Abstract
BACKGROUND
The impact of adherence enhancing interventions on the relationship between active drug use and adherence is largely unknown.
METHODS
We conducted a 24-week randomized controlled trial of antiretroviral directly observed therapy (DOT) versus treatment as usual (TAU) among HIV-infected methadone patients. Our outcome measure was pill count antiretroviral adherence, and our major independent variables were treatment arm (DOT v. TAU) and active drug use (opiates, cocaine, or both opiates and cocaine). We defined any drug use as ≥ one positive urine toxicology result, and frequent drug use as ≥50% tested urines positive. We used mixed-effects linear models to evaluate associations between adherence and drug use, and included a treatment arm-by-drug use interaction term to evaluate whether DOT moderates associations between drug use and adherence.
RESULTS
39 participants were randomized to DOT and 38 to TAU. We observed significant associations between adherence and active drug use, but these were limited to TAU participants. Adherence was worse in TAU participants with any opiate use than in TAU participants without (63% v 75%, p < 0.01); and worse among those with any polysubstance (both opiate and cocaine) use than without (60% v 73%, p=0.01). We also observed significant decreases in adherence among TAU participants with frequent opiate or frequent polysubstance use, compared to no drug use. Among DOT participants, active drug use was not associated with worse adherence.
CONCLUSIONS
Active opiate or polysubstance use decreases antiretroviral adherence, but the negative impact of drug use on adherence is eliminated by antiretroviral DOT.
Keywords: Directly observed therapy, HIV, medication adherence, methadone, randomized trial, substance use
1. Introduction
Adherence to antiretroviral therapy is critical to prevent HIV disease progression, drug resistance, morbidity, and mortality. Research has consistently shown that drug use is associated with poor adherence (Arnsten et al., 2002; Gifford et al., 2000; Gordillo et al., 1999; Halkitis et al., 2005; Haubrich et al., 1999; Hinkin et al., 2007; Ingersoll, 2004; Lucas et al., 2001; Malta et al., 2010; Palepu et al., 2006; Rigsby et al., 2000; Sharpe et al., 2004; Stein et al., 2000), but this relationship has not been described in detail. Specifically, though HIV-infected drug users have worse adherence than non-drug users, few studies have examined how specific drugs of abuse affect adherence. In addition, the majority of studies describing associations between drug use and antiretroviral adherence have been cross-sectional (Gifford et al., 2000; Gordillo et al., 1999; Ingersoll, 2004; Rigsby et al., 2000; Sharpe et al., 2004; Stein et al., 2000), and have relied on self-reported measures of both adherence (Gifford et al., 2000; Haubrich et al., 1999; Ingersoll, 2004; Lucas et al., 2001; Sharpe et al., 2004; Stein et al., 2000) and drug use (Arnsten et al., 2002; Gifford et al., 2000; Gordillo et al., 1999; Halkitis et al., 2005; Haubrich et al., 1999; Ingersoll, 2004; Lucas et al., 2001; Palepu et al., 2006; Sharpe et al., 2004; Stein et al., 2000). In sum, few studies have examined the adherence impact of active drug use using either longitudinal designs or objective measures of adherence or drug use (Arnsten et al., 2002; Hinkin et al., 2007; Lucas et al., 2001; Palepu et al., 2006).
Though many successful adherence enhancing interventions have been described, the impact of such interventions on the relationship between active drug use and adherence is largely unknown. In particular, methadone clinic-based antiretroviral directly observed therapy (DOT) has been shown to be feasible and efficacious in improving adherence and viral suppression (Berg et al., 2011; Berg et al., 2009; Conway et al., 2004; Lucas et al., 2006; Lucas et al., 2004), and provides a unique opportunity to study the impact of this intervention on active drug users. The Support for Treatment Adherence Research through Directly Observed Therapy (STAR*DOT) trial was a randomized controlled trial that demonstrated the efficacy of methadone-clinic based antiretroviral DOT for improving adherence and reducing HIV viral load among methadone maintenance patients (Berg et al., 2011). To our knowledge, no study has assessed the impact of antiretroviral DOT on the relationship between drug use and adherence.
The objectives of this analysis were: 1) to examine the impact of opiate, cocaine, or polysubstance (both opiate and cocaine) use on antiretroviral adherence, and 2) to determine the effect of DOT on the association between drug use and adherence. We hypothesized that: 1) opiate, cocaine and polysubstance use decrease antiretroviral adherence; and that 2) the association between drug use and adherence is moderated by antiretroviral DOT.
2. Methods
2.1 Design and setting
The STAR*DOT trial has been previously described (Berg et al., 2011; Berg et al., 2009). Briefly, participants were randomized to antiretroviral DOT or treatment as usual (TAU) for 24 weeks. Participants randomized to DOT received one dose of their antiretroviral medication concurrently with their usual methadone dose. This was administered by methadone clinic nurses at the clinic dispensing window 5 or 6 times weekly. For weekend or evening doses that could not be observed, participants were given single dose pillboxes, and were asked to return the pillboxes at their next clinic visit. TAU participants self administered their antiretroviral medications throughout the trial. Both TAU and DOT participants were offered adherence counseling by paraprofessional counselors based on principles of Motivational Interviewing (Cooperman et al., 2007).
