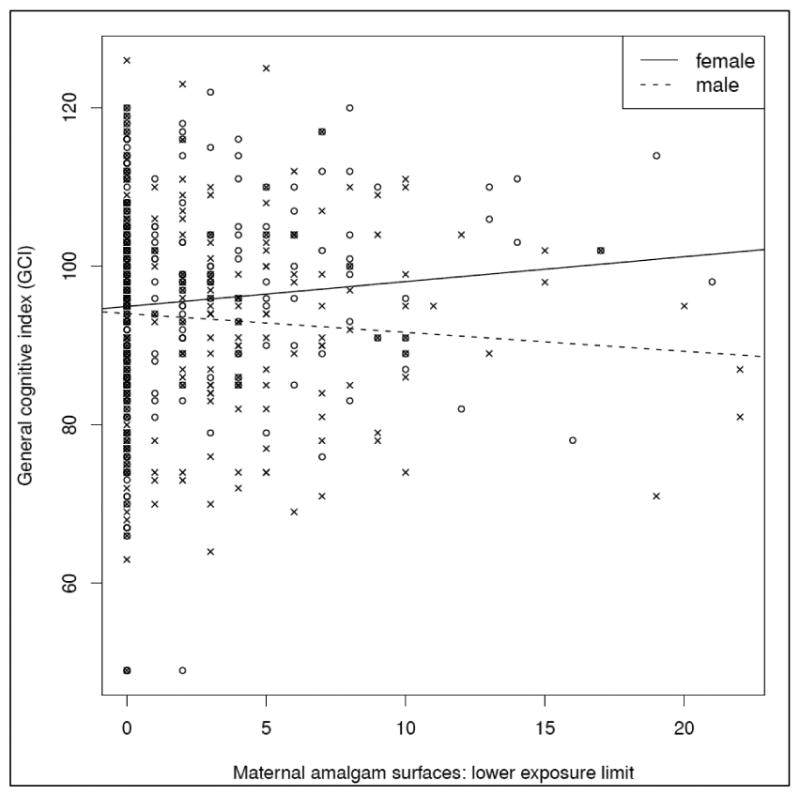
Relationship between amalgam surfaces (Hg0) and General Cognitive Index using the LEL. The plot shows individual data points for males (crosses) and females (circles). Lines show predicted values for males (dotted lines) and females (solid lines) from the linear regression described for model 2 that allows separate sex-specific slopes for amalgam surfaces and is adjusted for prenatal MeHg, postnatal MeHg, birth weight, mother's age, HOME score, Hollingshead SES, Caregiver Intelligence, and child's medical history and hearing status. Although the sex by prenatal Hg0 interaction is significant (p = 0.04), neither male (p = .19) nor female (p = .13) slopes were significantly different from zero.
Similar results (coefficients and p-values) were obtained using the UEL number of amalgam surfaces as the metric of Hg0 exposure (data not shown). The UEL amalgam surface metric was not a significant predictor for any outcomes. When adjusting for UEL amalgam surfaces, increasing recent postnatal MeHg exposure was associated with significant improvement in both sexes in the same outcomes: GCI (0.35, p = 0.02), PLS Total Score (0.22, p = 0.01), and W-J Applied Problems (0.57, p = 0.01). In addition there were fewer Bender Gestalt Errors with increasing postnatal MeHg exposure (-0.20, p = 0.01) in male children.
We next examined the association between our secondary metric, the occlusal amalgam point score, and the same outcomes. Results for both LEL and UEL adjusting for prenatal and recent postnatal MeHg and covariates (Model 2) are given in Table 5. The amalgam occlusal points-by-sex interaction term was significant for the GCI (LEL and UEL) and for three other outcomes at UEL (PLS Total Score and the W-J for both the Applied Problem and Letter-Word Recognition subtests). For these outcomes, the slope for amalgam points was significantly different for males and females. Males performed less well on each outcome compared to females as occlusal points increased. However, a significant interaction does not necessarily mean that the amalgam point slope was significantly different from zero (i.e. significantly adverse or beneficial) for either males or females. There was a significant adverse association of the UEL occlusal point score in males on a single outcome (W-J Letter Word Recognition; slope = -0.16; p = 0.04; 95% CI: -0.31, -0.01). There was also a significant improvement in scores for females on the GCI (with LEL and UEL), the PLS total score (UEL) and the W-J Applied Problem subtest (UEL).
Table 5.
