Abstract
We will provide a translational view of using the recent technological advances in dental research for predicting, monitoring, and preventing the development of oral diseases by investigating the diagnostic and therapeutic role of salivary proteins. New analytical state-of-the-art technologies such as mass spectrometry and atomic force microscopy have revolutionized the field of oral biology. These novel technologies open avenues for a comprehensive characterization of the salivary proteins followed by the evaluation of the physiological functions which could make possible in a near future the development of a new series of synthetic protein for therapeutic propose able to prevent global oral diseases such as periodontal disease and dental caries, the two most prevalent oral diseases in the World.
Keywords: Saliva, Proteins, Proteomics, Oral disease, Mass spectrometry, Atomic Force Microscopy
Introduction
Periodontal disease (i.e., gingivitis, chronic periodontitis) and dental caries are the two most globally prevalent chronic oral pathologies that affect children, youth, adults, and elders (Featherstone 2000; Albandar 2002). In the United States, gingivitis affects 50% of adults, while chronic periodontitis affects an estimated 35% of the adult population (Albandar et al. 1999). In addition, each year over 300,000 patients worldwide are diagnosed with oral cancer (Parkin et al. 1988), representing 2–3% of all malignancies (Parkin et al. 2005). More than 90% of these cases are categorized as oral squamous cell carcinoma (SCC), with high metastasis rates, resulting in high patient mortality (Neville and Day 2002; Parkin et al. 2005).
Recent advances in dental research have enforced the need to gain a more comprehensive understanding of the prevention, treatment, and management of oral diseases. Oral health is an essential component of an individual’s well-being because it is very closely related to general health. Throughout the years, oral diseases have been defined as localized oral disturbances, but recent research suggests that they can be considered as general health distal determinants, acting as comorbidities and risk factors for many systemic diseases. For instance, the association between diabetes mellitus and periodontal disease can be considered to be bidirectional: diabetes can be a risk factor for the development of periodontitis (diabetic patients are 2.1–3.0 times more at risk of developing periodontitis; Salvi et al. 1997), while patients with periodontitis are much more likely to develop diabetes (Grossi and Genco 1998; Deshpande et al. 2010). Considering the interconnectedness of oral diseases and general health, we must gain a complete understanding of the pathophysiology of oral diseases within the dynamic, complex oral cavity in order to successfully develop potential treatments and accurate patient risk assessments for the prediction of these diseases (i.e., biomarkers).
Periodontitis and dental caries are multi-factorial diseases primarily dependent on biofilm development. The oral cavity fosters an intricate microbial ecosystem, consisting of more than 700 bacterial species, many of which play an important role in maintaining oral health (Aas et al. 2005). However, when this ecosystem becomes disrupted, an increase in pathogenic microorganisms occurs, resulting in the initiation of disease. To initialize the growth of pathogenic biofilms and therefore the development of oral disease, microbial adhesion to the oral surface, such as dental enamel or denture resin is the first and essential step to prevent cells from being removed by salivary flow (Whittaker et al. 1996; Jenkinson and Lamont 1997). Human saliva plays a significant role in controlling microbial adhesion since its proteinaceous components, after adsorbed to the oral surface, result in the formation of salivary protein pellicles.
The acquired pellicle (AP) is a protein integument formed on the oral surface immediately after exposure of saliva to the oral environment. This protein film formed on the dental enamel is a result of specific physical bonds (i.e., hydrophobic, hydrogen bonding, ionic, and van der Waals bonds) between the substrata surface and the salivary molecules (i.e., salivary proteins, peptides, carbohydrates, lipids; Dawes et al. 1963; Rolla et al. 1983; Siqueira et al. 2007a), resulting in the development of a 100–1000 nm protein film on the oral surface for microorganisms to adhere (Kuboki et al. 1987; Skjorland et al. 1995).
