Abstract
Caffeine enhances cognition, but even high non-physiological doses have modest effects on synapses. A1 adenosine receptors (A1Rs) are antagonized by caffeine and are most highly enriched in hippocampal CA2, which has not been studied in this context. Here we show that physiological doses of caffeine in vivo or A1R antagonists in vitro induce robust, long-lasting potentiation of synaptic transmission in rat CA2 without effect in other regions of the hippocampus.
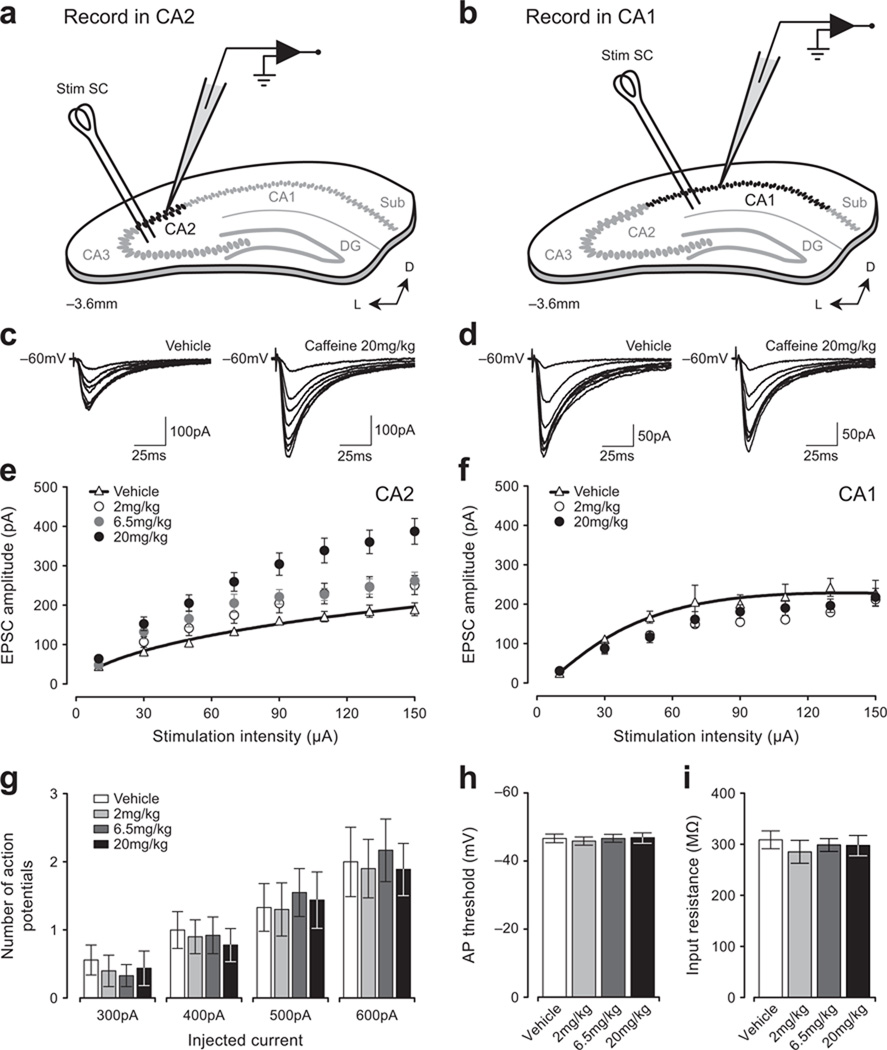
Caffeine is a widely-used naturally-occurring cognitive enhancer, yet none of its effects on synaptic transmission described thus far are overt. The effects of caffeine on cognition are mediated primarily by blockade of the A1 adenosine receptor (A1R), and antagonists of A1Rs enhance induction and stability of long-term potentiation (LTP) in hippocampal CA11,2. A1Rs, though, are most highly enriched in CA23, suggesting a unique role for this region in mediating the effects of caffeine. Schaffer collateral synapses in CA2 differ significantly from others in the hippocampus in that they enigmatically fail to exhibit activity-dependent LTP4 due largely to higher calcium buffering and extrusion, and presence of RGS14 in CA2 pyramidal neurons5,6. We therefore examined whether caffeine differentially influences synaptic strength in CA2. To do this, we prepared hippocampal slices from juvenile rats one hour after they had been orally administered one of three doses of caffeine representing different levels of human use: 2 mg/kg, or two large cups of coffee; 6.5 mg/kg, one highly-caffeinated energy drink; or 20 mg/kg, a dose that exceeds most people’s daily intake. Regardless of dose, caffeine induced a persistent and significant increase in synaptic responses in CA2, but not in CA1 (Fig. 1a–f). Additionally, neuronal excitability was unaffected by caffeine (Fig. 1g–i), suggesting that the enhanced transmission in CA2 was due to modifications at synapses. Although previous studies have shown that adenosine antagonists modulate LTP induction1,7, this is the first demonstration of long-lasting plasticity induced solely by in vivo exposure to caffeine. As important, these data identify synapses in CA2 as a target of caffeine.