Linear regression coefficients (and p-values) for covariate-adjusted models using occlusal POINTS to estimate Hg0 exposure, adjusted for pre- and recent postnatal MeHg exposure (Model 2). Results using both the lower exposure limit (LEL) and upper exposure limit (UEL) are given. Any model for which there is a significant sex by exposure interaction (p<0.05) shows separate exposure slopes for males and females. If this interaction is not significant, the overall exposure effect is shown.
| Regression coefficients (p-values) | ||||||
|---|---|---|---|---|---|---|
| Woodcock-Johnson Tests of Achievement | ||||||
| McCarthy GCI | PLS Total Score | Applied Problems | Letter and Word Recognition | Bender Gestalt Errors | CBCL Total T Score | |
| Sample Size (n) | 587 | 547 | 584 | 583 | 573 | 586 |
|
| ||||||
| Lower Exposure Limit (LEL) | ||||||
|
| ||||||
| Prenatal (Hg0) Amalgam Occlusal Points | N/A | 0.07 (0.19) | -0.01 (0.93) | -0.11 (0.17) | -0.04 (0.14) | 0.04 (0.56) |
|
| ||||||
| Prenatal Amalgam Occlusal Points:Females | 0.31 (0.02) | N/A | N/A | N/A | N/A | N/A |
|
| ||||||
| Prenatal Amalgam Occlusal Points:Males | -0.18 (0.14) | N/A | N/A | N/A | N/A | N/A |
|
| ||||||
| Prenatal MeHg Exposure | -0.08 (0.50) | 0.11 (0.06) | 0.11 (0.51) | -0.01 (0.91) | 0.02 (0.66) | -0.03 (0.79) |
|
| ||||||
| Recent Postnatal MeHg Exposure | 0.35 (0.02) | 0.22 (0.01) | 0.55 (0.01) | 0.20 (0.13) | N/A | 0.01 (0.93) |
|
| ||||||
| Recent Postnatal MeHg:Females | N/A | N/A | N/A | N/A | 0.02 (0.74) | N/A |
|
| ||||||
| Recent Postnatal MeHg:Males | N/A | N/A | N/A | N/A | -0.20 (0.01) | N/A |
|
| ||||||
| Upper Exposure Limit (UEL) | ||||||
|
| ||||||
| Prenatal (Hg0) Amalgam Occlusal Points | N/A | N/A | N/A | N/A | -0.04 (0.10) | -0.04 (0.52) |
|
| ||||||
| Prenatal Amalgam Occlusal Points:Females | 0.23 (0.02) | 0.13 (0.01) | 0.33 (0.02) | 0.14 (0.10) | N/A | N/A |
|
| ||||||
| Prenatal Amalgam Occlusal Points:Males | -0.15 (0.10) | -0.05 (0.36) | -0.14 (0.26) | -0.16 (0.04) | N/A | N/A |
|
| ||||||
| Prenatal MeHg Exposure | -0.08 (0.46) | 0.11 (0.06) | 0.09 (0.56) | -0.03 (0.78) | 0.01 (0.73) | -0.02 (0.80) |
|
| ||||||
| Recent Postnatal MeHg Exposure | 0.36 (0.02) | 0.22 (0.01) | 0.57 (0.01) | 0.22 (0.10) | N/A | 0.01 (0.96) |
|
| ||||||
| Recent Postnatal MeHg:Females | N/A | N/A | N/A | N/A | 0.02 (0.74) | N/A |
|
| ||||||
| Recent Postnatal MeHg:Males | N/A | N/A | N/A | N/A | -0.20 (0.01) | N/A |
Abbreviations: Hg0 (Mercury Vapor); MeHg (Methylmercury); LEL (Lower Exposure Limit); UEL (Upper Exposure Limit); N/A (Not Applicable). Higher GCI, PLS, and W-J Applied Problems/Letter Word scores indicate improved performance. Lower Bender Gestalt Errors and CBCL Total Scores indicate improved performance.
Secondary analyses
Among the 587 women with complete dental and covariate data, 78 had one or more amalgam surfaces placed during pregnancy, and 76 had at least one occlusal point placed during pregnancy. Placement of amalgam surfaces or occlusal points during pregnancy did not significantly predict any outcome after adjusting for other model covariates and exposures (data not shown).
