Abstract
Background
Naltrexone provides excellent opioid blockade, but its clinical utility is limited because opioid-dependent patients typically refuse it. An injectable suspension of naltrexone for extended release (XR-NTX) was recently approved by the FDA for treatment of opioid dependence. XR-NTX treatment may require concurrent behavioral intervention to maximize adherence and effectiveness, thus we sought to evaluate employment-based reinforcement as a method of improving adherence to XR-NTX in opiate dependent adults.
Methods
Opioid-dependent adults (N = 38) were detoxified and inducted onto oral naltrexone, then randomly assigned to contingency or prescription conditions. Participants received up to six doses of XR-NTX at four-week intervals. All participants could earn vouchers for attendance and performance at a therapeutic workplace. Contingency participants were required to accept XRNTX injections to access the workplace and earn vouchers. Prescription participants could earn vouchers independent of their acceptance of XR-NTX injections.
Results
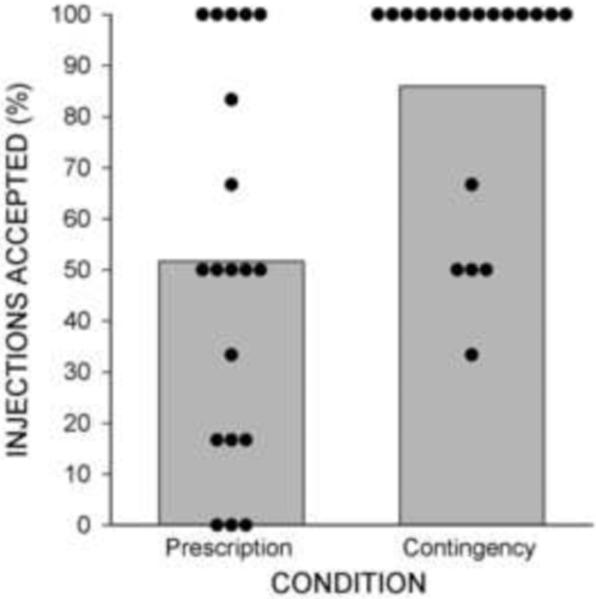
Contingency participants accepted significantly more naltrexone injections than prescription participants (87% versus 52%, p = .002), and were more likely to accept all injections (74% versus 26%, p = .004). Participants in the two conditions provided similar percentages of samples negative for opiates (72% versus 65%) and for cocaine (58% versus 54%).Opiate positivity was significantly more likely when samples were also cocaine positive, independent of naltrexone blockade (p = .002).
Conclusions
Long-term adherence to XR-NTX in unemployed opiate dependent adults is low under usual care conditions. Employment-based reinforcement can maintain adherence to XRNTX. Ongoing cocaine use appears to interfere with the clinical effectiveness of XR-NTX on opiate use.
Keywords: contingency management, incentives, therapeutic workplace, heroin, opioid pharmacotherapy, extended-release naltrexone
1. Introduction
Naltrexone is an opioid receptor antagonist that blocks the reinforcing, subjective, and physiological effects of opioids (Martin et al., 1973; Mello et al., 1981; Schuh et al., 1999; Walsh et al., 1996). Unlike agonist medications such as methadone and buprenorphine, naltrexone has no abuse liability or diversion potential, cannot directly cause overdose, and can be prescribed by any physician without the need for special waivers. Despite these attributes, non-adherence has severely limited the utility of naltrexone in the treatment of opioid dependence (Sullivan et al., 2007; Comer et al., 2007; Adi et al., 2007; Modesto-Lowe, 2002). Most experience is with oral naltrexone, which has been clinically available for decades.
Several injectable suspension formulations of naltrexone for extended release (XR-NTX) have been developed to reduce the frequency of dosing and improve adherence (Comer et al., 2007). Evaluations with an investigational XR-NTX formulation, Depotrex®, showed that XRNTX can provide long-lasting opioid blockade and may be useful in the treatment of opioid addiction (Comer et al., 2002; 2006). Because opioid dependence is a chronic condition (McLellan et al., 2000; Hser et al., 2001; Galai et al., 2003), it is likely that naltrexone treatment will need to be delivered as a long-term maintenance intervention for many patients. However, the initial clinical evaluation of Depotrex® showed that only 68% of patients completed an eight-week treatment episode.
