Abstract
The serotonin 5HTR2C receptor has been shown to mediate HPA axis activation during stress. We hypothesized that a functional polymorphism (rs6318) of the 5HTR2C gene would be associated with HPA axis response to a laboratory stress protocol. The present sample consisted of 41 men (22 African Americans, 19 Caucasians). We found that at rest men with the more active rs6318 Ser23 C allele had similar cortisol values compared to those with the less active sys23 G allele. During laboratory stress, however, men with the Ser23 C allele exhibited the predicted significantly higher cortisol levels (p < .001), as well as larger increases in anger (P=0.08) and depressive mood (P=0.006) ratings, compared to the sys23 G carriers. The increase in cortisol was significantly related to the increases in ratings of anger and depression assessed before and after the emotion induction, and these correlations became nonsignificant when rs6318 genotype was covaried. We conclude that genetic variation in 5HTR2C may be associated with HPA axis activation and stimulated by emotional stress, and also with both psychological and physiological endophenotypes that increase the risk of cardiovascular disease and type 2 diabetes.
Keywords: rs6318, 5HTR2C, cortisol, hypothalamic-pituitary axis, stress, emotion, cardiovascular disease, serotonin
Introduction
A growing body of research implicates psychosocial risk factors like hostility, depression, social isolation, job stress, and low socioeconomic status in the etiology, pathogenesis and course of cardiovascular disease (Williams, 2008). Enhanced hypothalamic-pituitary-adrenal (HPA) axis response to stress is prominent among the mechanisms regularly cited as potential mediators of psychosocial risk factors’ health-damaging effects. High levels of hostility have been found to be associated with increased cortisol responses to anger-inducing interpersonal challenge (Suarez, Kuhn, Shanberg, Williams, & Zimmerman, 1998). Increased stress in daily life is associated with a higher cortisol awakening response and higher mean day and evening cortisol levels (Kumari et al., 2010). Increased HPA activation by stress has been implicated in the impact of stress on the pathogenesis of the metabolic syndrome (Rosmond, 2005), and elevated cortisol levels has been shown to play a role in mediating the association between depressive symptoms and elevated blood glucose levels (Boyle et al., 2007). Recent prospective studies provide direct evidence for a causal role in that glucocorticoid excess predicts increased cardiovascular disease (CVD) incidence (Davey-Smith et al., 2005; Rosmond et al., 2003). Elevated cortisol levels have also been found associated with several indices of accelerated aging, including decreased bone mineral density (Raff et al., 1999), increased incident cognitive impairment (Karlamangla, Singer, Chodosh, McEwen, & Seeman, 2005) and increased frailty(Varadhan et al., 2008).
Considerable investigation has therefore been directed toward identifying the neurobiological mechanisms whereby psychosocial risk factors enhance the activity of the HPA axis. There is now a growing consensus that serotonin acts at multiple sites to contribute to stress-induced HPA axis activation. These sites include the limbic forebrain including the amygdala, where 5-HT stimulates inputs that drive cortocotropin-releasing hormone (CRH) release from the periventricular nucleus (PVN), direct innervation of CRH-containing neurons in the PVN itself by 5-HT through actions on 5-HT2C receptors, and possibly through some peripheral actions directly on the adrenal cortex to stimulate glucocorticoid production (Dinan, 1996; Heisler et al., 2007; Lowry, 2002).
Documentation of the 5HTR2C receptor’s key role in stress-induced CNS serotonin’s mediation of stress-induced HPA axis activation (Dinan, 1996; Heisler, et al., 2007; Lowry, 2002) has led to attempts to identify variants in the HTR2C gene that might moderate stress effects on the HPA axis. One such 5HTR2C variant that has received considerable attention is a single nucleotide polymorphism (SNP) -- rs6318; 68G>C – that leads to a substitution of serine for cysteine at codon 23 (Cys23Ser), with the frequency of the Ser23 C allele being approximately 0.13 in unrelated Caucasians (Lappalainen et al., 1995). Recent research indicates that the Ser23 C allele is constitutively more active than the Cys23 (Okada et al., 2004). In humans, compared to men carrying the 5HTR2C Cys23 G allele, those with the less common Ser23 C allele show a trend to faster and stronger ACTH responses to the 5HTR2C agonist mchlorophenylpiperazine (m-CPP) (Kuhn et al., 2002), as well as a different pattern of change in regional cerebral blood flow following m-CPP infusion (Kuhn et al., 2004). Cortisol response to oral m-CPP in women did not differ as a function of rs6318 (Cys23Ser) genotype (Quested et al., 1999)–a difference that could be due to X inactivation of the 5HTR2C gene variants on the X chromosome (Valley & Willard, 2006). Based on the foregoing review, we hypothesized that the more active 5HTR2C Ser23 C allele will be associated with a larger cortisol response to an acute emotional laboratory stressor in men.