Comment
In the primary analysis that included 48 models we found no adverse association between exposure using either surfaces or occlusal point scores and 46 endpoints. There was an adverse association between amalgam points and the W-J Letter Word score. Prenatal Hg0 exposure using our secondary metric, the occlusal point score, showed a significant slope of -0.16 (p = 0.04) in males indicating an adverse association. This finding was present only using the UEL and was present in two models [without (data not shown) and with (Table 5) adjustment for prenatal and recent postnatal MeHg exposure]. There were no other adverse associations between any of the 4 dental amalgam scores and the 6 endpoints either without or with adjustment for MeHg. The UEL carries a higher degree of uncertainty than the LEL since it is derived by including ‘possible’ restorations. Considering we fit 48 primary models, the presence of two adverse associations could be a chance finding and does not imply that prenatal exposure to Hg0 from dental amalgams results in neurobehavioral consequences. Accumulation of Hg in the fetal brain attributable to maternal inhalation of Hg0 is reported to be significantly less than in the maternal brain.48 This is likely due to ‘first pass’ oxidation of Hg0 in the fetal liver to divalent mercury (Hg++), which does not cross the blood-brain barrier as well as Hg0.49 However, several studies have found that males are more susceptible to the toxic effects of mercury than females.50,51,52 The two adverse findings in males are therefore intriguing and warrant further prospective investigation.
We found several significant interactions with sex, both using our primary surface metric (Table 4) and our secondary occlusal points metric (Table 5). Most of the significant interactions were with the UEL metric. As the number of prenatal occlusal points increased, females performed significantly better on the GCI (with both LEL and UEL), the PLS Total Score (with the UEL), and the W-J Applied Problems Score (with the UEL), in covariate-adjusted models, with and without adjustment for pre and postnatal MeHg. We know of no scientific reason to believe that maternal amalgam might improve neurodevelopmental outcomes in either sex. Dental care is free in Seychelles and access to services should be equivalent. However, higher SES mothers may place a greater value on regular dental care and optimally utilize restorative services. Although higher SES might favorably influence outcomes, it would not explain the apparent disparity between males and females. The findings could also be spurious.
A compelling reason for examining this cohort of children was to explore whether the risk of adverse neurodevelopmental outcomes was accentuated by co-exposure to MeHg and Hg0. Children in this cohort were exposed prenatally and postnatally to elevated levels of MeHg. Consumption of fish is the primary protein source for inhabitants of the island nation of Seychelles. Mothers in this study reported eating approximately 12 fish meals per week during pregnancy.21 This high level of fish consumption resulted in a mean prenatal MeHg (hair THg) exposure of 6.7 ppm. The children also consumed fish and their recent postnatal MeHg exposure was 6.4 ppm on average. In comparison, the 1999-2000 U.S. NHANES study found a mean exposure (hair THg) of 0.47 ppm in females of child bearing age (16-49 years of age) and postnatal exposure of 0.22 ppm in children aged 1-5 years.53 Compared to the US NHANES, the Seychelles mean prenatal MeHg exposure was 14 times higher and the postnatal exposure was 29 times higher. Our models of prenatal and recent postnatal MeHg exposure in this study (Tables 4 and 5) confirm the absence of any detectable adverse influence of prenatal and recent postnatal MeHg exposures, as reported in our earlier analyses of the entire cohort.21
To our knowledge, this is the first study to comprehensively examine the risk of prenatal Hg0 exposure from maternal amalgams. The study's strengths include a large, well-defined cohort, sensitive neurodevelopmental assessments, and the presumption that if adverse effects were present, they may be more readily detectable in subjects with other forms of mercury exposure. Moreover, we utilized two different metrics as biomarkers of Hg0 exposure from amalgam restorations and did find significant associations between covariates known to influence child development and endpoints. This suggests there was sufficient power to detect associations if they were present.
The most significant limitation of this study was its retrospective design. We determined the maternal amalgam status during gestation by examining the mothers 10 years after delivery and then reviewing their past dental records to complete the picture. Although dental records in Seychelles were very good, we adjusted for this uncertainty by utilizing two measures of exposure. The LEL and the UEL represent the most likely ‘minimum’ and ‘maximum’ respective numbers of amalgams present in the mother during pregnancy. The true exposure level presumably lies ‘bracketed’ between these levels. It is most likely closer to the LEL, which represents amalgams with documented presence. Consequently, some amalgam exposures could have been misclassified. In addition, there may have been covariates that were important that were not measured.
We do not consider results of this retrospective study to be definitive and are currently studying developmental outcomes in another cohort of children in which maternal amalgam status during gestation was recorded prospectively.