Our research group recently conducted a clinical trial to assess longer-term adherence to the Depotrex® investigational formulation of XR-NTX and to determine if adherence could be increased using a novel employment-based reinforcement intervention that could potentially be used to improve adherence to XR-NTX over extended periods of time (Everly et al., 2011). In that study, unemployed heroin-dependent adults were hired into a model employment setting in which they could earn wages for working in a job skills training program. Participants were prescribed a six-dose course of Depotrex® through subcutaneous injections given every three weeks at no cost to them. Participants assigned to a usual care prescription condition could work in the workplace and earn wages independent of their acceptance of XR-NTX. Participants assigned to the contingency condition were required to accept XR-NTX to access the training program and earn wages. Contingency participants were significantly more likely than prescription participants to complete the 18-week course of treatment (67% vs. 35% completers).
Depotrex® was never approved by the FDA, but the FDA recently approved another formulation of XR-NTX, Vivitrol®. This medication has a four-week inter-dose interval and is administered through intramuscular injections. A randomized trial of this XR-NTX formulation in Russia showed that almost half of the participants discontinued treatment prematurely; 53% completed 24 weeks of treatment (Krupitsky et al., 2011). The present study was conducted to assess rates of adherence to the FDA-approved formulation of XR-NTX, Vivitrol®, in a U.S. population of opioid dependent adults and to assess the efficacy of the employment-based reinforcement intervention for improving adherence with this clinically available XR-NTX formulation.
2. Method
2.1 Participants
Volunteers were recruited from detoxification programs in Baltimore, MD and through street outreach between September, 2008 and October, 2009. Individuals were eligible if they met the DSM-IV criteria for opioid dependence (American Psychiatric Association, 2000), reported using heroin at least 21 of the last 30 days while living in the community, were unemployed, were 18–65 years old, were medically approved for naltrexone, and lived in or near Baltimore, MD. Individuals were excluded if they were pregnant or breastfeeding, had serum aminotransferase levels over three times normal, had current hallucinations, delusions, or thought disorders, current suicidal or homicidal ideation, expressed interest in methadone treatment, were required to use opioids for medical purposes, earned over $200 in taxable income over the previous 30 days, had physical limitations that would prevent them from using a keyboard, or were incarcerated or under constant monitoring by the criminal justice system. Participants provided written consent. The study was approved by the Johns Hopkins Medicine Institutional Review Board, and is registered at: clinicaltrials.gov: NCT00684788.
2.2 General Therapeutic Workplace Procedures
The study was conducted in the therapeutic workplace, a model workplace in which employment-based reinforcement contingencies (i.e., incentives) are arranged to promote therapeutic behavior change. Participants could attend the therapeutic workplace for four hours each weekday and work on training programs that were almost fully automated. Participants were paid in vouchers that were exchangeable for goods and services. Earnings were based on hours worked and performance on the training programs. Participants could earn $8.00 per hour in base pay for time spent in the workplace, plus about $2.00 per hour in productivity pay for their performance on training programs. Detailed descriptions of the therapeutic workplace can be found elsewhere (Silverman et al., 2001; 2002; 2007; Donlin et al., 2008; DeFulio et al., 2009).
2.3 Assessments
Assessments were conducted at intake and every 30 days after random assignment for 6 months. The main assessments included the Addiction Severity Index — Lite (ASI — Lite; McLellan et al., 1985) for evaluating drug use, educational, employment, family, medical, and legal histories; the heroin, cocaine, alcohol, and nicotine sections of the Composite International Diagnostic Interview (CIDI; intake only; Compton et al., 1996), a diagnostic tool for psychiatric disorders; and the Wide Range Achievement Test — 4th edition (WRAT4; intake only) for assessing math, reading, and spelling skills (Wilkinson, 1993). Additional assessments of exploratory measures were collected but are not reported here.
