Abstract
The CCN family of proteins consists of six members with conserved structural features. These proteins play several roles in the physiology and pathology of cells. Among the pathological roles of the CCN family, one of the most important and controversial ones is their role in the expansion and metastasis of cancer. Up to now a number of reports have described the possible role of each CCN family member independently. In this study, we comprehensively analyzed the roles of all six CCN family members in cell growth, migration and invasion of breast cancer cells in vitro and in vivo. As a result, we found the CCN2/CCN3 ratio to be a parameter that is associated with the metastatic phenotype of breast cancer cells that are highly metastatic to the bone. The same analysis with cell lines from oral squamous carcinomas that are not metastatic to the bone further supported our notion. These results suggest the functional significance of the interplay between CCN family members in regulating the phenotype of cancer cells.
Keywords: CCN family, CCN2, CCN3, Bone metastasis, Breast cancer, Oral cancer
Introduction
Breast cancer is one of the major life-threatening diseases, especially for women, as it often involves metastasis to the bones. Bone metastasis is a frequent complication of several common human malignancies, including breast, prostate, and lung cancer (Yoneda 1998; Guise and Mundy 1998) and is associated with a high morbidity rate because of intractable bone pain, pathological fracture, hypercalcemia, and nerve compression (Guise and Mundy 1998). Recently, it was reported that highly malignant breast cancer cells express CCN family 2/connective tissue growth factor gene (CCN2/CTGF), indicating that this particular molecule is one of the important factors for bone metastasis of breast cancer cells (Kang et al. 2003; Shimo et al. 2006).
CCN2 is a major member of the CCN family of proteins. CCN proteins consist of six members: CCN1/CYR61, CCN2/CTGF, CCN3/NOV, CCN4/Wnt-induced secreted protein WISP-1, CCN5/WISP-2, and CCN6/WISP-3 (Brigstock 2003; Perbal 2004; Perbal and Takigawa 2005; Leask and Abraham 2006; Chen and Lau 2009). These proteins are composed of four distinct modules, i.e., IGF-binding protein-like module (IGFBP), von Willebrand factor type C repeat (VWC), thrombospondin type-1 repeat (TSP1), and C-terminal module (CT). The only exception is CCN5, which lacks the CT module. With these modules, CCN2 interacts with a number of intra- and extracellular molecules, acting as a signal conductor. Indeed, CCN2 plays physiological roles in bone formation, angiogenesis, and wound healing (Perbal and Takigawa 2005; Chen and Lau 2009; Kubota and Takigawa 2007, 2011). On the other hand, under certain pathological situations, this factor has been known to be involved in arthritis, fibrosis, and cancer metastasis (Perbal and Takigawa 2005; Leask 2011).
Up to now, several reports have described the involvement of a few CCN family members in breast cancer invasion and metastasis to bone and in tumor angiogenesis (Babic et al. 1998; Xie et al. 2001; Kang et al. 2003; Jiang et al. 2004). Especially, the critical importance of CCN1 as a prognostic indicator and therapeutic target in breast cancer has been indicated as well as CCN2 (Tsai et al. 2002; Menendez et al. 2005; Espinoza et al. 2011). However, there has been no study that analyzed the role of all of the CCN family protein members in the context of breast cancer invasion and metastasis. Therefore, in the present study, we comprehensively analyzed the expression and role of all six CCN family members among breast and oral cancer cells and thereby found a new regulatory parameter that may be related to bone metastatic breast cancer phenotype.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-(MB)-231 and MCF-7 were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The human oral squamous cell carcinoma cell lines HSC-2 and SAS were cultured in a equal mixture of DMEM and Ham F-12 containing 10% FBS under the same air condition. Normal human umbilical vein endothelial cells (HUVEC) were cultured in endothelial cell growth medium-2 (Cambrex BioScience, Verviers, Belgium).
Antibodies
For the specific detection of CCN2 and CCN3 proteins, we prepared five different antibodies. All of them were evaluated for specificity by Western blotting of the recombinant human CCN proteins described below. Eventually we selected two antibodies that showed highest specificity: 8–86 for CCN2 (Kawaki et al. 2003; Kubota et al. 2004) and SC-18678 (lot. L15) for CCN3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For the detection of glutathione S-transferase (GST) and GST-CCN2, an anti-GST antibody was obtained from GE Healthcare (Waukesha, WI, USA).