Conclusion
We carried out a retrospective study of prenatal Hg0 exposure from maternal dental amalgams to determine if there were adverse associations with children's neurodevelopment. We found two adverse associations present only in males after examining two Hg0 exposure measures, six neurodevelopmental endpoints, and 48 models. We do not believe these data support the hypothesis that prenatal exposure to Hg0 from amalgam is harmful to the developing fetus.
Acknowledgments
We are especially grateful to members of the Oral Health Directorate, Ministry of Health, Seychelles (Elisabeth Arissol, Marie Helene Dogley, Agnes Elizabeth, Helena Elizabeth, Kathleen Ernesta, Dr. Harold Pothin, and Dr. Eric Van Holle). We thank Margaret Langdon for technical assistance.
Funding/Support: This research was supported by National Institute of Environmental Health Sciences (NIEHS) and National Institute of Dental and Craniofacial Research (NIDCR) (ES-15578, ES-05497, ES-01247, and ES-07271), by a grant from the United States Food and Drug Administration (FDA), and by the Ministry of Health (MOH), Victoria, Mahé, Republic of Seychelles.
Biographies
Gene E. Watson, D.D.S., Ph.D. is an associate professor, Eastman Institute for Oral Health, Department of Environmental Medicine, and Department of Pharmacology and Physiology, University of Rochester, Rochester, NY. He contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Miranda Lynch, Ph.D. was a student in the Department of Biostatistics and Computational Biology, University of Rochester, Rochester, NY. She now is a post-doctoral student in the Department of Biostatistics, Harvard School of Public Health, Boston, MA. She contributed to the analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and statistical analysis.
Gary J. Myers, M.D. is a professor, Department of Neurology, University of Rochester, Rochester, NY. He contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Conrad F. Shamlaye, M.D. is an epidemiologist, Republic of Seychelles Ministry of Health, Victoria, Mahé, Seychelles. He contributed to the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, and study supervision.
Sally W. Thurston, Ph.D. is an associate professor, Department of Biostatistics and Computational Biology, University of Rochester, Rochester, NY. She contributed to the analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and statistical analysis.
Grazyna Zareba, Ph.D. is a research assistant professor, Department of Environmental Medicine, University of Rochester, Rochester, NY. She contributed to the acquisition of data, critical revision of the manuscript, and technical support.
Thomas W. Clarkson, Ph.D. is professor emeritus, Department of Environmental Medicine, University of Rochester, Rochester, NY. He contributed to the study concept and design, critical revision of the manuscript, and technical support.
Philip W. Davidson, Ph.D. is a professor, Department of Pediatrics, University of Rochester, Rochester, NY. He contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Footnotes
Conflict of Interest Disclosures: The authors declare no conflicts of interest.
References
- 1.Nascimento MM, Gordan VV, Qvist V Dental Practice-Based Research Network Collaborative Group. Reasons for placement of restorations on previously unrestored tooth surfaces by dentists in The Dental Practice-Based Research Network. J Am Dent Assoc. 2010 Apr;141(4):441–8. doi: 10.14219/jada.archive.2010.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeRouen TA, Cunha-Cruz J, Hilton TJ Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry (PRECEDENT) What's in a dental practice-based research network? Characteristics of Northwest PRECEDENT dentists, their patients and office visits. J Am Dent Assoc. 2010 Jul;141(7):889–99. doi: 10.14219/jada.archive.2010.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. (WHO) Environmental Health Criteria 118: Inorganic Mercury. Geneva, Switzerland: WHO; 1991. [Google Scholar]
- 4.Conseil d'Evaluation des Technologies de la Sante du Quebec (CETS) The safety of dental amalgam: a state-of-the-art review. Int J Technol Assess Health Care. 1997;13:639–642. [PubMed] [Google Scholar]
- 5.World Health Organization. WHO consensus statement on dental amalgam. [Accessed March 4, 2011];1997 http://www.fdiworldental.org/sites/default/files/statements/English/WHO-consensus-statement-on-dental-amalgam-1997.pdf.
- 6.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) The safety of dental amalgam and alternative dental restoration materials for patients and users. [Accessed March 4, 2011];2008 http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_016.pdf.
- 7.Health Canada. Mercury and human health. [Accessed March 4, 2011];2008 http://www.hc-sc.gc.ca/hl-vs/alt_formats/pacrb-dgapcr/pdf/iyh-vsv/environ/merc2008-eng.pdf.