The AP has important binding sites for oral microbiota; the protein-microbial adhesion process involves stero-specific interaction between receptors on the pellicles and adhesins on the microbial cell surfaces (Scannapieco et al. 1994). The AP may control the adhesion of pathogenic microbes to oral surfaces because some salivary pellicle proteins found in vivo can inhibit or enhance growth of oral microbiota (Scannapieco et al. 1994). For instance, the carboxyl-terminal of histatin 5 demonstrates potent fungistatic and fungicidal effects against pathogenic fungi, C. albicans, at concentrations found in salivary secretions of healthy individuals (15–30 μM) (Oppenheim et al. 1986; Xu et al. 1991). The antimicrobial affect of histatin 5 is due to its composition of multiple basic amino acid residues (arginine and lysine), allowing this salivary protein to possess a basic character capable of disrupting the cell membrane by forming membrane pores, inducing membrane permeability (increased loss of K+ from cell) and resulting in cell death (Pollock et al. 1984).
In contrast, the carboxyl-terminal of acidic proline-rich proteins (PRPs) promotes the attachment of various oral bacteria (i.e., Streptococcus and Actinomyces sp.) to the AEP, thus enhancing microbial colonization of the tooth surface (Gibbons and Hay 1988). Specifically, the ProGln terminal of acidic PRPs is the preferred protein-binding site for microorganisms including S. gordonii (Gibbons et al. 1991). Similarly to acidic PRPs, the carboxyl-terminal of statherin binds a variety of potentially invasive oral microbiota, including P. gingivalis (Amano et al. 1994) and C. albicans (Cannon et al. 1995). In addition, at concentrations of 100 μg/mL (healthy individuals), statherin is capable of inducing the transition from virulent, hyphael C. albicans to the cocci form (Leito et al. 2009).
Recent studies have shown that pathogenic microorganisms have increased their resistance to natural host defenses and to antimicrobial treatments, resulting in more persistent and serious infections (Ramage et al. 2006; Tsang et al. 2007). This enforces the need for the development of novel antimicrobial treatments that would inhibit and/or kill pathogenic microbes, preventing further colonization and development of oral diseases. Since certain salivary proteins affect the growth of pathogenic oral microbes, their potential role in treatment/prevention of oral diseases must be considered.
Challenges
In order to evaluate the effectiveness of antimicrobial salivary proteins as a potential novel therapeutical approach for the combat of oral diseases, it is important to gain a comprehensive understanding of the inhibitory effects salivary proteins exhibit on pathogenic oral microbiota. One approach is to design larger-scale reaction systems that can allow us to control variables of interest (i.e., microbial consortia) and target specific questions about salivary protein-microbial interactions. Throughout the years, many in vitro model systems that model the oral cavity have been designed involving either flow cells (Christersson et al. 1987; Larsen and Fiehn 1995; Guggenheim et al. 2001) and even chemostats (Herles et al. 1994; Bradshaw et al. 1996; Kinniment et al. 1996; Bowden 1999). However, some of these models yield contradictory results due to the selection of different parameters. Since the oral cavity is an extremely complex and dynamic system, many different components need to be considered when designing these systems, including multi-species biofilms, flow rate, temperature, pH, nutrient fluxes, and choice of proteins. Considering that human saliva consists of 2290 proteins (Loo et al. 2010) and 130 proteins in the AP (Siqueira et al. 2007b) it becomes incredibly challenging to reproduce the in vivo environment.