Methods
Participants
Participants were recruited to take part in a study designed to examine the moderating effects of genetic, behavioral, and environmental mechanisms on health disparities. The study was conducted at Duke University Medical Center, and all subjects gave informed consent prior to their participation in the study using a form approved by the Duke University Medical Center Institutional Review Board. Those enrolled in the study received $500 for their participation. The study protocol required that participants be in good current health because of the study procedures, see below and (Williams et al., 2001, 2003) therefore all participants underwent a comprehensive psychological examination, as well as medical history, physical exam, electrocardiogram, chest radiograph, hemoglobin, hematocrit, white cell count, and blood chemistries to rule out current medical and psychiatric disorders. Use of any prescription drugs as well as use of illegal drugs (as detected by a urine screen prior to entry into study) were grounds for exclusion (Burroughs et al., 2003). The current study sample consisted of 79 participants (43 males and 36 females) who were randomized to a tryptophan enhancement condition. Because the 5HTR2C gene is located on the X chromosome, only men were examined. Of the 43 males, two did not have adequate cortisol data, resulting in a final sample of 41 males, with a mean age of 34 years (range 18 – 49).
Procedure
Upon evening admission to the General Research Unit at Duke University Medical Center, sociodemographic and personality data were gathered, and blood was drawn for assessment of biological parameters. Test day one– the focus of the present study--consisted of a sham tryptophan (saline) infusion, followed by an emotional stress reactivity protocol (see, (R.B. Williams, et al., 2001). On the day of the sham infusion, beginning at 6:30 a.m. participants were not allowed food or liquids, nor were they allowed to smoke, until the completion of all study procedures at approximately 1:30 p.m. At 7:00 a.m. an IV (D5 W/.5 N saline) was started and kept running at 50 cc per hour till 1:30. All participants were seated in a reclined position and activity was limited to watching videos provided by the investigator or playing cards. Bathroom visits were allowed at any point up to 11:00 A.M., and then participants continued to sit in a reclined position until 12:00 PM. A basal blood sample for cortisol assay, prior to the reactivity protocol, was obtained at 12:00 A.M (following the lengthy reclining rest period). Following this blood draw, participants were informed they would begin the stress protocol soon. The 45-minute mental stress protocol began at approximately 12:45. The protocol timing is described below:
-
•
7:00 a.m. – 11:00 a.m. reclining rest period with bathroom breaks allowed
-
•
11:00 a.m. – Noon reclining rest period with no break allowed – basal cortisol blood sample drawn at noon.
-
•
Noon, participants were allowed a brief break and informed that the stress protocol would begin shortly.
-
•
12:45 the 45-minute stress protocol begins and proceeds as follows, with bloods drawn for cortisol assay after the initial baseline and each task and rest period thereafter:
-
❖
5-minute anticipatory baseline
-
❖
5-minute public speaking task during which the participant reads aloud in the presence of the experimenter, followed by a 5-minute rest
-
❖
5-minute anger induction, 5-minute rest period
-
❖
2nd 5-minute public reading task, 5-minute rest period
-
❖
Sadness induction
-
❖
Recovery
Emotion Measurement
The Profile of Mood States (POMS) (McNair & Lorr, 1964) was used to assess the emotional response to the anger and sadness inductions during the stress protocol. The POMS asks participants to rate “How you are feeling right now” in response to 65 affective adjectives rated on a 5-point Likert-type scale with ordered response options ranging from from “not at all” to “extremely.” Participants in the present study completed the POMS at the basal baseline, prior to the stress protocol, and again at the end of the protocol. The POMS factor derived subscales Anger (POM_A) and Depression (POM_D) were used to assess emotional responses to anger and sadness inductions, respectively, in the present study.