- 8.Brownawell AM, editor. Life Science Research Office. Review and analysis of the literature on the potential adverse health effects of amalgam. Rockville, MD: Life Sciences Research Office; 2004. [Google Scholar]
- 9.DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitão J, Castro-Caldas A, Luis H, Bernardo M, Rosenbaum G, Martins, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006 Apr 19;295(15):1784–92. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Trachtenberg F, Barregard L, et al. Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. JAMA. 2006;295:1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Dental Amalgam: a scientific review and recommended public health service strategy for research, education and regulation. [Accessed March 4, 2011];1993 http://www.health.gov/environment/amalgam1/ct.htm.
- 12.Frederiksson A, Dencker L, Archer T, Danielsson BRG. Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol. 1996;18:129–134. doi: 10.1016/0892-0362(95)02059-4. [DOI] [PubMed] [Google Scholar]
- 13.Danielsson BRG, Frederiksson A, Dahlgren L, et al. Behavioral effects of prenatal metallic mercury inhalation exposure in rats. Neurotoxicol Teratol. 1993;15:391–396. doi: 10.1016/0892-0362(93)90056-t. [DOI] [PubMed] [Google Scholar]
- 14.Morgan DL, Chandra SM, Price HC, et al. Disposition of inhaled mercury vapor in pregnant rats: maternal toxicity and effects on developmental outcome. Toxicol Sci. 2002;66:261–273. doi: 10.1093/toxsci/66.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Newland MC, Warfinge K, Berlin M. Behavioral consequences of in utero exposure to mercury vapor: alterations in lever-press durations and learning in squirrel monkeys. Toxicol Appl Pharmacol. 1996;139:374–386. doi: 10.1006/taap.1996.0178. [DOI] [PubMed] [Google Scholar]
- 16.Ishitobi H, Stern S, Thurston SW, et al. Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ Health Perspect. 2010;118:242–248. doi: 10.1289/ehp.0900956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 872. Dental devices: Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental amalgam, mercury, and amalgam alloys; final rule. [Accessed March 4, 2011];2009 http://edocket.access.gpo.gov/2009/pdf/E9-18447.pdf.
- 18.Shamlaye CF, Marsh DO, Myers GJ, et al. The Seychelles child development study on neurodevelopmental outcomes following in utero exposure to methylmercury from a maternal fish diet: background and demographics. Neurotoxicology. 1995;16:597–612. [PubMed] [Google Scholar]
- 19.Myers GJ, Marsh DO, Davidson PW, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology. 1995;16:653–664. [PubMed] [Google Scholar]
- 20.Davidson PW, Myers GJ, Cox C, et al. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion: outcomes at 19 and 29 months. Neurotoxicology. 1995;16:677–688. [PubMed] [Google Scholar]
- 21.Davidson PW, Myers GJ, Cox C, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 22.Myers GJ, Davidson PW, Cox C, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 23.Davidson PW, Cory-Slechta DA, Thurston SW, et al. Fish Consumption and Prenatal Methylmercury Exposure: Cognitive, Scholastic and Behavioral Outcomes in the Main Cohort at 17 Years from the Seychelles Child Development Study. doi: 10.1016/j.neuro.2011.08.003. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingman A, Albertini T, Brown LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US population. J Dent Res. 1998;77:461–471. doi: 10.1177/00220345980770030501. [DOI] [PubMed] [Google Scholar]
- 25.Kingman A, Albers JW, Arezzo JC, et al. Amalgam exposure and neurological function. Neurotoxicology. 2005;26:241–255. doi: 10.1016/j.neuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Pesch A, Wilhelm M, Rostek U, et al. Mercury concentrations in urine, scalp hair, and saliva in children from Germany. J Expo Anal Environ Epidemiol. 2002 Jul;12:252–8. doi: 10.1038/sj.jea.7500228. [DOI] [PubMed] [Google Scholar]
- 27.Factor-Litvak P, Hasselgren G, Jacobs D, et al. Mercury derived from dental amalgams and neuropsychologic function. Environ Health Perspect. 2003;111:719–723. doi: 10.1289/ehp.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luglie PF, Campus G, Chessa G, et al. Effect of amalgam fillings on the mercury concentration in human amniotic fluid. Arch Gynecol Obstet. 2005;271:138–142. doi: 10.1007/s00404-003-0578-6. [DOI] [PubMed] [Google Scholar]