The study was conducted in twelve methadone clinics administered by the Division of Substance Abuse at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. This network treats approximately 3,500 opioid dependent patients, of whom 10–15% are HIV positive.
The protocol for the STAR*DOT trial was approved by the Albert Einstein College of Medicine Committee on Clinical Investigations. We obtained a Certificate of Confidentiality from the National Institutes on Drug Abuse to protect against disclosure of research information in federal, state or local civil, criminal, or administrative proceedings.
2.2 Participants
Eligible participants for this study shared the following characteristics: they were HIV positive, prescribed antiretroviral therapy, attended methadone maintenance treatment at least 5 or 6 days per week, received their HIV treatment at their methadone clinic or a closely affiliated clinic, were on a stable dose of methadone for at least 2 weeks prior to the trial, and were documented to be sensitive to their antiretroviral regimen via genotypic analysis of HIV-1 virus. Individuals were excluded if they could not provide informed consent, were not English-speaking, were already on DOT for HIV treatment, or had a primary care physician who did not agree with their participation.
2.3 Research visits and data collection
The research visit schedule for all participants included a baseline visit, followed by eight weekly and then four monthly visits (weeks 1, 2, 3, 4, 5, 6, 7, 8, 12,16, 20 and 24); there were therefore 13 data collection points. At each visit, data were collected using Audio Computer Assisted Self Interview (ACASI), which has been shown to improve reporting of stigmatized behaviors (DesJarlais et al., 1999; Macalino et al., 2002; Mizuno et al., 2007).
2.4 Measures
2.4.1 Major dependent variable: antiretroviral adherence
Adherence was assessed during the 24 week trial using pill counts. For DOT participants, pills that were never dispensed (e.g., because of missed methadone clinic visits or patient refusal), as well as those returned to nurses in pillboxes, were counted weekly at each methadone clinic by research assistants. We assumed that unreturned take-home pillboxes contained pills that had not been ingested. For TAU participants, pill counts occurred at all visits (weeks 1, 2, 3, 4, 5, 6, 7, 8, 12, 16, 20 and 24).
2.4.2 Major independent variables: drug use and treatment arm
At the baseline visit, self-reported drug use occurring in the prior 30 days was assessed using the Recent Drug Abuse Survey (Arnsten et al., 2002; Berg et al., 2004). Urine toxicology specimens were collected at each of 13 research visits. Specimens were sent to a commercial laboratory, where they were tested for amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, methadone, and phencyclidine, using the EMIT procedure (Siemens Healthcare Diagnostics, Deerfield Illinois).
Drug use variables were defined by urine toxicology test results. Types of drug use included cocaine, opiates, or polysubstance (both cocaine and opiate) use. We chose to focus on cocaine and opiate use because of their high prevalence in our setting, and because of the documented impact of these drugs on adherence. For each type of drug, any drug use was defined as one or more positive urine toxicology result over the 24 week period. Frequent drug use was defined as positive results on ≥50% of urine toxicology tests, as this metric is commonly used to assess outcomes of substance abuse treatment. Therefore, we analyzed six drug use variables: three reflecting any drug use (cocaine, opiate or polysubstance use) and three reflecting frequent drug use (cocaine, opiate, or polysubstance use).
Treatment arm was defined as antiretroviral directly observed therapy (DOT) or antiretroviral treatment as usual (TAU). Analyses are intent-to-treat, with participants analyzed in the treatment arms to which they were randomized.
2.4.3 Additional covariates
Sociodemographic and clinical characteristics, including age, sex, race/ethnicity, education, marital status, employment, and insurance, were assessed at baseline. Self-reported alcohol use was assessed at baseline, week 8 and week 24 using the Alcohol Use Disorders Identification Test [AUDIT; (Saunders et al., 1993)], with hazardous alcohol use defined as an AUDIT score ≥ 4 for women and ≥ 8 for men (Bradley et al., 1998; Fiellin et al., 2000; Steinbauer et al., 1998). Baseline methadone dose was assessed via review of methadone clinic records. Because methadone doses ≥100 mg daily are associated with reduced opioid use and greater retention in care (Donny et al., 2005; Fareed et al., 2009; Maxwell and Shinderman, 1999; Strain et al., 1999), methadone dose was dichotomized at ≥100 mg daily. Baseline antiretroviral adherence was assessed via self-report, and dichotomized at 100%, as has been done previously (Chesney et al., 2000; Simoni et al., 2006). HIV viral load and CD4+ T-cell counts were also determined at baseline, and weeks 8, 16, 20 and 24. Psychiatric symptoms were assessed at baseline, and weeks 8, 16, and 24 with the Brief Symptom Inventory [BSI; (Derogatis and Melisaratos, 1983)] a self-report inventory designed to assess global severity of general psychiatric symptoms. We evaluated both the BSI global measure of emotional distress (the Global Severity Index), and the BSI depression scale, and dichotomized scores at a T score ≥ 63.