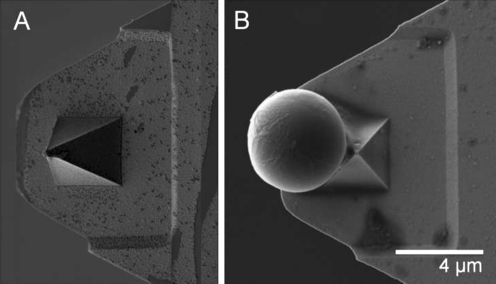
Another challenge when investigating the role of salivary proteins on oral biofilms is being able to view the world of a microbe- on a small, micro-meter-scale. The advancements in high-resolution microscopy instruments have facilitated the investigation of microbial interactions (i.e., scanning electron microscopy, confocal microscopy, transmission electron microscopy). In addition to these tools, atomic force microscopy (AFM) has revolutionized the field of oral microbiology, enabling us to make a variety of protein/cell surface measurements on the atomic magnitude, directly in aqueous solution. Unlike conventional microscopy, AFM allows us to study adhesive (Lodish et al. 2004), mechanical (Greenleaf et al. 2007), electrostatic (Barkai et al. 2004), and immunochemical (Horber and Miles 2003) nanoscale-level properties. In order to successfully conduct these measurements, the AFM cantilever-tip is typically functionalized with the protein/cell of interest and then used to probe a substrate (Zhang et al. 2009). However, the attachment of a pre-functionalized microsphere to the cantilever provides a much higher surface area when probing the substrate of interest, therefore greatly expanding the spectrum of adhesive interactions that can be obtained by a single cantilever (Ounkomol et al. 2009). For instance, streptavidin-coated microspheres can be attached to the AFM cantilever-tip (Fig. 1), and then reacted with biotinylated protein of interest to obtain an AFM functionalized, high-surface area probe (Zhang et al. 2009). This novel AFM-based force spectroscopy approach allows us to determine biophysical properties of cells and proteins, and also enables us to measure single cell-protein adhesion interactions; the first pathophysiologic phenomena that occurs prior to the development of biofilms on oral surfaces. By manipulating microbial-protein interactions, we could essentially control oral disease development at the pellicle level, to prevent initial microbial adherence that leads to the development of oral diseases.
Fig. 1.
Scanning electron micrograph of AFM cantilever (a) and of streptavidin-coated silica microsphere (~5 μm) adhered to AFM cantilever-tip via Araldite® epoxy glue (b). This functionalized microsphere can be coated with biotinylated proteins of interest to create an effective AFM probe for protein-adhesion measurements
In addition to the use of these microscopic techniques, the development of revolutionary mass spectrometry has allowed for the investigation of microbial-protein interactions using a proteomic approach, allowing us to understand how salivary proteins affect metabolic pathways inside a cell. Moreover, the analysis of the composition of the AP can lead to the establishment of biomarkers and therefore the prevention of oral diseases that can impact general health. These powerful microscopic and proteomic techniques should be combined when investigating protein-microbial interactions in order to obtain a representative and comprehensive understanding of microbial responses to antimicrobial agents.
Salivary components as diagnostic tools for oral diseases
The advancement of new technology and instrumentation will enable us to obtain reliable protein fingerprints based on saliva and/or the acquired pellicle. Combining this information with a patient’s oral microbiome can provide health care professionals with a comprehensive grasp of each patient’s pathophysiological state.
A patient’s saliva sample could potentially have individual proteins or groups of proteins that could be powerful biomarkers for oral diseases, including oral cancer. This is because saliva contains secretions from gingival crevicular fluid along with major and minor salivary glands, and is much less invasive compared to blood sampling (Spielmann and Wong 2011; Edgar 1992; Siqueira and Dawes 2011). For example, it has been demonstrated that patients diagnosed with head and neck squamous cell carcinoma exhibit elevated levels of soluble CD44 (solCD44), a family of salivary isoforms, compared to cancer-free patients (Franzmann et al. 2007). In addition, patients with early childhood caries exhibit higher levels of glycoprotein, while caries-free patients demonstrate elevated amounts of proline-rich protein in saliva (Bhalla et al. 2010). Therefore, analyzing the protein composition of a patient’s saliva could provide a protein profile for that patient, which could contain information as to which salivary proteins are upregulated/downregulated. This information could ultimately be compared to protein profiles of healthy patients, and those with different stages of various oral diseases, in order to accurately diagnose a patient (Blicharz et al. 2009).
When considering oral diseases that initiate on hard surfaces (i.e., dental caries), sampling the acquired pellicle and obtaining corresponding protein profile can likely be much more important compared to saliva (Siqueira et al. 2007a; Siqueira and Oppenheim 2009). Pellicle formation is a highly selective process since only a fraction of proteins found in human saliva (130/2290 proteins) are present in the in vivo AP on dental enamel surface (Siqueira et al. 2007b). Since more than 51% of the recently identified pellicle proteins have unknown biological functions (Siqueira et al. 2007b), future research should focus on identifying the biological function of the remainder of the pellicle proteins. There may in fact be additional proteins, or protein complexes, that could be even stronger biomarkers/predictors for various oral diseases.