- 29.Bellinger DC, Trachtenberg F, Daniel D, Zhang A, Tavares MA, McKinlay S. A dose-effect analysis of children's exposure to dental amalgam and neuropsychological function: The New England Children's Amalgam Trial. JADA. 2007;138:1210–1216. doi: 10.14219/jada.archive.2007.0345. [DOI] [PubMed] [Google Scholar]
- 30.Maserejian NN, Trachtenberg FL, Assmann SF, Barregard L. Dental amalgam exposure and urinary mercury levels in children: The New England Children's Amalgam Trial. Environ Health Perspect. 2008;116:256–262. doi: 10.1289/ehp.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olstad ML, Holland RI, Wandel N, Pettersen AH. Correlation between amalgam restorations and mercury concentrations in urine. J Dent Res. 1987;66:1179–1182. doi: 10.1177/00220345870660061701. [DOI] [PubMed] [Google Scholar]
- 32.Sällsten G, Thorén J, Barregård L, Achütz A, Skarping G. Long term use of nicotine chewing gum and mercury exposure from dental amalgam fillings. J Dent Res. 1996;75:594–598. doi: 10.1177/00220345960750011301. [DOI] [PubMed] [Google Scholar]
- 33.Cernichiari E, Toribara TY, Liang L, et al. The biological monitoring of mercury in the Seychelles study. Neurotoxicology. 1995;16:613–628. [PubMed] [Google Scholar]
- 34.Cernichiari E, Brewer R, Myers GJ, et al. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology. 1995;16:705–710. [PubMed] [Google Scholar]
- 35.Cernichiari E, Myers GJ, Ballatori N. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology. 2007;28:1015–1022. doi: 10.1016/j.neuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Ott KH, Grimmeisen J, Alt F, Messerschmidt J, Tölg G. Mercury in the hair of dentists and dental personnel. Dtsch Zahnarztl Z. 1991;46:154–158. [PubMed] [Google Scholar]
- 37.Morton J, Mason HJ, Ritchie KA, et al. Comparison of hair, nails and urine for biological monitoring of low level inorganic mercury exposure in dental workers. Biomarkers. 2004;9:47–55. doi: 10.1080/13547500410001670312. [DOI] [PubMed] [Google Scholar]
- 38.Gibb HJ, Kozlov K, Buckley JP, et al. Biomarkers of mercury exposure at a mercury recycling facility in Ukraine. J Occ Env Hyg. 2008;5:483–489. doi: 10.1080/15459620802174432. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy D. McCarthy Scales of Children's Abilities. New York, NY: The Psychological Corp; 1972. [Google Scholar]
- 40.Zimmerman I, Steiner V, Pond R. Preschool Language Scale. Rev ed. Columbus, Ohio: CE Merril; 1979. [Google Scholar]
- 41.Woodcock R, Johnson M. Woodcock-Johnson Tests of Achievement. Allen, Tex: DLM; 1989. [Google Scholar]
- 42.Koppitz EM. The Bender Gestalt Test for Young Children. London, England: Grune & Stratton; 1963. [Google Scholar]
- 43.Achenbach TM. Manual for the Child Behavior Checklist and 1991 Child Behavior Profile. Burlington: University of Vermont Dept of Psychiatry; 1991. [Google Scholar]
- 44.Caldwell B, Bradley R. Home Observation of Measurement of the Environment. Little Rock: University of Arkansas at Little Rock; 1984. [Google Scholar]
- 45.Hollingshead AB. Four factor index of social status. New Haven, CT: Hollingshead; 1975. [Google Scholar]
- 46.Raven J. Standard Progressive Matrices Cambridge. England: HK Lewis; 1958. [Google Scholar]
- 47.Weisberg S. Applied linear regression. 3rd. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 48.Clarkson TW, Magos L, Greenwood MR. The transport of elemental mercury into fetal tissues. Biol Neonate. 1972;21:239–244. doi: 10.1159/000240512. [DOI] [PubMed] [Google Scholar]
- 49.Clarkson TW. The three faces of mercury. Environ Health Perspect. 2002;110:11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida M, Suzuki M, Satoh M, Yasutake A, Watanabe C. Neurobehavioral effects of combined prenatal exposure to low-level mercury vapor and methylmercury. J Toxicol Sci. 2011;36:73–80. doi: 10.2131/jts.36.73. [DOI] [PubMed] [Google Scholar]
- 51.Grandjean P, Weihe P, White RF, et al. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Env Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- 52.Vahter M, Akesson A, Liden C, et al. Gender differences in the disposition and toxicity of metals. Env Res. 2007:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Mcdowell MA, Dillon CF, Osterloh J, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]