2.5 Statistical Analysis
2.5.1 Baseline characteristics
Baseline characteristics are described by means and standard deviations or medians and interquartile range for continuous variables, and by percentages for categorical variables. Comparisons of participant characteristics between treatment arms (DOT vs. TAU) were conducted by t-tests, Wilcoxon rank sum tests, or chi-square tests.
2.5.2 Adherence
Pill count adherence was assessed as a continuous measure. Pill count adherence rates were derived by first computing the mean pill count adherence rate for each antiretroviral medication in the participant’s regimen. Second, we averaged the adherence rates for each antiretroviral medication to get an overall adherence rate for each participant at each timepoint. We then computed the mean pill count adherence rate for each participant during the following four assessment periods: baseline to week 4, weeks 5–8, weeks 12–16, and weeks 20–24 (e.g., week 8 pill count adherence represents the mean of pill count adherence rates at weeks 5, 6, 7, and 8). Seventy seven percent of pill count adherence rates were between 0 and 100%. We modified pill count adherence rates that were <0% or >100% according to specified rules (Berg et al., 2011). Rates of missing pill count data were comparable between the two treatment arms at weeks 8, 16, and 24, and were not included in analyses.
2.5.3 Drug use
We first measured the prevalence of drug use during the 24 week intervention period by examining each urine toxicology test. We tested the difference in prevalence of both any and frequent drug use between study arms with chi square tests.
We operationalized frequent drug use using 4 distinct time periods: weeks 0–4 (during which 5 urine specimens were collected), weeks 5–8 (4 specimens collected), weeks 12–16 (2 specimens collected) and weeks 20–24 (2 specimens collected). Those with ≥50% of tested urine toxicology results positive for the time period were categorized as “frequent drug use” in that time period for use in repeated measures analyses, described below.
2.5.4 Associations between adherence and drug use
We first tested the hypotheses that opiate, cocaine, and polysubstance are associated with lower adherence. To this end, we applied mixed effects linear models based on adherence rate = drug use + treatment arm + time. We then tested whether the effect of drug use on adherence is different between treatment arms, using mixed-effect linear models in the form of: adherence rate = drug use + treatment arm + drug use x treatment arm + time. The primary parameter of interest in this analysis was the interaction term, drug use-by-treatment arm, which quantifies a differential effect of drug use on adherence between the DOT and TAU arms. We then tested differences in adherence among participants with and without drug use, within each treatment arm. We also evaluated the association between hazardous alcohol use and adherence, with and without testing for interaction effects of study arm.
Across all applied mixed effects models, the covariance of the within subjects adherence outcome was assumed to be unstructured, the most conservative assumption. Two-sided significance levels were set at 0.05 for all tests. We used SAS v9.2 (SAS Institute Inc., Cary, NC) for the conduct of all analyses.
3. Results
3.1 Participant characteristics
77 participants were enrolled in the STAR*DOT trial, of whom 39 were randomized to DOT and 38 to TAU (Table 1). Participants were 53% male and 47% female, 45% Hispanic and 40% non Hispanic black. Their mean age was 47 years. The median duration of methadone treatment was 10 years, and the median dose of methadone was 125 mg. At baseline, 55% had urine toxicology results positive for cocaine, and 31% positive for opiates. Sociodemographic, substance use, and HIV clinical characteristics were evenly distributed between the two treatment arms. DOT and TAU participants attended a similar number of adherence counseling visits (3.33 for DOT versus 4.92 visits for TAU; p= 0.11). We observed no significant difference in the proportion of participants in the DOT and TAU study arms with a Brief Symptom Index depression subscale or Global Severity Index T score ≥ 63 at baseline, week 8, 16, or 24.
Table 1.