Recombinant CCN proteins
The recombinant human CCN2 (rCCN2) was prepared as described previously (Nakanishi et al. 2000). All of the other CCN ones were purchased from Pepro Tech EC (London, UK). These proteins were diluted with 0.1% BSA-PBS to a concentration of 50 μg/ml as stock solutions. A recombinant CCN3 tagged with GST and GST alone were produced in E. coli BL21 (DE3) strain and were purified through a glutathione Sepharose 4B column (GE Healthcare), according to an established methodology.
RNA extraction and reverse transcription
The total RNAs of the cells were isolated by using an RNeasy Mini Kit (QIAGEN) or Trizol reagent (Invitrogen, Carlsbad, CA, USA) as instructed by the manufacturer. Then, 500 ng of total RNA of each sample was reverse-transcribed with avian myeloblastosis virus (AMV) reverse transcriptase (Takara Bio, Otsu, Japan) at 42°C for 30 min.
Quantitative real-time PCR
Quantitative real-time PCR was performed as described previously (Yanagita et al. 2007; Kawaki et al. 2008a). Sequences of the primer sets used were as follow: CCN1 (forward, 5′-AAC AAC TTC ATG GTC CCA GT-3′; reverse, 5′-CTC AAA CAT CCA GCG TAA GT-3′); CCN2 (forward, 5′-GCA GGC TAG AGA AGC AGA GC-3′; reverse, 5′-ATG TCT TCA TGC TGG TGC AG-3′); CCN3 (forward, 5′-GCC CAG ATG AGG AGG ATT-3′; reverse, 5′-GCA TCT CAC ATT GAC GGT TC-3′); CCN4 (forward, 5′-CCA CTC GGA TCT CCA ATG TT-3′; reverse, 5′-ACT TGG GTT GAT AGG AGC GT-3′); CCN5 (forward, 5′-TGA GAG GCA CAC CGA AGA CC-3′; reverse, 5′-AGC AGC CAC AGC CAT CCA-3′); CCN6 (forward, 5′-TTA CAT TCA GCC TTG CGA C-3′; reverse, 5′-CAG CAT CTC TTA TCC AAG CAT-3′); β-actin (forward, 5′-GAT CAT TGC TCC TCC TGA GC-3′; reverse, 5′-ACT CCT GCT TGC TGA TCC AC-3′); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward, 5′-GCC AAA AGG GTC ATC ATC TC-3′; reverse, 5′-GTC TTC TGG GTG GCA GTG AT-3′).
Migration assay
The rhCCNs at the final concentration of 100 ng/ml in 700 μl of EBM-2 containing 10% FBS were placed in the lower chamber. Next, a Boyden chamber was set on the plate; then HUVEC in the logarithmic growth phase were seeded at a density of 1 × 105 cells/200 μl onto polycarbonate membranes of the Boyden chambers (pore size: 8.0 mm; Becton Dickinson, Franklin Lakes, NJ, USA). The migration assay was carried out essentially as described earlier (Shimo et al. 1999).
[3H] Thymidine incorporation assay
The proliferation of HUVEC was assessed by measuring the incorporation of [3H]-thymidine into the cells. HUVEC cells (2 × 104) were seeded in each well of a 48-well plate containing EBM-2 medium supplemented with 10% FBS. One day after cell seeding, a given rhCCN at 100 ng/ml was applied to each of several wells. Eighteen hour later, these cultures were pulse-labeled for the last 4 h before harvest with 20 μCi/well of [3H] thymidine. The radioactivity incorporated into acid-precipitated materials was counted by a scintillation counter (Micro Beta Plus; Wallac, Gaithersburg, MD, USA).
In vitro pull-down assay
For the evaluation of direct binding of CCN3 to heparin, 2.5 fmol of GST or GST-CCN3 was mixed with 20 μl of heparin-agarose suspension (Sigma) in a co-IP buffer (20 mM Tris, 2.5 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, 10% glycerol; pH 8.0), which was swirled for 1 h. Thereafter, the precipitate was washed with co-IP buffer 5 times, and bound proteins were extracted in a sodium dodecylsulfate (SDS) sample buffer. Detection of GST or GST-CCN3 was performed by Western blotting analysis against the anti-GST antibody after SDS-polyacrylamide gel electrophoresis, as described previously (Yanagita et al. 2007; Kawaki et al. 2008b).