In addition to obtaining saliva and AP protein patient profiles, sampling the oral microbial community (microbes adhered to oral surfaces) could produce a microbial patient profile, which could also be used as a biomarker for oral disease. Certain oral microbes adhered to the pellicle become more prevalent in patients suffering from certain oral diseases. For instance, patients exhibiting dental caries have an oral microbiota dominated by acidogenic and acid-tolerant gram-positive bacteria (i.e., Streptococcus and Lactobacilli sp) (Marsh 2003). Meanwhile, patients with periodontal disease have an increased proportion of obligately anaerobic bacteria (i.e., gram-negative species) (Socransky et al. 1998). The presence/absence or quantity of certain individual microbes, or even microbial community composition, adhered to the pellicle can correspond to various stages of oral disease development. Health care professionals could employ techniques such as the Human Microbial Identification Microarray to determine the microbial community of the oral cavity, and determine ‘predictor’ microbes of oral diseases.
Future direction
Gaining a comprehensive understanding of interactions between oral microbiota and salivary antimicrobial proteins could potentially result in the development of novel treatments for a variety of oral pathologies. It would be very interesting if a potential treatment for oral diseases could be currently in our oral cavity in the form of salivary proteins. Therefore, biofilm-dependent oral diseases can be controlled at the pellicle level- the interface between pathogenic microbes and the solid oral surfaces. By controlling, or perhaps altering, the composition of the pellicle, we could potentially interfere the adhesion process of the oral microbiota to oral surfaces. Therefore, the future of oral therapeutics should focus on the interaction between salivary proteins and microorganisms.
In addition, the oral cavity contains biomarkers for oral diseases hidden within its complex oral fluids, AP integuments, and microbial consortia. The use of these proposed salivary biomarkers would promote health professionals to change their focus from disease diagnosis to monitoring and detecting oral disease at onset. Longitudinal studies should be conducted to better understand if such biomarkers could be used to identify progression of oral diseases. Ultimately, multiple biomarkers should be combined to achieve optimum specificity and sensitivity for detection of oral diseases.
Conclusion
Saliva is a complex fluid that possesses many important functions that relate directly to oral health. Accurate analysis of salivary components is a relatively new tool for assessing biological markers (hormones, immunoglobulins and antimicrobial proteins) for oral diseases as dental caries, periodontitis and oral candidiasis. The fingerprint profile of immunological compounds, such as immunoglobulin and other antimicrobial proteins in saliva samples can be an indicator of the host immune system’s stress response to acute systemic disturbances, whereas assessment of the pellicle salivary constituents can identify susceptibility to local infections. Assessing proteins physical properties (i.e., adhesion forces) on different surfaces, cells or even to other proteins (protein-protein complexes) would assist us in understanding the role of salivary proteins and the pathophysiology of oral diseases. Subsequently, this new knowledge would help in developing innovative and effective therapeutics approaches to maximize the prevention of pathologic biofilm development. In conclusion, assessing and understating salivary composition can be applied as a feasible and reliable tool for predicting and treating several oral infections, diagnosing systemic diseases and determining the state of patient’s immune systems. Therefore, collecting and analyzing saliva would not only help to better monitor and maintain the oral health of patients, but it could also significantly improve the health care system.
Acknowledgments
Preparation of this article was supported by the CIHR (grant # 106657 and grant # 97577) and NSERC grant #371813. WLS is a recipient of a CIHR New Investigator Salary Award (grant # 113166). The authors have declared no conflict of interest
Conflicts of interest The authors have declared no conflict of interest.
Contributor Information
Dusa Vukosavljevic, Phone: +1-519-6612111, Email: dvukosav@uwo.ca.
William Custodio, Phone: +55-19-21065294, FAX: +55-19-21065211, Email: wcust@fop.unicamp.com.br.