Baseline characteristics of the study population
| Sociodemographic characteristics | Total (n=77) | DOT arm (n=39) | TAU arm (n=38) | p value* |
|---|---|---|---|---|
| Age, mean (sd) | 47 (7) | 45 (7) | 49 (7) | 0.05 |
| Sex, n (%) | ||||
| Male | 41 (53) | 19 (49) | 22 (58) | 0.42 |
| Race/ethnicity, n (%) | ||||
| Hispanic | 35 (45) | 20 (51) | 15 (39) | 0.30 |
| Black, non-Hispanic | 31 (40) | 14 (36) | 17 (45) | |
| White, non-Hispanic | 8 (10) | 5 (13) | 3 (8) | |
| Other | 3 (4) | 0 (0) | 3 (8) | |
| Education, n (%) | ||||
| Less than high school | 38 (49) | 19 (49) | 19 (50) | 0.89 |
| High school (partial or completed) | 20 (26) | 11 (28) | 9 (24) | |
| College (partial or completed) | 19 (25) | 9 (23) | 10 (26) | 0.74 |
| Marital status, n (%) | ||||
| Married or living with partner | 34 (44) | 17 (44) | 17 (45) | 0.14 |
| Widowed, separated or divorced | 22 (29) | 8 (20) | 14 (37) | |
| Single | 21 (27) | 14 (36) | 7 (18) | |
| Employment status, n (%) | ||||
| Employed | 2 (3) | 1 (3) | 1 (3) | 1.00 |
| Unemployed or unable to work, n (%) | 75 (97) | 38 (97) | 37 (97) | |
| Insurance, n (%) | ||||
| Medicaid | 69 (90) | 33 (85) | 36 (95) | 0.26 |
| Medicare | 16 (21) | 10 (26) | 6 (16) | 0.40 |
| Private insurance | 6 (8) | 3 (8) | 3 (8) | 1.0 |
| Substance use characteristics | ||||
| Median duration methadone maintenance, years (IQR) (n=48) | 10 (5–16) | 9 (4–15) | 10 (6–16) | 0.93 |
| Median methadone dose, mg (IQR) (n=74) | 125 (90–180) | 135 (90–170) | 120 (90–185) | 0.85 |
| Methadone dose ≥ 100, n (%) (n=74) | 50 (68) | 24 (65) | 26 (70) | 0.62 |
| Self-reported illicit drug use in prior 30 days, n (%) | ||||
| Heroin | 20 (26) | 9 (24) | 11 (29) | 0.60 |
| Cocaine | 41 (54) | 21 (55) | 20 (53) | 0.82 |
| Marijuana | 11 (14) | 7 (19) | 4 (11) | 0.30 |
| Amphetamine | 3 (4) | 1 (3) | 2 (5) | 1.00 |
| Hazardous alcohol use | 18 (24) | 9 (24) | 9 (24) | 0.95 |
| Positive baseline urine toxicology report, n (%) | ||||
| Opiate (non-methadone) | 24 (31) | 11 (30) | 13 (35) | 0.62 |
| Cocaine | 42 (55) | 19 (51) | 23 (62) | 0.35 |
| Benzodiazepine | 7 (9) | 3 (8) | 4 (11) | 0.71 |
| Marijuana | 13 (17) | 6 (16) | 7 (19) | 0.72 |
| Amphetamine | 0 (0) | 0 | 0 | 1.00 |
| Lifetime history of injection drug use | 46 (61) | 27 (71) | 19 (50) | 0.06 |
| Clinical characteristics | ||||
| Self-reported antiretroviral adherence in prior seven days | ||||
| 100%, n (%) | 55 (72) | 25 (66) | 30 (79) | 0.20 |
| <100%, n (%) | 21 (27) | 13 (34) | 8 (21) | |
| Median duration of HIV infection, years (IQR) | 13 (9–17) | 12 (8–15) | 15 (10–18) | 0.09 |
| HIV viral load < 75 copies/ml, n (%) | 41 (53) | 19 (49) | 22 (58) | 0.42 |
| Median CD4+ T cell count, cells/mm3, (IQR) (n=74) | 345 (151–494) | 367 (189–509) | 277 (110–453) | 0.34 |
| BSI depression subscale T≥63, n (%) (n=69) | 31 (45) | 17 (50) | 14 (40) | 0.40 |
| BSI global severity index T≥63, n (%) (n=69) | 35 (51) | 18 (53) | 17 (49) | 0.72 |
p value for difference between DOT and TAU groups
3.2 Prevalence of drug use
During the 24-week trial, a median of 11 of 13 scheduled urine toxicology specimens was collected among all participants [interquartile range (IQR) 10–13], with a median of 11 in the DOT arm (IQR 9–13), and 12 in the TAU arm (IQR 10–13). Despite methadone maintenance treatment and trial enrollment, there was a high prevalence of ongoing drug use in both arms. Among DOT and TAU participants respectively, 65.8% and 81.6% of urine toxicology tests showed any use of cocaine, 68.4% and 65.8% any opiate use, and 44.7% and 39.5% any polysubstance use, but differences between arms were not significant (Table 2).
Table 2.
Prevalence of drug use throughout the 24 week intervention period*
| Total n (%) |
DOT n (%) |
TAU n (%) |
p-value | |
|---|---|---|---|---|
| Any cocaine use† | 56 (73.7) | 25 (65.8) | 31 (81.6) | 0.12 |
| Frequent cocaine use‡ | 42 (55.3) | 22 (57.9) | 20 (52.6) | 0.64 |
| Any opiate use | 51 (67.1) | 26 (68.4) | 25 (65.8) | 0.81 |
| Frequent opiate use | 20 (26.3) | 9 (23.7) | 11 (29.0) | 0.60 |
| Any polysubstance use§ | 32 (42.1) | 17 (44.7) | 15 (39.5) | 0.64 |
| Frequent polysubstance use | 12 (15.8) | 5 (13.2) | 7 (18.4) | 0.53 |
Maximum number of possible tests per participant = 13; median number of tests collected=11 in DOT arm, 12 in TAU arm, and 11 in both study arms.