Mouse model of bone metastasis and bone immunohistochemistry
A mouse model of bone metastasis was prepared as described previously (Sasaki et al. 1995). The lower limb with a bone joint obtained from a model mouse was prepared at 28 days after the injection of MDA231 cells. Immunostaining for CCN family proteins was performed on 5-μm frozen serial sections that had been placed on glass slides and fixed with 4% paraformaldehyde. To reduce nonspecific binding, we submerged the slides in Histofine Blocking Reagent (Nichirei Bioscience, Tokyo, Japan) for 60 min. The samples were incubated with the primary antibodies described above in blocking reagent at the suppliers recommended concentration at 4°C overnight. Positive signals were visualized by using a Histofine Simple Stain Mouse MAX-PO(R) (for rabbit primary antibodies), or Histofine Simple Stain Mouse MAX-PO(G) (for goat primary antibodies), all of which were purchased from the same vendor (Nichirei Bioscience). Positive signals were visualized by using 3, 3-diaminobenzidine tetrachloride (DAB: Sigma). Finally, the sections were counterstained with methyl green solution (Wako Pure Chemical Industries, Osaka, Japan). After staining, all sections were examined with a motorized microscope Bx61 and a DP71 digital camera (Olympus, Tokyo, Japan). All animal experiments in this study were conducted according to the Guidelines for Animal Research, and approved by the Animal Committee, of Okayama University.
Statistical analysis
Statistical analysis was performed, where required, by using Student’s paired t-test.
Results
Comparative analysis of the gene expression levels of all CCN family members between highly metastatic MDA-231 and nonmetastatic MCF-7 breast cancer cells
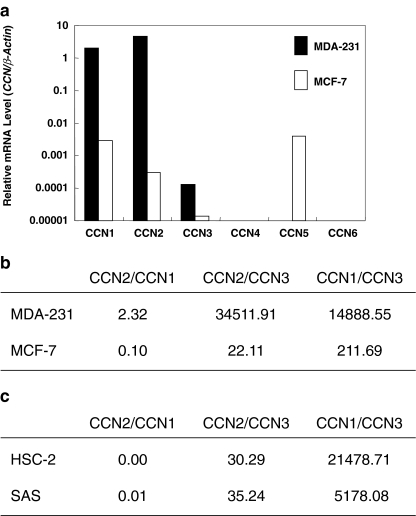
To evaluate the multiple involvement of CCN family members in the phenotype of breast cancer cells, we initially analyzed the CCN gene expression in highly malignant MDA-231 cells comprehensively by quantitative RT-PCR. As a result, CCN1, CCN2 and CCN3 were found to be expressed in MDA-231 cells, whereas the other CCN members were not (Fig. 1a). Comparatively, we next analyzed MCF-7 cells, whose phenotype is known to be less malignant. Interestingly, CCN5 gene was specifically expressed in MCF-7; and CCN1, CCN2 and CCN3 genes were also expressed. Subsequently, to further find out a parameter that would determine the metastatic phenotype of breast cancer, we evaluated the mRNA expression ratio among the three CCN family proteins that were commonly expressed in both cells (Fig. 1b). Consequently, we found that the CCN2/CCN3 and CCN1/CCN3 ratios were strikingly higher in MDA-231 cells than in MCF-7 cells. Indeed, the difference in CCN2/CCN3 between the two cell lines was more than 1,500-fold, whereas that in the CCN1/CCN3 ratio was approximately 70-fold. Therefore, high CCN2/CCN3 and CCN1/CCN3 ratios can be a parameter representing the highly metastatic phenotype of breast cancer.
Fig. 1.