Walter L. Siqueira, Phone: +1-519-6612111, FAX: +1-519-8502459, Email: walter.siqueira@schulich.uwo.ca
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar JM. Periodontal diseases in North America. Periodontology. 2002;2000(29):31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- Amano A, Sojar HT, Lee JY, Sharma A, Levine MJ, et al. Salivary receptors for recombinant fimbrillin of Porphyromonas gingivalis. Infect Immun. 1994;62:3372–3380. doi: 10.1128/iai.62.8.3372-3380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkai E, Jung YJ, Silbey R. Theory of single-molecule spectroscopy: beyond the ensemble average. Annu Rev Phys Chem. 2004;55:457–507. doi: 10.1146/annurev.physchem.55.111803.143246. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Tandon S, Satyamoorthy K. Salivary proteins and early childhood caries: a gel electrophoretic analysis. Contemp Clin Dent. 2010;1:17–22. doi: 10.4103/0976-237X.62515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicharz TM, Siqueira WL, Helmerhorst EJ, Oppenheim FG, Wexler PJ, et al. Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva. Anal Chem. 2009;81:2106–2114. doi: 10.1021/ac802181j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden GH. Controlled environment model for accumulation of biofilms of oral bacteria. Methods Enzymol. 1999;310:216–224. doi: 10.1016/S0076-6879(99)10019-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw DJ, Marsh PD, Schilling KM, Cummins D. A modified chemostat system to study the ecology of oral biofilms. J Appl Bacteriol. 1996;80:124–130. doi: 10.1111/j.1365-2672.1996.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Cannon RD, Nand AK, Jenkinson HF. Adherence of Candida albicans to human salivary components adsorbed to hydroxylapatite. Microbiology. 1995;141:213–219. doi: 10.1099/00221287-141-1-213. [DOI] [PubMed] [Google Scholar]
- Christersson CE, Fornalik MS, Baier RE, Glantz P. In vitro attachment of oral microorganisms to solid surfaces: evaluation of a controlled flow method. Scand J Dent Res. 1987;95:151–158. doi: 10.1111/j.1600-0722.1987.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Dawes C, Jenkins GN, Tonge CH. The nomenclature of the integuments of the enamel surface of the teeth. Br Dent J. 1963;115:65–68. [Google Scholar]
- Deshpande K, Jain A, Sharma R, Prashar S, Jain R. Diabetes and periodontitis. J Indian Soc Periodontol. 2010;14:207–212. doi: 10.4103/0972-124X.76917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar WM. Saliva: its secretion, composition and functions. Brit Dent J, London. 1992;172(8):305–312. doi: 10.1038/sj.bdj.4807861. [DOI] [PubMed] [Google Scholar]
- Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- Franzmann E, Reatgui E, Pernas F, Karakullukcu B, Carraway K, et al. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1348–1355. doi: 10.1158/1055-9965.EPI-06-0011. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Hay DI. Human salivary acidic proline-rich proteins and statherin promote the attachment of actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RJ, Hay DI, Schlesinger DH. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991;59:2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf WJ, Woodside MT, Block SM. High-resolution, singlemolecule measurements of biomolecular motion. Annu Rev Biophys Biomol Struct. 2007;36:171–190. doi: 10.1146/annurev.biophys.36.101106.101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:20–29. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- Guggenheim M, Shapiro S, Gmür R, Guggenheim B. Spatial arrangements and associative behavior of species in an in vitro oral biofilm model. Appl Environ Microbiol. 2001;67:1343–1350. doi: 10.1128/AEM.67.3.1343-1350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herles S, Olsen S, Afflitto J, Gaffar A. Chemostat flow cell system: an in vitro model for the evaluation of antiplaque agents. J Dent Res. 1994;73:1748–1755. doi: 10.1177/00220345940730111101. [DOI] [PubMed] [Google Scholar]
- Horber JK, Miles MJ. Scanning probe evolution in biology. Science. 2003;302:1002–1005. doi: 10.1126/science.1067410. [DOI] [PubMed] [Google Scholar]
- Jenkinson HF, Lamont RJ. Streptococcal adhesion and colonization. Crit Rev Oral Biol Med. 1997;8:175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- Kinniment SL, Wimpenny JW, Adams D, Marsh PD. Development of a steady-state oral microbial biofilm community using the constant-depth film fermenter. Microbiology. 1996;142:631–638. doi: 10.1099/13500872-142-3-631. [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Teraoka K, Okada S. X-ray photoelectron spectroscopic studies of the adsorption of salivary constituents on enamel. J Dent Res. 1987;66:1016–1019. doi: 10.1177/00220345870660050401. [DOI] [PubMed] [Google Scholar]