Any drug use is defined as having one or more positive urine toxicology test results over the 24 week period.
Frequent drug use is defined as having ≥ 50% positive urine toxicology test results over the 24 week period.
Polysubstance use is defined as having a urine toxicology test positive for both cocaine and opiates.
3.3 Association between drug use and adherence
Table 3 describes adherence rates for participants with and without drug use over the 24 week study period. Among all participants, every measure of drug use was associated with worse adherence, though those effects were small and only some were statistically significant. Participants with any opiate use had lower adherence than participants with no opiate use (74% v 79%; p=0.04), and those with frequent opiate use had lower adherence than those without frequent opiate use (72% v 79%; p=0.01). Further, adherence was 73% among participants with any polysubstance use, compared to 78% among participants without any polysubstance use (p=0.07), and 71% among participants with frequent polysubstance use, compared to 78% among participants without frequent polysubstance use (p=0.06).
Table 3.
Impact of drug use on antiretroviral adherence, with and without testing for interaction of drug use and study arm
| Estimated adherence among all participants* | Estimated adherence among DOT arm participants accounting for interaction effects† | Estimated adherence among TAU arm participants accounting for interaction effects† | |||||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Any cocaine use | No | 0.78 | 0.73–0.83 | 0.83 | 0.76–0.90 | 0.72 | 0.66–0.79 |
| Yes | 0.76 | 0.71–0.80 | 0.84 | 0.78–0.90 | 0.68 | 0.61–0.74 | |
| p value‡ | 0.50 | 0.86 | 0.25 | ||||
| Any opiate use | No | 0.79 | 0.75–0.84 | 0.85 | 0.79–0.90 | 0.75 | 0.69–0.82 |
| Yes | 0.74 | 0.69–0.78 | 0.83 | 0.77–0.89 | 0.63 | 0.56–0.70 | |
| p value‡ | 0.04 | 0.55 | 0.004 | ||||
| Any polysubstance use | No | 0.78 | 0.74–0.82 | 0.84 | 0.79–0.90 | 0.73 | 0.67–0.79 |
| Yes | 0.73 | 0.67–0.78 | 0.82 | 0.75–0.89 | 0.60 | 0.52–0.69 | |
| p value‡ | 0.07 | 0.56 | 0.01 | ||||
| Frequent cocaine use | No | 0.78 | 0.73–0.83 | 0.83 | 0.76–0.89 | 0.74 | 0.67–0.80 |
| Yes | 0.76 | 0.71–0.80 | 0.85 | 0.79–0.91 | 0.66 | 0.60–0.73 | |
| p value‡ | 0.38 | 0.61 | 0.07 | ||||
| Frequent opiate use | No | 0.79 | 0.75–0.83 | 0.85 | 0.80–0.90 | 0.74 | 0.69–0.80 |
| Yes | 0.72 | 0.66–0.77 | 0.83 | 0.76–0.90 | 0.60 | 0.52–0.67 | |
| p value‡ | 0.01 | 0.63 | 0.0005 | ||||
| Frequent polysubstance use | No | 0.78 | 0.74–0.82 | 0.84 | 0.79–0.89 | 0.72 | 0.67–0.78 |
| Yes | 0.71 | 0.65–0.78 | 0.83 | 0.73–0.92 | 0.60 | 0.51–0.69 | |
| p value‡ | 0.06 | 0.77 | 0.01 | ||||
Antiretroviral adherence was evaluated in linear mixed effects models: adherence = drug use + study arm + week.
Antiretroviral adherence was evaluated in linear mixed effects models: adherence = drug use + study arm + drug use * study arm + week.
Tests the difference in adherence between participants with and without positive urine toxicology test results.