Expression profile of CCN family members in breast and oral cancer cells. a Comprehensive comparison of gene expression of all of the CCN family members between highly metastatic MDA-231 and non-metastatic MCF-7 breast cancer cells. To minimize the influence of different metabolic activities, β-actin was employed as an internal control rather than GAPDH. Note that the ordinate is scaled in a logarithmic manner. b The mRNA ratios among the major CCN family members, CCN1, CCN2, and CCN3, in breast cancer MDA-231 and MCF-7 cells. The greatest difference observed between the two cell lines is in the CCN2/CCN3 ratio. c The mRNA ratios among CCN1, CCN2 and CCN3 in oral squamous cell carcinoma cell lines HSC-2 and SAS cells. Note that CCN2/CCN3 ratios are lower than MDA-231, whereas CCN1/CCN3 ratios are comparable to that of MDA-231. Representative data of three evaluations, which yielded comparable results, are displayed
Low CCN2/CCN3 ratio observed in human oral squamous cell carcinoma cell lines
As represented by the phenotype of MDA231 cells, breast cancer is one of the major types of solid tumors that frequently form bone metastatic lesions (Yoneda 1998). In contrast, although being invasive to bones, oral squamous carcinomas generally do not metastasize to bones (Yoneda 1998). Therefore, we performed analyses similar to the described above with a few oral squamous cell carcinoma cell lines. Evaluation of gene expression levels of CCN family members and subsequent computation of data revealed quite low CCN2/CCN3 ratio, despite both cell lines have been known to be highly invasive (Fig. 1c). Interestingly, CCN1/CCN3 ratio was quite high in both cell lines, suggesting the association of this parameter with invasiveness. These findings further support the relationship between CCN2/CCN3 ratio and bone metastatic phenotype of carcinomas.
Different angiogenic functions between CCN2 and CCN3 as evaluated by the migration and proliferation assays with HUVEC
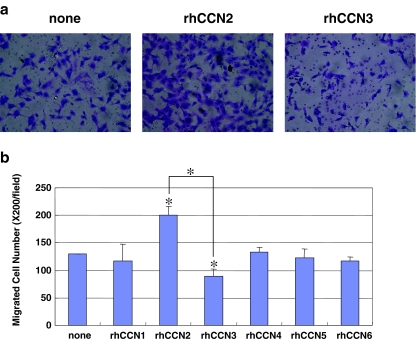
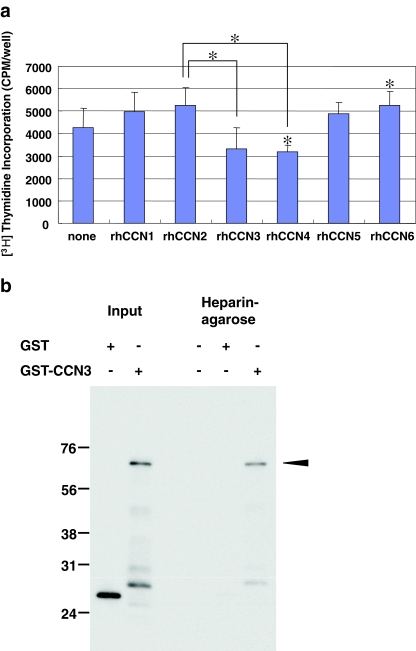
Angiogenesis is the one of the important events involved in tumor expansion and metastasis, as a number of reports have reported in the past (Babic et al. 1998; Shimo et al. 2006). Therefore, we evaluated the biological functions of CCN proteins in terms of the migration and proliferation of vascular endothelial cells, HUVEC. In the migration assay, we found that the CCN2 protein promoted the migration most strongly. In contrast, CCN3 had a rather repressive effect on the migration of the HUVEC (Fig. 2a and b). Moreover, similar effects of CCN2 and CCN3 on the cell proliferation were confirmed with the same HUVEC. Namely, CCN2 up-regulated the proliferation of HUVEC, whereas CCN3 down-regulated it (Fig. 3a). In addition, CCN4 or CCN6 significantly repressed or enhanced the proliferation of HUVEC, respectively (Fig. 3a). Collectively, these findings indicate that enhanced CCN2 production with repressed CCN3 production may provide an ideal microenvironment for tumor angiogenesis (Figs. 2b and 3a), supporting the significance of CCN2/CCN3 ratio in tumor biology. It is also suggested that, in terms of cell proliferation, contribution of CCN4 and CCN6 may not be ruled out. The major functions of CCN2 are known to be exerted via the interaction with heparan sulfate proteoglycans (HSPGs), which is represented by haparin binding ability. Therefore, in order to gain insights into the molecular mechanism for the counteracting functions of CCN2 and CCN3, heparin binding ability of CCN3 was evaluated in vitro. As a result, GST-tagged recombinant CCN3 was found to bind to heparin, whereas GST alone did not (Fig. 3b). These results together suggest the involvement of competitive binding to HSPG in the observed counteracting effects of CCN2 and CCN3.
Fig. 2.