Figure 1. Orally-administered caffeine potentiates synaptic responses in CA2.
The placement of stimulating and recording electrodes in coronal slices is shown in the schematic diagrams for areas CA2 (a) and CA1 (b). Sample currents evoked by increasing stimulation intensity from single neurons in slices from rats dosed with 20 mg/kg caffeine or vehicle (CA2, c; CA1, d). EPSCs evoked by a range of stimulation intensities in slices following oral administration of caffeine are enhanced in CA2 relative to vehicle (e), but not in CA1 (f). Note: bars indicate mean ± SEM in this and subsequent figures, and experiments were approved by the NIEHS Animal Care and Use Committee. No change in the intrinsic excitability of CA2 neurons was detected at any dose of caffeine (g–i).
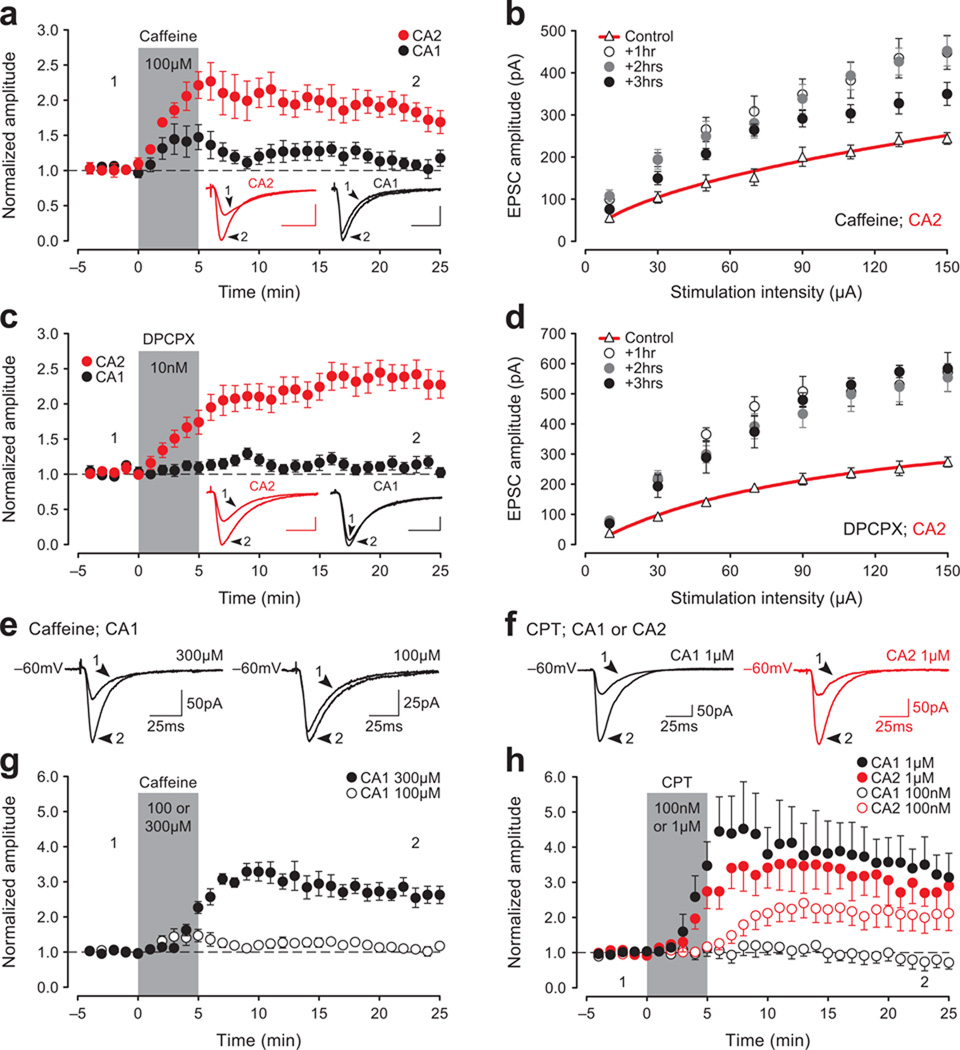
To control for possible indirect effects of caffeine, such as those on blood flow, and to further address the mechanisms of the enhancement, we applied caffeine directly to hippocampal slices and found that even a brief exposure induced an immediate and lasting facilitation of CA2 responses (juvenile slices, Fig. 2a; adult slices, Supplementary Fig. 1a,c). Similar results were not observed in CA1. To better assess the persistence of the facilitation, slices were exposed to caffeine for 5-min and then returned to a holding chamber for either one, two or three hours prior to whole-cell recordings. Synaptic responses were enhanced for at least three hours post-treatment (Fig. 2b). We attribute the caffeine-induced potentiation in CA2 to blockade of A1Rs because other, more selective, A1R antagonists, such as DPCPX (10 nM; juvenile, Fig. 2c; adult, Supplementary Fig. 1b,d), PSB 36 (10 nM; not shown) or CPT (100 nM, Fig. 2h), induced a similar potentiation (hereafter termed A1R-P). As with caffeine, DPCPX failed to induce lasting effects on transmission in CA1 or CA3 (CA1, black circles, Fig. 2c and Supplementary Fig. 1b,d; CA3, open circles, Supplementary Fig. 1e,f), but both caffeine and CPT can indeed potentiate responses in CA1 if the concentrations applied are significantly higher, as shown previously8 (caffeine 300 µM, Fig. 2e,g; CPT 1 µM, Fig. 2f,h). These findings suggest that when low concentrations of A1R antagonists are used, enhancement is observed only in CA2.