For safety purposes, blood samples for liver function testing were taken prior to each of the first three naltrexone injections and at the sixth month of the study, and females received urine pregnancy tests prior to each naltrexone injection. If a participant had aminotransferase levels that were over two times normal, then additional blood draws were conducted prior to all remaining injections.
Urine samples were collected under observation upon arrival at the therapeutic workplace on Mondays, Wednesdays, Fridays, and at each 30-day assessment. Urine samples were screened using an Abbott AxSYM® for opiates and cocaine. Samples collected at 30-day assessments were also screened for methadone, buprenorphine, benzodiazepines, and amphetamines. Samples were considered positive for opiates and cocaine if the concentration of the metabolite (morphine and benzoylecgonine, respectively) was ≥ 300 ng/ml. Opiate urinalysis detects the subset of opioids that produce morphine-positive urine samples, most commonly heroin.
2.4 XR-NTX Treatment
Participants were required to complete opioid detoxification and were then invited to attend the therapeutic workplace for induction onto oral naltrexone (Depade®; from Mallinckrodt, Inc.). All opioid detoxifications and naltrexone inductions were overseen by a physician and were guided solely by clinical judgment. Participants who completed opioid detoxification through an extended inpatient detoxification program were immediately inducted onto oral naltrexone. Participants who completed a brief inpatient detoxification were required to complete an outpatient detoxification prior to being inducted onto oral naltrexone. The outpatient portion of the detoxifications was conducted while participants attended the therapeutic workplace, and lasted one to two weeks. During outpatient detoxification participants were required to provide opioid negative urine samples to gain access to the workplace. During oral naltexone induction, participants were required to take scheduled oral naltrexone doses to gain access to the therapeutic workplace. Oral naltrexone induction continued until a maintenance dose of 100 mg on Monday and Wednesday and 150 mg on Friday was reached. The maintenance routine was maintained until three consecutive doses were ingested, after which the induction period ended and oral naltrexone treatment was discontinued. The oral naltrexone induction period was completed in two weeks or less for all participants.
After completing oral naltrexone induction, participants were randomly assigned to one of the study conditions described below, invited to attend the workplace for 26 weeks, and offered a 24-week course of XR-NTX at no cost. Vivitrol®, the XR-NTX formulation used in this study, contains 380 mg of naltrexone in a microsphere formulation and 3.4 mL diluent. The drug was administered as an intramuscular gluteal injection via customized needles provided by the manufacturer. Injections were administered at a facility located on the same campus, 0.3 miles from the therapeutic workplace. Participants could receive a total of six injections, once every four weeks. After 24 weeks (the XR-NTX blockade period), participants were encouraged, but not required, to resume oral naltrexone treatment. To minimize opioid overdose risk, reminders of these risks were provided routinely and frequently throughout treatment, and at monthly lunch-time overdose prevention seminars that included free pizza to encourage seminar attendance.
In order to insure that participants did not forget to take XR-NTX, all participants were required to complete a form that notified them that their next scheduled XR-NTX administration was due. This form also specified whether they were required to take XR-NTX in order to maintain their access to the therapeutic workplace. Participants were required to circle either “Yes” or “No” in response to the question, “Do you want to take your next depot naltrexone injection?” and initial the form. The form was also initialed by the staff member who administered it.
2.5 Experimental Design and Conditions
Prior to random assignment, participants were stratified according to whether or not they 1) attended the workplace every day of the last three workdays; 2) submitted one or more opiate-positive urine samples out of the last 3 samples; and 3) submitted one or more cocaine-positive urine samples out of the last 3 samples. Participants were randomly assigned, via computer, to either a prescription condition or a contingency condition in a manner that ensured that the levels of each stratification variable were evenly distributed among the conditions (Kernan et al., 1999). Prescription participants were offered XR-NTX injections, but were allowed access to the therapeutic workplace independent of whether they accepted it. Contingency participants were required to accept XR-NTX injections to gain and maintain access to the workplace. If a contingency participant had not taken XR-NTX within 3 days of the scheduled date of administration, then the participant could not access the workplace; access to the workplace could be reinstated by resuming XR-NTX treatment. Additionally, missing a scheduled injection resulted in a base pay reset from $8 per hour to $1 per hour. After the reset, the participant's base pay increased by $1 per hour to the maximum of $8 per hour for every day the participant attended the workplace for at least 5 minutes. One contingency participant who was medically discontinued from XR-NTX treatment was exempt from these rules.