- Larsen T, Fiehn NE. Development of a flow method for susceptibility testing of oral biofilms in vitro. APMIS. 1995;103:339–344. doi: 10.1111/j.1699-0463.1995.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Leito JTD, Ligtenberg AJ, Nazmi K, Veerman EC. Identification of salivary components that induce transition of hyphae to yeast in Candida albicans. FEMS. Yeast Res. 2009;9:1102–1110. doi: 10.1111/j.1567-1364.2009.00575.x. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, et al. Molecular cell biology. 5. New York: W. H. Freeman and Company; 2004. p. 1344. [Google Scholar]
- Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J Dent Res. 2010;89:1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microb. 2003;149:1990–1995. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Yang YC, Diamond RD, Hyslop D, Offner GD, et al. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. I Biol Chem. 1986;261:1177–1182. [PubMed] [Google Scholar]
- Ounkomol C, Xie H, Heinrich V. Versatile horizontal force probe for mechanical tests on pipette-held cells, particles, and membrane capsules. Biophys J. 2009;96:1218–1231. doi: 10.1016/j.bpj.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Laara E, Muir CS. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Pollock WK, Armstrong RA, Brydon LJ, Jones RL, MacIntyre DE. Thromboxane-induced phosphatidate formation in human platelets. Relationship to receptor occupancy and to changes in cytosolic free calcium. Biochem J. 1984;219:833–842. doi: 10.1042/bj2190833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials; a clinically significant problem. FEMS Yeast research. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Rolla G, Ciardi JE, Schultz SA. Adsorption of glucosyltransferase to saliva-coated hydroxyapatite possible mechanism for sucrose-dependent bacterial colonization of teeth. Scand J Dent Res. 1983;91:112–117. doi: 10.1111/j.1600-0722.1983.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontites. Periodontol. 1997;2000(14):173–201. doi: 10.1111/j.1600-0757.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Solomon L, Wadenya RO. Emergence in human dental plaque and host distribution of amylasebinding streptococci. J Dent Res. 1994;73:1627–1635. doi: 10.1177/00220345940730100701. [DOI] [PubMed] [Google Scholar]
- Siqueira WL, Dawes C (2011) The salivary proteome: challenges and perspectives. Proteomics Clin Appl. (in press) [DOI] [PubMed]
- Siqueira WL, Oppenheim FG. Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle. Arch Oral. 2009;54:437–444. doi: 10.1016/j.archoralbio.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Helmerhorst EJ, Zhang W, Salih E, Oppenheim FG. Acquired enamel pellicle and its potential role in oral diagnostics. Ann NY Acad Sci. 2007;1098:504–509. doi: 10.1196/annals.1384.023. [DOI] [PubMed] [Google Scholar]
- Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP, Oppenheim FG. Identification of protein components in in vivo human acquired enamel pellicle using LC-ESI-MS/MS. J Proteome Res. 2007;6:2152–2160. doi: 10.1021/pr060580k. [DOI] [PubMed] [Google Scholar]
- Skjorland KK, Rykke M, Sonju T. Rate of pellicle formation in vivo. Acta Odontol Scand. 1995;53:358–362. doi: 10.3109/00016359509006001. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Hafferjee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Peridontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Spielmann N, Wong D. Saliva: diagnostics and therapeutic perspectives. Oral Diseases. 2011;17:345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CS, Ng H, McMillan AS. Antifungal susceptibility of Candida albicans biofilms on titanium discs with different surface roughness. Clin Oral Investig. 2007;11:361–368. doi: 10.1007/s00784-007-0122-3. [DOI] [PubMed] [Google Scholar]
- Whittaker CJ, Klier CM, Kolenbrander PE. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- Xu T, Levitz SM, Diamond RD, Oppenheim FG. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rico F, Xu AJ, Moy VT (2009) Atomic force microscopy of protein-protein interactions. Handbook of single-molecule biophysics doi:10.1007/978-0-387-76497-9_19: 555–570