Cortisol
Blood samples were spun for 15 minutes in a refrigerated centrifuge and plasma was transferred into polypropylene tubes containing .05 ml glutathione and then frozen at −70°c. Blood samples were processed at the Clinical Research Unit at DUMC under the supervision of Dr. Cynthia Kuhn. Cortisol was measured by specific radioimmunoassay (RIA) using Coat-A-Count kit from Diagnostic Products Inc. Inter-/Intra-assay coefficients of variation were less than 10% and 5%, respectively. Cortisol was transformed using natural logarithm in all analyses to adjust for skewed distributional properties.
Genotyping
Genomic DNA was extracted for genotyping by standard procedure (Puregene D-50K isolation kit, Gentra, Minneapolis, MN) from fresh or frozen samples of peripheral blood collected from the participants. The SNP, rs6318, was identified from NCBI’s Single Nucleotide Polymorphism database (dbSNP) (http://www.ncbi.nlm.nih.gov/SNP). Genotyping methods and quality control approaches were carried out as previously described (Kring et al., 2010). The rs6318 Ser23 coding (C) allele has been shown to be constitutively more active than the Cys23 coding (G) allele (Okada, et al., 2004). Due to the X-chromosomal location of the 5-HT2C gene and the fact that the sample was restricted to males only (=hemizygous), no test for Hardy-Weinberg-Equilibrium was performed.
Statistical Analyses
A repeated measures mixed model (PROC MIXED, SAS, version 9.2, Cary NC) was used to examine the association of rs6318 genotype with cortisol changes over the course of the stress protocol. Specifically, rs6318 allelic variation (C vs G) was examined as a predictor of the course of cortisol reactivity across the nine alternating stress and rest time periods, adjusted for basal cortisol levels (obtained at 12:00 noon following the lengthy rest period). The model also included age and race as adjustment variables. The present statistical approach varies from a conventional repeated measures analysis in which all measures of cortisol, including the baseline measure, serve as the repeated response. In conventional repeated measures models, the time by allele interaction would be of primary interest as this carries information about whether the groups diverge after the baseline period. In the baseline adjusted model used in the present analysis, however, the effect of primary interest is the main effect for allele type. The main effect for allele carries information about the difference between the allele types averaged across the cortisol measurements subsequent to the baseline. That is, it is a test of group difference in the average response to the tasks, adjusted for initial (resting) differences. The time by allele type interaction term was also tested in this latter model as a matter of course in examining model assumptions, but was removed from the model if not substantial.