Effect of each CCN protein on the migration of HUVEC as evaluated by the Boyden chamber method. a Typical microscopic views of migrated HUVECS in the presence of exogenous CCN2 and CCN3. b Comprehensive analysis of HUVEC migration in response to recombinant CCN proteins (t-test, *p < 0.01). Asterisks without brackets indicate significant differences against the control (none). The values represent the mean values with standard deviations (SDs)
Fig. 3.
Effect of each CCN protein on the proliferation of HUVEC and interaction of CCN3 with haparin. a Effects of recombinant CCN family proteins on [3H] thymidine incorporation. A highly significant difference between the effects of CCN2 and CCN3 was observed (t-test, *p < 0.01). Asterisks without brackets indicate significant differences against the control (none). The values represent the mean values with SDs. b Binding of CCN3 to heparin. GST or GST-CCN3 precipitated by heparin-agarose beads was detected by Western blotting with an antibody against GST. Positive controls before precipitation experiments are shown as well (left lanes)
Expression of CCN4, 5 and 6 by HUVEC
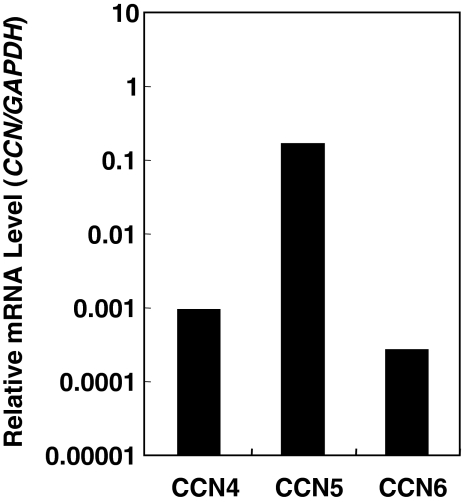
Since CCN family members are matricellular proteins that can be supplied and shared by any cell in the microenvironment, we next examined possible supply of CCN family members that were not provided by MDA-231 cells, but were produced by HUVEC itself. Quantitative mRNA analysis of HUVEC revealed significant expression of all of the members tested (Fig. 4). The obtained data suggest the presence of CCN4, 5 and 6 supplied by vascular endothelial cells in metastatic environment as well as other CCN family members from tumor cells.
Fig. 4.
Expression of CCN4, 5 and 6 in HUVEC. Quantitative evaluation of mRNAs was performed under the same methods as employed for Fig. 1. Representative data of two sets of experiments with comparable results are shown
Differential production of CCN2 and CCN3 in bone metastasis in vivo
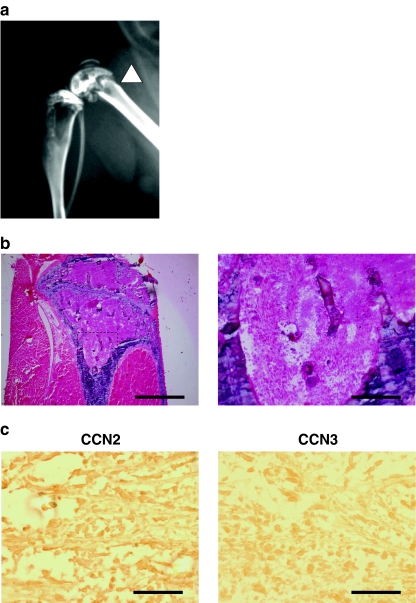
Finally, to confirm the in vitro data shown above, we analyzed CCN2 and CCN3 protein accumulation in the region of metastasis in a bone metastasis model in vivo. First, to confirm the osteolytic bone metastasis of breast cancer cells, MDA-231, we performed radiographs and H & E staining (Fig. 5a and b). Since we recognized successful bone metastasis, we subsequently evaluated the production of CCN2 and CCN3 by immunohistochemical analysis. As expected, the signal for CCN2 was observed in the area that had been invaded by the tumor cells (Fig. 5c). In contrast, the signal for CCN3 was not significant. Therefore, the significance of CCN2/CCN3 gene expression ratio as a parameter representing the bone metastatic phenotype was indicated in vitro and here confirmed in vivo.
Fig. 5.