Figure 2. A1R antagonists induce a long-lasting increase in synaptic strength in CA2 neurons in vitro.
Bath-application of caffeine (100 µM, a) or DPCPX (10 nM, c) for 5-min potentiates EPSCs in CA2, but not in CA1. Duration of drug perfusion is indicated by the gray bar in (a) and (c) and in subsequent panels. Inset traces recorded at the time-points marked by the numbers (calibration; 25 pA, 25 msec). The A1R-P in CA2, plotted as a function of stimulation intensity, persists in slices previously exposed to caffeine (b) or DPCPX (d) for 5-min and then returned to standard ACSF for either one, two or three hours prior to recording. A high concentration of caffeine potentiates responses in CA1 (300 µM, e and g). Similarly, The selectivity of the A1R antagonist CPT to potentiate responses in CA2 and not in CA1 is lost when a higher concentration is applied (100 nM versus 1 µM; f and h).
Phosphodiesterases (PDEs) can be inhibited by caffeine, albeit at much higher doses than used here9, so we tested whether a broad-spectrum PDE inhibitor would mimic the effects observed with caffeine. It did not, indicating that caffeine’s actions were not likely the result of preferential PDE inhibition in CA2 (Supplementary Fig. 2a). A1Rs acting presynaptically are similarly unlikely to account for A1R-P in CA2: differences in paired-pulse facilitation in CA2 during DPCPX application differed little from effects observed in CA12,10 (not shown), consistent with the finding that A1R immunoreactivity in CA2 does not co-localize with presynaptic markers3. Additionally, blockade of GABAA-mediated transmission or performing experiments at 32 °C did not impact the magnitude of A1R-P induced by caffeine or DPCPX in CA2 (not shown).