Change in emotional response was assessed using baseline adjusted change scores for the POMS_A and POMS_D subscales, and genotype group (C vs G) differences were examined using regression analyses. In addition, we examined the relations among the change in POMS_A and POMS_D, and the change in cortisol response from baseline to the period following the anger and sadness inductions using correlation analyses. Finally, to determine whether rs6318 genotype effects on cortisol and emotion responses to the stress protocol are accounting for the correlations between cortisol and emotion responses to the stress protocol, we tested the effect of covarying rs6318 genotype on those correlations.
Results
Cortisol Response
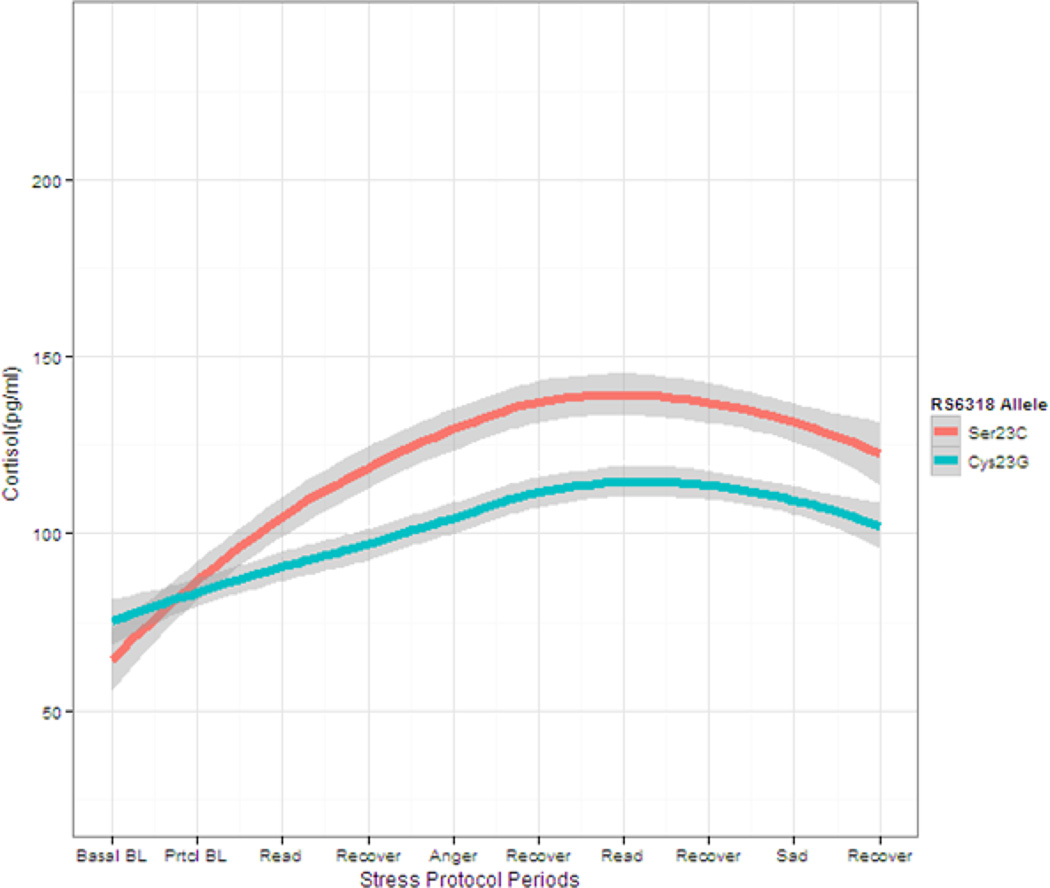
The rs6318 allele frequencies, presented by race in Table 1, are consistent with prior reports (Drago & Serretti, 2009). In our initial analysis, the rs6318 by time was not statistically significant (F(8,303) = 0.95, p = .479) and was therefore removed from the model. In our primary model, baseline cortisol (F(1,36) = 119.3, p = .001), time (F(8,311) = 12.2, p = .001), and rs6318 (F(1,36) = 25.9, p = .001) were statistically significant predictors of cortisol response over the course of the protocol. The effects of age (F(1,36)= 0.11, p = .747), race (F(1,36) = 0.202, p = 0.164) were not statistically significant. Figure 1 displays the mean cortisol levels for each allele group over the course of the stress protocol. The peak cortisol increase from baseline during the course of the stress protocol was about twice as large in C carriers than G carriers. At the basal baseline period men with the more active rs6318 C allele have similar cortisol values (baseline log cortisol C carriers = 4.01 (SD, 0.24) as compared to those with the less active G allele (G carriers =4.20 (SD, 0.43). Following this time point, however, the C carriers significantly diverge from the G carriers for the remainder of the protocol, with effects that remain fairly consistent over time (mean predicted reactivity log cortisol C carriers =4.85 (SE, 0.06); G carriers =4.54 (SE, 0.03).1 These values equate to an average increase of 72 pg/ml for the C carriers over the course of the protocol, as compared to an average increase of 27 pg/ml for G carriers.
Table 1.
Distribution of 5HTR2C rs6318 Ser23 C and Cys23 G alleles.
| Ser23 C | Cys23 G | |
|---|---|---|
| All men, N (%) | 9 (22.0) | 32 (78.0) |
| Caucasian, N (%) | 2 (10.5) | 17 (89.5) |
| African American, N (%) | 7 (31.8) | 15 (68.2) |
Figure 1.
Plasma cortisol levels (means and 95% CIs) during each period of the stress protocol: by rs6318 allele. Basal BL = Basal Baseline taken after 1 hour uninterrupted rest period; Prtcl B = Baseline measurement taken 45 after basal baseline assessment and immediately prior to commencement of stress protocol. Basal baseline was used in the statistical model as an adjustment covariate, while the remaining measures were models as repeated measures responses.
Emotional Response
Increases in POMS_A anger ratings from baseline to the end of the protocol were larger for the Ser23 C group (+5.0, SD=1.30) than the Cys23 G group (2.3, SD = 0.64). This difference, however, did not reach conventional statistical significance levels, (F(1,38) = 3.21, p = .082). For depression (POMS_D), the difference between Ser23 C and Cys23 G genotype groups was statistically significant, (F(1,39) = 8.59, p < .006), with the Ser23 C group having larger increases in depression ratings (9.4, SD=1.60) compared to the Cys23 G group (4.1,SD = 0.80).