2.6 Sample Size
Sample size was determined by a power analysis based on the magnitude of the effect on the percentage of doses taken in a similar study of voucher reinforcement of oral naltrexone adherence assuming an alpha of .05 and power of .80 (Preston et al., 1999). The resulting sample size was 40 participants per condition. After enrolling 35 participants in our first study, (Everly et al., 2011), Depotrex® became unavailable, and the study was ended. We conducted the present study using Vivitrol® until the sponsor institution's support for the project was exhausted. At the end of the study, there were 19 participants in the contingency condition and 19 in the prescription condition.
2.7 Outcome Measures
The primary outcome measure was the percentage of XR-NTX doses accepted. Secondary outcome measures included the percentage of urine samples negative for opiates and cocaine. Also analyzed were the correlation between naltrexone adherence and opiate use, the percentage of days participants attended the therapeutic workplace, voucher earnings, retention in naltrexone treatment and the therapeutic workplace, and the relationship between opiate urinalysis results and condition, naltrexone blockade, and cocaine urinalysis results.
2.8 Data Analyses
Participant characteristics at intake were analyzed using Fisher's Exact tests for dichotomous variables and t-tests for continuous variables. The main outcome analyses were based on data collected during the first 24 weeks after random assignment, the weeks that the XR-NTX could block the effects of opioids. Analyses of urine samples were based on the first five monthly assessments and the first 72 thrice weekly urine samples after random assignment. Missing samples were treated as positive for opiates and cocaine (missing positive). An alternative method of handling missing urine samples was analyzed in which missing samples were not imputed as positive (missing missing). This method of handling missing samples produced essentially the same results. Dichotomous measures were analyzed using the method of generalized estimating equations (Zeger et al., 1988). Mean voucher earnings were analyzed using a linear mixed-effects model (Singer, 1998). Retention in XR-NTX treatment and the therapeutic workplace were analyzed using a Cox proportional hazards model. A Chi Square test was used to compare the percentage of participants in the two conditions that took all scheduled XR-NTX injections. All analyses were intent-to-treat. Two-tailed tests were used and results were considered statistically significant if p ≤ .05. Statistical analyses were conducted using SAS software version 9.1.
3. Results
3.1 Participant Characteristics and Flow through the Study1
Of the 132 participants assessed for eligibility, 72 did not qualify and seven declined participation. Of the remaining 53 participants, nine failed to complete detoxification, four did not complete the required induction onto oral naltrexone, and two were discontinued prior to random assignment due to unrelated medical issues. The remaining 38 participants were randomly assigned to the prescription (n = 19) and contingency (n = 19) conditions. Data analyses include all 38 participants. Table 1 shows that there were no significant differences between the conditions on any of the characteristics assessed at intake.
Table 1.
Participant characteristics at intake.