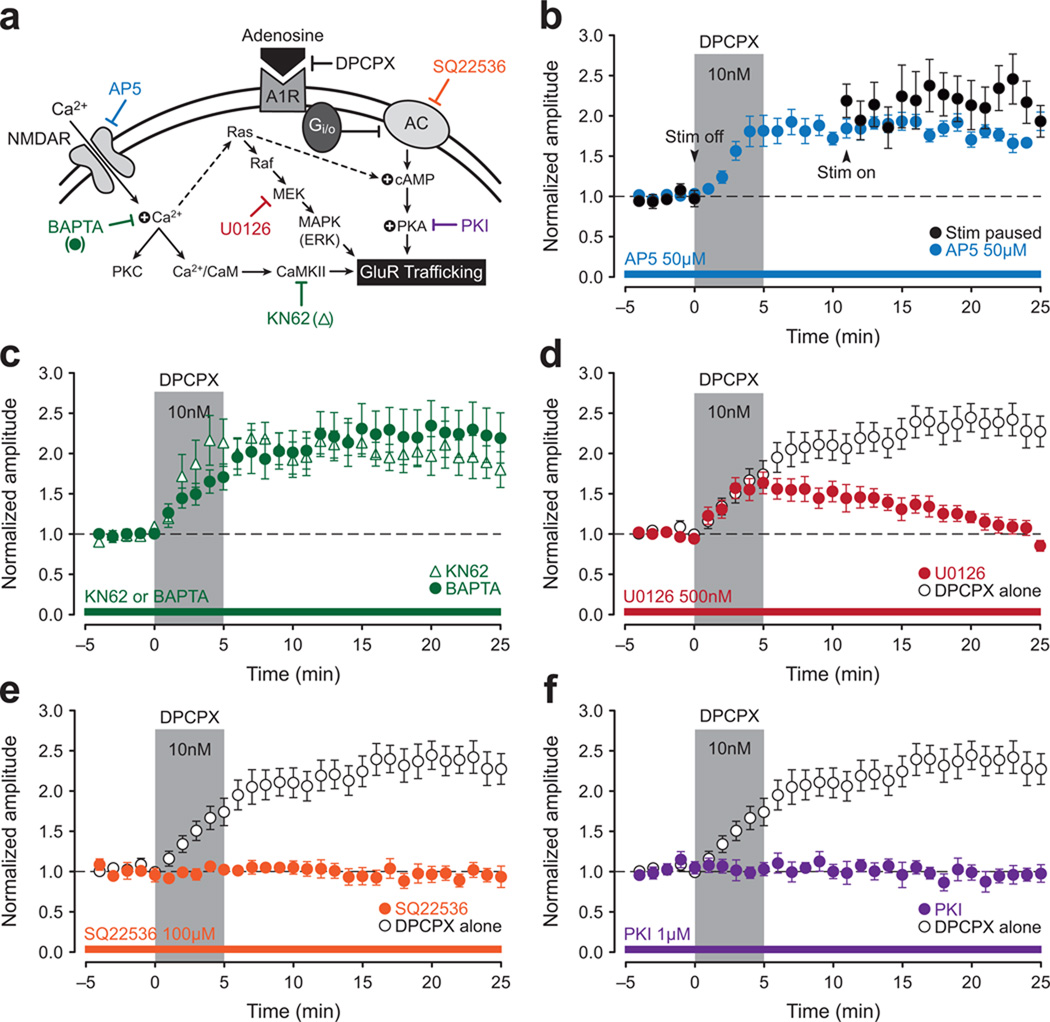
To investigate further the mechanisms underlying A1R-P at CA2 synapses (Fig. 3a), we tested whether A1R-P shared properties with tetanus-induced LTP. A1R-P did not require synaptic stimulation during drug application, nor was it sensitive to the NMDAR antagonist AP5 or intracellular calcium chelation with BAPTA (Fig. 3b,c). Inhibition of CaMKII, PKC and PKM or PI3 kinase with KN62 (Fig. 3c), Go6983 (500 nM; not shown) or Wortmannin (100 nM; not shown), respectively, was also ineffective at blocking A1R-P. These data indicate that the calcium-dependent mechanisms required for LTP induction in CA1 are either bypassed or are unnecessary in A1R-P in CA2. They also argue against a critical role for ryanodine receptor-mediated increases in postsynaptic calcium11.
Figure 3. A1R-potentiation at CA2 synapses is mediated by cAMP-dependent activation of PKA.