The age and race adjusted correlation between the change in emotion and the change in cortisol from baseline to Anger was r = 0.38, p = 0.028. Similarly, that for Sadness was r = 0.40, p = 0.019. In order to determine whether the association between changes in emotion and change in cortisol during the stress protocol is accounted for, in part, by the observed influence of rs6318 genotype on emotion and cortisol responses, we recalculated the correlations among the emotion and cortisol change scores with the additional adjustment for rs6318 genotype. As reported above, with adjustment only for age and race, the correlation between change in cortisol and change in anger ratings was r = 0.38, p = 0.028, and for depressive mood was r = 0.40, p = 0.019. When adjusted for age, race, and rs6318 these correlations are no longer significant: Cortisol with Anger is r = 0.287, p = 0.105; cortisol with Sadness is r = 0.251, p = 0.159. Thus, the relation between change in cortisol and change in anger, and between change in cortisol and change in depressive mood, was substantially reduced when accounting for effects of rs6318 genotype on cortisol and emotion changes.
Exploratory Analyses
Although the number of Caucasian participants was too small in the C-allele group (N = 2) to interpret a statistical test of a race by genotype interaction term, we examined the predicted log cortisol for each genotype by race combination in order to better understand the extent to which the effect of genotype might differ by race. The predicted mean log cortisol response for the Caucasian C allele group was 5.00 (SE, 0.13) and the Caucasian G allele group was 4.58 (SE, 0.02). Within the African American C allele group the predicted log cortisol response was 4.74 (SE, 0.07) and the G allele group was 4.47 (SE, 0.05). Therefore, within the sample, C vs G allele group cortisol response difference was larger among Caucasians than among African Americans. To explore the extent to which the larger allele difference among whites might be influencing the results of our primary model, we reestimated the mixed model using data from only the 22 African Americans (N = 7 for the C allele, and 15 for the G allele). The result was essentially unchanged from the analysis with the full sample, i.e., effect of rs6318 (F(1,18) = 10.1, p = 0.005). Thus, the somewhat larger genotype effect among Caucasians does not appear to have much impact on the results observed in the primary model with the entire sample.
Finally, although the present study focused on the known functional SNP rs6318, we also had data available for 8 additional 5HTR2C SNPs that are in strong linkage disequilibrium with rs6318 (i.e., rs12838742, rs11167435, rs50886, rs1023574, rs12847225, rs518147, rs543229, and rs17260565). We tested these additional 8 SNPs for an association with cortisol response and as expected, the results for these SNPs were similar to those of the functional SNP, with each one being statistically significantly related to cortisol response, with a similar pattern of group differences (p’s 0.04 – 0.001).
Discussion
Consistent with our study hypothesis, these results show that the cortisol response to a stress task was significantly larger in men hemizygous for the Ser23 C allele compared to those carrying the Cys23 G allele, i.e., an increase of 72 pg/ml vs 27 pg/ml, respectively. This finding adds to the evidence reviewed in the introduction that the 5HTR2C receptor plays a key role in the activation of the HPA axis by acute stress. The significantly larger cortisol in Ser23 C than Cys23 G carriers in both race/ethnic groups is consistent with prior research (Okada et al., 2004) showing the Ser23 C allele is constitutively more active than the Cys23 G allele.
The fact that, in the present study, men hemizygous for the Ser23 C allele of the 5HT2C rs6318 SNP had both larger cortisol responses and larger increases in anger and depressive mood during the stress protocol suggests that the 5HT2C receptor is at least partially responsible for the increase in both HPA axis function and negative moods under stress. Further support for this interpretation comes from the significant correlations between cortisol increase and the increase in anger during the anger recall task and between cortisol increase and sadness increase during the sadness recall task. That is, in the setting of anger/sadness mood elicitation men with the rs6318 ser23 C allele exhibited larger increases in both HPA axis function and anger/sadness. This can be interpreted as showing that the known function of 5HT2C receptors in the hypothalamic PVN to release CRH when stimulated may be mediating, during anger/sadness recall, an increase in both HPA axis function and angry/sad mood. Support for this interpretation is provided by the finding that correlations between the cortisol increase and increases in anger and sadness become nonsignificant when rs6318 is covaried.