3.4 Association between drug use and treatment arm and adherence
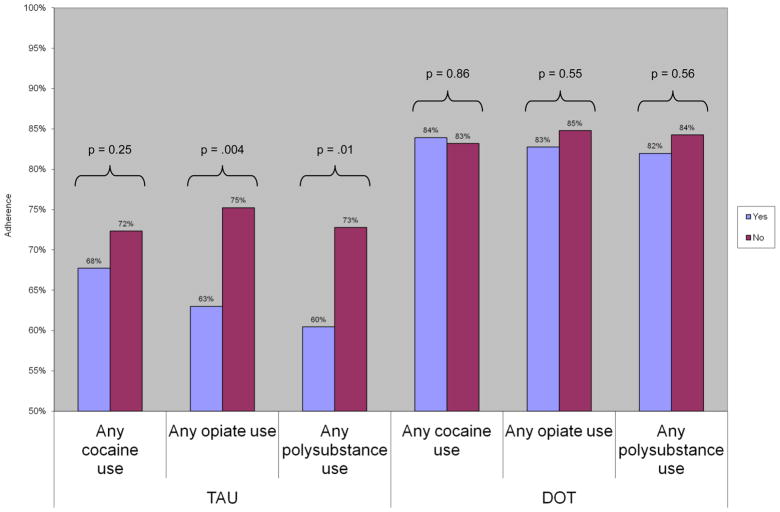
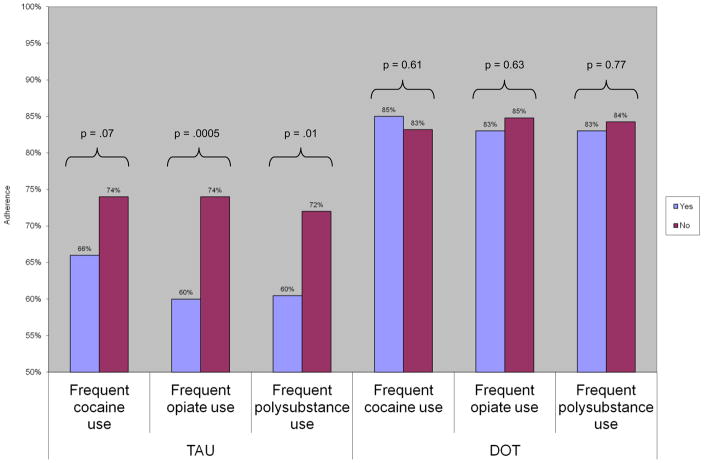
At all post-baseline assessment points, adherence was higher among DOT than TAU participants; by week 24, mean adherence was 86% among DOT participants and 56% among TAU arm participants (p<0.0001). During the 24 week intervention, the proportion of DOT participants with an undetectable VL increased from 51% to 71% (Berg, et al., 2011). Because of a significant interaction between treatment arm and drug use, we evaluated the association between drug use and adherence in each treatment arm, to assess the moderating effect of DOT. Among TAU participants, any opiate or polysubstance use was associated with decreased adherence compared to no drug use: any opiate use (63 v 75%; p=0.04), and any polysubstance use [60 v 73%; p = 0.01 (Figure 1)]. Similarly, frequent opiate or polysubstance use was also associated with lower adherence among TAU participants: frequent opiate use (60 v 74%; p<0.001), and frequent polysubstance use [60 v 72%; p=0.01 (Figure 2)]. Among TAU participants, adherence was also decreased with any cocaine use (68 v 72%; p=0.25) or frequent cocaine use (66 v 74%; p=0.07), compared to no cocaine use, though these effects were not statistically significant. Conversely, in all measures of drug use, we observed no association between drug use and adherence among participants receiving antiretroviral DOT.
Figure 1.
Association between any drug use, treatment arm, and adherence
DOT: directly observed therapy; TAU: treatment as usual
Antiretroviral adherence was evaluated in linear mixed effects models: adherence = drug use + study arm + any drug use * study arm + week
Figure 2.
Association between frequent drug use, treatment arm, and adherence
DOT: directly observed therapy; TAU: treatment as usual
Antiretroviral adherence was evaluated in linear mixed effects models: adherence = drug use + study arm + frequent drug use * study arm + week
In mixed effects linear models, we found no significant association between hazardous alcohol use and adherence, both in the study population overall, and in testing for interactions between hazardous alcohol use and study arm. Though participants without active cocaine, opiate or polysubstance use and DOT participants were more likely to have undetectable viral loads, there was no significant moderating effect of DOT on the association between drug use and virologic outcomes (data not shown).
4. Discussion
In this sample of HIV-infected opiate dependent adults prescribed highly active antiretroviral therapy, we found that both any use and frequent use of opiates were associated with small but significant decreases in antiretroviral adherence. However, when we examined the moderating effect of treatment arm (DOT v. TAU), we found that decreases in adherence were limited to participants self-administering antiretrovirals. Specifically, we observed that both opiate use and polysubstance use were associated with decreased antiretroviral adherence among TAU participants, but among subjects receiving antiretroviral DOT, the decrease in adherence associated with drug use was eliminated. These findings strongly support the use of DOT as a tool for optimizing HIV care for drug users.