Accumulation of CCN2 and absence of CCN3 in bone metastatic region of breast cancer cells in vivo. a Representative radiograph of a hind limb from a mouse 28 days after tumor inoculation. The arrowhead indicates the osteolytic lesion. b Bone histology of the midtibial metaphyses. Tumor cells have filled the marrow cavity. The right panel is a high-power magnification of the rectangular area in the left panel. Scale bar: 1 mm (left), and 200 μm (right). c Immunostaining for CCN2 and CCN3 in the bone metastatic lesions. Scale bar: 100 mm
Discussion
It is well known that various factors are related to cancer metastasis, invasion, and tumor angiogenesis. In a recent report, it was proven that highly metastatic cancer cells already have metastatic ability when the tumor occurs (Kang et al. 2003); and thus comprehensive analysis of the gene expression profile has been actively undertaken. In this research, we initially aimed at analyzing at the molecular level the expression of all of the CCN family members in breast cancer cells having different phenotype. At the same time, we analyzed the angiogenic ability of individual CCN family members. Based on the data obtained in vitro, we have defined a parameter representing the metastatic phenotype of breast cancer cells, which has been also supported by the data in vivo and also by those with oral squamous cell carcinoma cell lines in vitro.
CCN1, CCN2, and CCN3 are the major members of the CCN family, and several reports have suggested the involvement of these molecules in the invasion and metastasis of cancers. Since the angiogenic ability is closely related to the invasive and metastatic phenotype, we performed migration and proliferation assays using recombinant CCN proteins and HUVEC. According to our results, CCN2 was the strongest promoter of HUVEC migration, whereas CCN3 was a repressor of it, which is consistent with all other data presented here. However, CCN3 was reported to be a pro-angiogenic molecule in a past report (Lin et al. 2003). The apparent discrepancy between our data and the previous report may be ascribed to the difference in the method used to prepare the recombinant CCN3 protein and in the final concentration of CCN3 applied in these experiments. It is known that growth factors often display opposite functions under different doses. Additionally, Lin et al. employed bovine adrenal capillary endothelial cells, which yielded different results from ours. In either case, it is important to recognize that particular type of endothelial cells, including HUVEC, may not necessarily represent the endothelial cells associated with tumor angiogenesis. Finally, since the structure of CCN family proteins is so similar that the molecular behavior of CCN2 and CCN3 can be highly complicated, depending upon the conditions, when they co-exist. In fact, we evaluated the endogenous expression of CCN family genes in HUVEC cells and found that CCN2 was also abundantly produced by HUVEC itself (data not shown), whereas CCN3 was not. This finding indicates two biological issues. One is that CCN2 can be supplied not only by tumor cells, but also by vascular endothelial cells in an autocrine manner. The other is that the repressive activity of recombinant CCN3 may be exerted in the co-presence of CCN2. As indicated by the data in Fig. 3b, not only CCN2, but also CCN3 interacted with heparin. In analogous to CCN2, CCN3 might display angiogenic effects at a weaker level than CCN2 under the interaction with HSPG. If so, CCN3 could repress the effect of comparative amount of CCN2. However, when CCN3 was provided in excess, the net angiogenic effect given by CCN3 itself might overwhelm the repressive effect caused by the displacement of limited amount of CCN2. As such, it is possible that the conditional repressive action of CCN3 on angiogenesis is exerted by the molecular interaction via HSPG to inhibit the CCN2 action.
Apart from CCN2 and CCN3, it should be noted that CCN5 was found to be expressed only in non-metastatic MCF-7 cells; however, no repressive effect of CCN5 on angiogenic behavior of HUVEC was observed. Of note, a previous report also showed that CCN5 represses the malignant phenotype of breast cancer cells (Fritah et al. 2008). Therefore, it is hypothesized that the anti-invasive effect of CCN5 is conferred through a mechanism independent from angiogenesis. In contrast, although gene expression was not detected in the breast cancer cell lines, CCN4 and CCN6 exerted repressive and enhancing effects on the proliferation of HUVEC, respectively. Even if these members are not produced by breast cancer cells by themselves (Fig. 1), they may be supplied from other cells in the microenvironment, such as endothelial cells (Fig. 4). Thus, roles of CCN4 and 6 in breast cancer progression may not be overlooked. Also, the higher CCN1/CCN3 ratio in highly invasive MDA-231 may be noted, since it is consistent with a previous finding representing the role of CCN1 as a tumor promoter (Tsai et al. 2002). Indeed, CCN1/CCN3 ratio was strikingly high in aggressive oral squamous cell carcinoma cell lines, although significant angiogenic effects by CCN1 were not confirmed in our hands. Obviously, further investigation is necessary to clarify these points.