In addition to its association with enhanced activation of the HPA axis and larger increases in anger and sadness during emotion arousal in the lab in the present study, the ser23 C variant has also been found to be associated with affective disorders (Lerer et al., 2001). Specifically, in a sample consisting of European populations, there were significantly larger proportions of ser23 C allele carriers in patients with major depressive and bipolar disorder, as compared to normal controls (Lerer et al., 2001). Depression has long been linked to dysregulated HPA axis function, and depressed men exhibit higher levels of salivary cortisol across the day compared to healthy men (Hinkelmann et al., 2011). The present findings, along with studies linking the Ser23 C allele with affective disorders, suggest, therefore, that the rs6318 effects on 5HT2C receptor-mediated effects on both emotions and the HPA axis may be accounting, at least in part, for the linkages between depression and dysregulated HPA axis function.
Another important implication of the present findings is that men carrying the 5HTR2C Ser23 C allele may be more likely than Cys23 G allele carriers to express elevated levels of a broad range of endophenotypes that have been found associated (Reynolds et al., 2010; Rosmond, et al., 2003) with elevated cortisol levels, including central obesity, elevated glucose, insulin and insulin resistance, higher blood pressure and lipids, as well as diseases – hypertension, coronary heart disease, type-2 diabetes – that are increased in persons with these endophenotypes. Further evidence that increased HPA axis function is associated with increased risk of coronary heart disease comes from very recent studies showing increased risk for myocardial infarction in men with elevated hair cortisol levels (Pereg et al., 2011) and increased levels of coronary artery calcification in men and women with larger salivary cortisol responses to mental stress (Hamer, O'Donnell, Lahiri, & Steptoe, 2010).
An important limitation of the current study is the small sample size and consequent limited number of participants carrying the rs6318 C allele. Small sample sizes can result in unstable regression estimates and also can limit the number of adjustment covariates that can be included in a multivariable model without introducing further instability (Steyerberg, 2009). Thus, future work should include attempts at direct replication of the present finding, as well as continued elucidation of the underlying biology. It is reassuring, however, that we hypothesized, based on prior research, that the 5HT2C rs6318 Ser23 C allele will be associated with a larger cortisol response to an emotional stressor in men.
In conclusion, we found that a previously documented functional SNP, rs6318, on the 5HTR2C gene is associated with increased cortisol and emotional responses to acute mental stress in men carrying the Ser23C allele. Research is currently under way to determine whether this variant is associated with psychosocial traits, cardiovascular disease and type-2 diabetes endophenotypes, and disease endpoints.
Highlights.
The serotonin 5HTR2C receptor mediates HPA axis activation during stress.
A functional polymorphism of the 5HTR2C gene predicted HPA axis response to stress.
Specifically, in our study rs6318 moderated cortisol levels in response to stress.
Genetic variation in 5HTR2C may be associated with risk for cardiovascular disease.
Acknowledgments
Supported by a grant (P01-HL036587) from the National Heart, Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We also conducted a more conventional repeated measures ANOVA model (with the baseline cortisol level modeled as part of the serial response rather than as a covariate. Consistent with our primary analysis, this model yielded a significant time by allele interaction (p = 0.0009).