A small number of studies to date have evaluated the differential effects of specific drugs of abuse on adherence. Our finding that opiate use was associated with decreased adherence is consistent with data from a prospective cohort of HIV/HCV co-infected injection drug users, in which use of heroin at least weekly had an independent negative association with antiretroviral adherence (Palepu et al., 2006). In addition, a number of studies have described a significant, negative association between cocaine use and antiretroviral adherence (Arnsten et al., 2002; Halkitis et al., 2005; Hinkin et al., 2007; Ingersoll, 2004; Sharpe et al., 2004). Though we observed decreased adherence among cocaine users in the TAU arm, these findings were not statistically significant. This may reflect insufficient statistical power, given that there were few participants without cocaine use. Alternately, it is possible that participants episodically used cocaine at a level that did not decrease adherence. Finally, these findings may reflect different social or clinical characteristics among methadone patients who continue to use cocaine, compared to methadone patients who continue to use opiates. Because methadone maintenance treatment has been associated in many studies with improvements in adherence, HIV RNA suppression, and CD4+ T-cell count (Kapadia et al., 2008; Palepu et al., 2006; Sambamoorthi et al., 2000; Uhlmann et al., 2010), persons who are abstinent from opiates but continue to use cocaine may be engaged in methadone treatment in a way that supports or improves medication adherence. In such persons, opiate abstinence may be associated with improved drug treatment outcomes despite ongoing cocaine use, while continued opiate use during methadone maintenance may reflect inadequate opiate blockade or poor methadone treatment adherence. Poor methadone clinic attendance may in turn decrease the effect of methadone clinic-based antiretroviral DOT. Finally, antiretroviral medications that induce methadone metabolism, such as efavirenz and lopinavir/ritonavir, may contribute to both active opiate use and suboptimal antiretroviral adherence.
Our findings confirm and extend research demonstrating the effectiveness of antiretroviral DOT. Prior research has shown methadone clinic-based antiretroviral DOT programs to be feasible and effective in improving adherence and viral suppression (Berg et al., 2011; Berg et al., 2009; Conway et al., 2004; Lucas et al., 2006; Lucas et al., 2004). One prior study to date has evaluated antiretroviral DOT effects in the context of ongoing drug use. In a cohort of 54 persons receiving methadone clinic-based antiretroviral DOT, frequent cocaine use did not affect virologic response to antiretroviral therapy (Conway et al., 2004). Our findings extend that research by demonstrating that DOT is associated with improved adherence despite ongoing opiate, cocaine, or polysubstance use. Though we observed a deleterious association between active drug use and adherence, this was entirely absent among DOT participants, further supporting the utility of DOT in substance abuse treatment settings.
To guide understanding of adherence behavior change, our finding that DOT promotes adherence despite active drug use may be viewed within the Information, Motivation, Behavioral Skills (IMB) model (Fisher et al., 2006). The IMB model predicts specific moderating factors, including substance abuse, which can be expected to have adverse effects on adherence. Without adjuvant support to change such moderating factors, skill-based adherence interventions may fail. Among active drug users, structural interventions such as DOT may provide the necessary level of adjuvant adherence support to promote adherence behavioral skills.
Despite its strengths, our study has some limitations. First, we do not know if the opiate positive urine toxicology results were due to illicit opiate use, or to prescribed non-methadone opiate use. However, given that treatment arm assignment was randomized, illicit opiate use was likely equivalent between arms. Second, our small sample size limits our statistical power. Despite the marked difference in adherence rates among those who did and did not use drugs in the TAU arm, and the lack of such difference in the DOT arm, we had limited ability to detect drug use x treatment arm interaction effects. Third, we only assessed methadone dose at baseline, and cannot evaluate the effect of methadone dose over time. Given comparable methadone doses at baseline, and a comparable prevalence of opiate use during the intervention period between the two study arms, we believe that methadone dose effects were similar between arms. Fourth, generalizability outside of methadone programs, or to treatment settings without resources such as existing DOT infrastructures, may be limited. Nonetheless, over 250,000 people are enrolled in methadone programs in the US (Kresina et al., 2009), and a significant number of methadone and other substance abuse treatment programs offer linked medical care services, including HIV care (Brown et al., 2007; Brown et al., 2006).
In sum, active drug users may derive particular benefit from methadone clinic-based antiretroviral directly observed therapy. Among HIV-infected, methadone-maintained individuals, use of opiates or both opiates and cocaine, regardless of frequency, is associated with worse antiretroviral adherence, but this effect is eliminated by directly observed therapy. To promote adherence, antiretroviral DOT should be strongly considered among drug users in methadone treatment.
Acknowledgments
Role of Funding Source
This work was funded by National Institutes of Health grants R01 DA015302 and R25 DA14551 awarded to Dr. Arnsten, K23 DA025736 awarded to Dr. Nahvi, K23 DA022454 awarded to Dr. Litwin, K23 DA021087 awarded to Dr. Berg, and a Center for AIDS Research grant (P30 AI051519) awarded to the Albert Einstein College of Medicine of Yeshiva University. The funding sources had no further role in the study design; data collection, analysis, or interpretation; manuscript preparation; or the decision to submit the paper for publication.