The CCN2/CCN3 mRNA ratio is a parameter that can be determined relatively easily with clinical samples. Therefore, this value may be considered for one of the parameters to predict the risk of bone metastasis of breast cancers and other related malignancies. Nevertheless, contribution of local microenvironments to metastaic events may not be overlooked, as typically represented by the fact that CCN2 and other growth factors can be supplied by the cells and ECM surrounding the tumor (Fig. 4; Shimo et al. 2006). Further investigation with clinical samples is underway for the examination of the diagnostic utility of this parameter in breast cancer cases.
Acknowledgments
This work was supported by the programs Grants-in-Aid for Scientific Research (S)[to M.T.] and (C) [to S.K.] from Japan Society for the Promotion of Science, and by a grant from the program Grants-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [to M.T.]. Work performed in B. Perbal’s laboratory was funded by French Ministry of Education: EA1556; and by European PROTHETS (Prognosis and Therapeutic Targets of Ewing Family of Tumors, FP6 Contract 503036). We thank Dr. Takanori Eguchi for useful discussions, and Ms. Yoko Tada for valuable secretarial assistance.
Contributor Information
Satoshi Kubota, Phone: +81-86-2356646, FAX: +81-86-2356649, Email: kubota1@md.okayama-u.ac.jp.
Masaharu Takigawa, Phone: +81-86-2356646, FAX: +81-86-2356649, Email: takigawa@md.okayama-u.ac.jp.
References
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza I, Liu H, Busby R, Lupu R (2011). CCN1 a candidate target for zoledronic acid treatment in breast cancer. Mol Cancer Ther in press [DOI] [PMC free article] [PubMed]
- Fritah A, Saucier C, Wever O, Bracke M, Bièche I, Lidereau R, Gespach C, Drouot S, Redeuilh G. Role of WISP-2/CCN5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol Cell Biol. 2008;28:1114–1123. doi: 10.1128/MCB.01335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/er.19.1.18. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, Mansel RE. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endcr Relat Cancer. 2004;11:781–791. doi: 10.1677/erc.1.00825. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel P, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;5:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Minato M, Moritani NH, Hattori T, Hanagata H, Kubota M, Miyauchi A, Nakanishi T, Takigawa M. Novel enzyme-linked immunosorbent assay systems for the quantitative analysis of connective tissue growth factor (CTGF/Hcs24/CCN2): detection of HTLV-1 tax-induced CTGF from a human carcinoma cell line. DNA Cell Biol. 2003;22:641–648. doi: 10.1089/104454903770238111. [DOI] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Yamada T, Matsumura T, Mandai T, Yao M, Maeda T, Lyons KM, Takigawa M. Functional requirement of CCN2 for intramembranous bone formation in embryonic mice. Biochem Biophys Res Commun. 2008;366:450–456. doi: 10.1016/j.bbrc.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M (2011). The role of CCN2 in cartilage and bone development. J Cell Commun Signal in press [DOI] [PMC free article] [PubMed]
- Kubota S, Kawata K, Yanagita T, Doi H, Kitoh T, Takigawa M. Abundant retention and release of connective tissue growth factor (CTGF/CCN2) by platelets. J Biochem. 2004;136:279–282. doi: 10.1093/jb/mvh126. [DOI] [PubMed] [Google Scholar]
- Leask A (2011). Possible strategies for anti-fibrotic drug intervention in scleroderma. J Cell Commun Signal in press [DOI] [PMC free article] [PubMed]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;2005(24):761–779. doi: 10.1038/sj.onc.1208238. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M. Effect of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/en.141.1.264. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal B, Takigawa M. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. [Google Scholar]
- Sasaki A, Boyce BF, Story B, Wright KR, Chapman M, Boyce R, Mundy GR, Yoneda T. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–3557. [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kubota S, Yoshioka N, Ibaragi S, Isowa S, Eguchi T, Sasaki A, Takigawa M. Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J Bone Miner Res. 2006;21:1045–1059. doi: 10.1359/jbmr.060416. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Bogart DF, Castañeda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:817881–817885. doi: 10.1038/sj.onc.1205682. [DOI] [PubMed] [Google Scholar]
- Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–8923. [PubMed] [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, Tanaka S, Takigawa M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007;274:1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Yoneda T. Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur J Cancer. 1998;34:240–245. doi: 10.1016/S0959-8049(97)10132-0. [DOI] [PubMed] [Google Scholar]