References
- Boyle SH, Surwit RS, Georgiades A, Brummett BH, Helms MJ, Williams RB, Barefoot JC. Depressive symptoms, race and glucose concentrations: the role cortisol as mediator. Diabetes Care. 2007;30:2484–2488. doi: 10.2337/dc07-0258. [DOI] [PubMed] [Google Scholar]
- Burroughs AR, Visscher AW, Haney TL, Efland JR, Barefoot JC, Williams RB, Siegler IC. Community recruitment process by race, gender, and ses gradient: lessons learned from the community health and stress evaluation (chase) study experience. Journal of Community Health. 2003;28:421–437. doi: 10.1023/a:1026029723762. [DOI] [PubMed] [Google Scholar]
- Davey-Smith G, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone and coronary heart disease: Prospective evidence from the Caephilly Study. Circulation. 2005;112:332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Science. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Hamer M, O'Donnell K, Lahiri A, Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. European Heart Journal. 2010;31(4):424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. Journal of Neuroscience. 2007;27(26):6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelmann K, Botzenhardt J, Muhtz C, Agorastos A, Wiedemann K, Kellner M, Otte C. Sex differences of salivary cortisol secretion in patients with major depression. Stress. Stress. 2011 doi: 10.3109/10253890.2011.582200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiology of Aging. 2005;26 Suppl 1:80–84. doi: 10.1016/j.neurobiolaging.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Kring SI, Brummett BH, Barefoot J, Garrett ME, Ashley-Koch AE, Boyle SH, Williams RB. Impact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controls. Psychosomomatic Medicine. 2010;72(5):427–433. doi: 10.1097/PSY.0b013e3181de30ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KU, Joe AY, Meyer K, Reichmann K, Maier W, Rao ML, Quednow BB. Neuroimaging and 5-HT2C receptor polymorphism: a HMPAO-SPECT study in healthy male probands using mCPP-challenge of the 5-HT2C receptor. Pharmacopsychiatry. 2004;37(6):286–291. doi: 10.1055/s-2004-832685. [DOI] [PubMed] [Google Scholar]
- Kuhn KU, Quednow BB, Bagli M, Meyer K, Feuchtl A, Westheide J, Rao ML. Allelic variants of the serotonin(2C) receptor and neuroendocrinological responses to the serotonin(2C) receptor agonist m-chlorophenylpiperazine in healthy male volunteers. Pharmacopsychiatry. 2002;35(6):226–230. doi: 10.1055/s-2002-36388. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Sacker A, Kirschbaum C, Marmot M, Chandola T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 2010;35(7):1091–1099. doi: 10.1016/j.psyneuen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, Goldman D. Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C) Genomics. 1995;27(2):274–279. doi: 10.1006/geno.1995.1042. [DOI] [PubMed] [Google Scholar]
- Lerer B, Macciardi F, Segman RH, Adolfsson R, Blackwood D, Blairy S, Mendlewicz J. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Molecular Psychiatry. 2001;6:579–585. doi: 10.1038/sj.mp.4000883. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of Neuroendocrinology. 2002;14(11):911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M. An analysis of mood in neurotics. Journal of Abnormal and Social Psychology. 1964;69:620–627. doi: 10.1037/h0040902. [DOI] [PubMed] [Google Scholar]
- Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D. Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Molocular Psychiatry. 2004;9(1):55–64. doi: 10.1038/sj.mp.4001357. [DOI] [PubMed] [Google Scholar]
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 2011;14(1):73–81. doi: 10.3109/10253890.2010.511352. [DOI] [PubMed] [Google Scholar]
- Quested DJ, Whale R, Sharpley AL, McGavin CL, Crossland N, Harrison PJ, Cowen PJ. Allelic variation in the 5-HT2C receptor (HTR2C) and functional responses to the 5-HT2C receptor agonist, m-chlorophenylpiperazine. Psychopharmacology (Berl) 1999;144(3):306–307. doi: 10.1007/s002130051010. [DOI] [PubMed] [Google Scholar]
- Raff H, Raff JL, Duthie EH, Wilson CR, Sasse EA, Rudman I, Mattson D. Elevated salivary cortisol in the evening in healthy elderly men and women: correlation with bone mineral density. Journal of Gerontology A Biological Science and Medical Science. 1999;54(9):M479–M483. doi: 10.1093/gerona/54.9.m479. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Labad J, Strachan MWJ, Braun A, Fowkes FGR, Lee AJ, Frice JF. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: The Edinburgh type 2 diabetes study. Journal of Clinical Endocrinology and Metabolism. 2010;94:1602–1608. doi: 10.1210/jc.2009-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Wallerius S, Wanger P, Martin L, Holm G, Bjorntorp P. A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. Journal of Internal Medicine. 2003;254(4):386–390. doi: 10.1046/j.1365-2796.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW. Clinical Prediction Models. New York: Springer; 2009. [Google Scholar]
- Suarez E, Kuhn C, Shanberg S, Williams R, Zimmerman E. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosomatic Medicine. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. Journal of Gerontology Series A Biolological Science and Medical Science. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- Williams RB. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annual Review of Clinical Psychology. 2008;4:349–365. doi: 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Siegler IC. Central nervous system serotonin function and cardiovascular responses to stress. Psychosomatic Medicine. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]