The authors thank Metta Cantlo, Amanda Carter, Hillel Cohen, Nina Cooperman, Elise Duggan, Noam Fast, Uri Goldberg, Harris Goldstein, Joseph Hecht, Laxmi Modali, Jennifer Mouriz, Tanya Nahvi, Yuming Ning, Francesca Parker, Megha Ramaswamy, and Maite Villanueva for assistance with protocol development, pharmacy coordination, data collection and management, manuscript preparation, and laboratory analyses. The trial was dependent on the cooperation of the medical providers, nurses, and patients at the Albert Einstein College of Medicine and Montefiore Medical Center Division of Substance Abuse. We also thank the Center for AIDS Research of the Albert Einstein College of Medicine, and staff members at Melrose Pharmacy, Bendiner & Schlesinger, Inc., and Bio-Reference laboratories, Inc.
Footnotes
Contributors
Drs. Nahvi, Litwin, Berg and Arnsten designed the study. Authors Heo and Li conducted the statistical analysis. Dr. Nahvi conducted literature searches and summaries of previous related work, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113:192–199. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Mouriz J, Li X, Duggan E, Goldberg U, Arnsten JH. Rationale, design, and sample characteristics of a randomized controlled trial of directly observed antiretroviral therapy delivered in methadone clinics. Contemp Clin Trials. 2009;30:481–489. doi: 10.1016/j.cct.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women. JAMA. 1998;280:166–171. doi: 10.1001/jama.280.2.166. [DOI] [PubMed] [Google Scholar]
- Brown LS, Jr, Kritz S, Goldsmith RJ, Bini EJ, Robinson J, Alderson D, Rotrosen J. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Rep. 2007;122:441–451. doi: 10.1177/003335490712200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LS, Jr, Kritz SA, Goldsmith RJ, Bini EJ, Rotrosen J, Baker S, Robinson J, McAuliffe P. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2006;30:315–321. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG Adherence Instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, Smith N, Mead A, DeVlaming S. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38(Suppl 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Parsons JT, Chabon B, Berg KM, Arnsten JH. The development and feasibility of an intervention to improve HAART adherence among HIV-positive patients receiving primary care in methadone clinics. J HIV/AIDS Soc Serv. 2007;6:101 – 120. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- DesJarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, Shi Q, Hagan H, Friedman SR. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet. 1999;353:1657–1661. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- Fareed A, Casarella J, Roberts M, Sleboda M, Amar R, Vayalapalli S, Drexler K. High dose versus moderate dose methadone maintenance: is there a better outcome? J Addict Dis. 2009;28:399 – 405. doi: 10.1080/10550880903183042. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: substance use, health care and psychological states. J Health Psychol. 2005;10:545–558. doi: 10.1177/1359105305053422. [DOI] [PubMed] [Google Scholar]
- Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall GN, Johnson D, Dube MP, Hwang JY, McCutchan JA. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS. 1999;13:1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- Hinkin C, Barclay T, Castellon S, Levine A, Durvasula R, Marion S, Myers HF, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16:199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Wu Y, Cohen MH, Greenblatt RM, Howard AA, Cook JA, Goparaju L, Golub E, Richardson J, Wilson TE. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse. 2008;34:161–170. doi: 10.1080/00952990701877052. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Litwin A, Marion I, Lubran R, Clark HW. United States government oversight and regulation of medication assisted treatment for the treatment of opioid dependence. J Drug Policy Analysis. 2009;2:2. [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38 (Suppl 5):S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Celentano DD, Latkin C, Strathdee SA, Vlahov D. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ Prev. 2002;14:367–378. doi: 10.1521/aeap.14.6.367.24075. [DOI] [PubMed] [Google Scholar]
- Malta M, Magnanini M, Strathdee S, Bastos F. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- Maxwell S, Shinderman M. Optimizing response to methadone maintenance treatment: use of higher-dose methadone. J Psychoactive Drugs. 1999;31:95–102. doi: 10.1080/02791072.1999.10471730. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Purcell DW, Mackenzie S, Tobin KE, Wunch T, Arnsten JH, Metsch LR for the INSPIRE Study Team. Acceptability of A-CASI by HIV-positive IDUs in a multisite, randomized, controlled trial of behavioral intervention (INSPIRE) J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S48–S54. doi: 10.1097/QAI.0b013e3181576795. [DOI] [PubMed] [Google Scholar]
- Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, Hogg RS, Montaner JS. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O’Malley SS, Dieckhaus KD, Rounsaville BJ. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamoorthi U, Warner LA, Crystal S, Walkup J. Drug abuse, methadone treatment, and health services use among injection drug users with AIDS. Drug Alcohol Depend. 2000;60:77–89. doi: 10.1016/s0376-8716(99)00142-8. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004;29:117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, Clarke J. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. Am J Drug Alcohol Abuse. 2000;26:195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- Steinbauer JR, Cantor SB, Holzer CE, III, Volk RJ. Ethnic and sex bias in primary care screening tests for alcohol use disorders. Ann Intern Med. 1998;129:353–362. doi: 10.7326/0003-4819-129-5-199809010-00002. [DOI] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, Hogg RS, Montaner JS, Wood E. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–913. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]