A schematic overview of a likely mechanism for A1R-P in CA2 (a). Attempts to induce A1R-P in CA2 neurons were made using DPCPX (10 nM, 5-min, gray bar, b–f), and open circles, where present, show the DPCPX response in the absence of any other manipulation. A temporary pause in delivery of test stimulation at the start of DPCPX application (black circles; b) or bath application of AP5 (50 µM, blue circles; b) fails to block A1R-P. Loading cells with BAPTA (10 mM, green circles) or bath application of the CaMKII inhibitor KN62 (10 µM, open triangles) also fails to block A1R-P (c). Although the MEK inhibitor U0126 did not block induction of A1R-P (500 nM, red circles; d), it did block its consolidation. Loading cells with the adenylyl cyclase inhibitor SQ22536 (100 µM, orange circles; e) or the PKA inhibitor PKI (1 µM, purple circles; f) blocks A1R-P in CA2.
In agreement with A1Rs being coupled to the Gi/o family of G-proteins, which, when activated, inhibit adenylyl cyclases, several variants of adenylyl cyclase are highly enriched in CA2 (Supplementary Fig. 2b). Thus as might be expected, A1R-P was blocked by loading CA2 neurons with inhibitors of adenylyl cyclase or PKA (SQ22536 or PKI, respectively; Fig. 3e,f; 10 µM MDL-12,330A or 1 µM KT 5720, respectively, not shown). Bath application of U0126, a specific inhibitor for MEK (or the ERK inhibitor FR 180204, 2 µM, not shown) blocked the stabilization of A1R-P, but not its earliest phase (Fig. 3d), suggesting a possible role of the Ras-MAPK pathway in A1R-P consolidation. Additionally, the DPCPX-mediated potentiation of synaptic responses in CA2 is correlated with an increase in the volume of spines located on the branches of secondary and tertiary apical dendrites in CA2 neurons (Supplementary Fig. 2c–e). Together, these findings indicate that induction of A1R-P in CA2 neurons is postsynaptic and is mediated through cAMP-dependent activation of PKA. ERK-MAP kinase is likely required for its consolidation.
Adenosine in the brain is thought to rise throughout the day and be cleared during sleep2. Our results support the hypothesis that the use of caffeine provides temporary increases in mental acuity by blocking the normal inhibitory effects of adenosine. The role of CA2 in brain function is unknown, but clues may be gleaned by the finding that the number of non-pyramidal (inhibitory) neurons are reduced in the CA2 of patients with schizophrenia12 and that caffeine has been observed to worsen symptoms of psychosis13. Interestingly, knockout of a vasopressin receptor enriched in CA2 produces mice with impairments in social recognition memory14 and caffeine enhances this kind of memory in rodents15. Thus, the robust potentiation induced by caffeine exposure both in vivo and in vitro strongly implicates A1R-P in CA2 as the physiological substrate for the cognitive enhancement provided by caffeine consumption.
Supplementary Material
ACKNOWLEGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, Z01 ES 100221. We thank David Armstrong and members of the Dudek lab for their input on the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
SBS and SMD conceived and designed the study. Experiments were conducted and analyzed by SBS, DAC and MZ. SBS, DAC and SMD wrote the manuscript. SMD supervised the project.
REFERENCES
- 1.Arai A, Lynch G. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 2.Dunwiddie TV, Masino SA. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Ochiishi T, et al. Neuroscience. 1999;93:955–967. doi: 10.1016/s0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Choi Y, Obrietan K, Dudek SM. J. Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons SB, Escobedo Y, Yasuda R, Dudek SM. Proc. Natl. Acad. Sci. USA. 2009;106:14080–14084. doi: 10.1073/pnas.0904775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SE, et al. Proc. Natl. Acad. Sci. USA. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forghani R, Krnjevic K. Hippocampus. 1995;5:71–77. doi: 10.1002/hipo.450050109. [DOI] [PubMed] [Google Scholar]
- 8.Garaschuk O, Kovalchuk Y, Krishtal O. Neurosci. Lett. 1999;135:10–12. doi: 10.1016/0304-3940(92)90124-p. [DOI] [PubMed] [Google Scholar]
- 9.Choi OH, Shamim MT, Padgett WL, Daly JW. Life Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 10.Wu LG, Saggau P. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 11.McPherson PS, et al. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- 12.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. Biol. Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 13.Lucas PB, et al. Biol. Psychiatry. 1990;28:35–40. doi: 10.1016/0006-3223(90)90429-6. [DOI] [PubMed] [Google Scholar]
- 14.Wersinger SR, et al. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 15.Prediger RD, Takahashi RN. Neurosci. Lett. 2005;376:160–165. doi: 10.1016/j.neulet.2004.11.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.