| Characteristica | Naltrexone Prescription (n=19) | Naltrexone Contingency (n=19) | Fisher's Exact (p) | t-test (P) |
|---|---|---|---|---|
| Age, mean (SEM), years | 42 | 45 | 0.46 | |
| Female, % | 58 | 26 | 0.10 | |
| Black/white, % | 84/16 | 95/5 | 0.60 | |
| Married, % | 11 | 11 | 1.00 | |
| High school diploma or GED, % | 63 | 68 | 1.00 | |
| Opioid dependent, %b | 100 | 100 | - | |
| Cocaine dependent, %b | 80 | 88 | 0.73 | |
| Usually unemployed past 3 years, % | 74 | 58 | 0.50 | |
| Past 30 days income, mean (SEM), $ | ||||
| Employment | 4 (18) | 0 | 0.33 | |
| Welfare | 127 (148) | 98 (142) | 0.55 | |
| Pension, benefits, Social Security | 37 (159) | 268 (729) | 0.18 | |
| Mate, family, friends | 217 (397) | 190 (462) | 0.85 | |
| Illegal | 2489 (4349) | 1089 (1984) | 0.21 | |
| Total income | 2874 (4202) | 1646 (2007) | 0.26 | |
| $ spent on drugs, mean (SEM), past 30 days | 2608 (4329) | 1529 (1779) | 0.32 | |
| Currently on parole/probation, % | 58 | 32 | 0.19 | |
| Lifetime felony conviction, % | 95 | 84 | 0.60 | |
| Grade levels, mean (SEM)c | ||||
| Reading | 9 (3) | 9 (3) | 0.94 | |
| Spelling | 7 (3) | 8 (4) | 0.42 | |
| Arithmetic | 6 (3) | 8 (3) | 0.23 |
Note. Results for Fisher's Exact tests and Mests are based on two-tailed tests with an alpha of .05.
Unless otherwise noted, characteristics are taken from the Addiction Severity Index – Lite.
Taken from the Composite International Diagnostic Interview.
Taken from the Wide Range Achievement Test.
3.2 Naltrexone Adherence and Retention
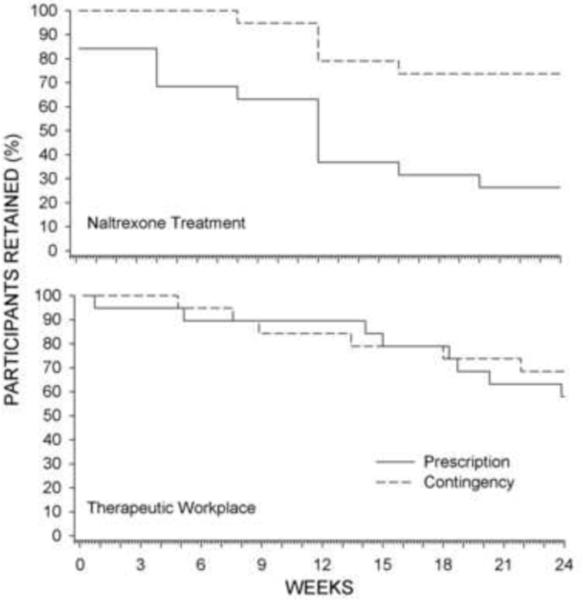
Contingency participants took significantly more naltrexone injections than prescription participants (Figure 1 and Table 2). Retention in the contingency condition was significantly greater than in the prescription condition [χ2 (1) = 7.11, p = .008; HR = 0.27; 95% CI = 0.1 – 0.71]. Figure 2 (top panel) shows that 84% of prescription participants took their first scheduled injection and 100% of contingency participants took their first scheduled injection. The entire course of medication was completed by 74% of contingency participants, compared to 26% of prescription (χ2 (1) = 8.53, p = .004). This difference in naltrexone retention occurred despite similar retention in the workplace (χ2 (1) = 0.3, p = .59; Fig. 2 bottom panel). One contingency participant was discontinued from naltrexone treatment due to his potential need for surgery as a function of an unrelated diagnosis of prostate cancer.
Figure 1.
The percentage of XR-NTX doses accepted by participants in the prescription and contingency conditions. Bars show condition percentages and circles show individual percentages.
Table 2.
Naltrexone injections, opiate and cocaine urinalysis results, and workplace attendance for participants in the two study conditions during the XR-NTX treatment phase of the study.
| Percentage |
||||
|---|---|---|---|---|
| Prescription | Contingency | OR (95% CI) | P | |
| Injections Received | 51.8 | 86.8 | 6.0(1.44–25.05) | 0.002 |
| Monthly Urinalysis | ||||
| Opiate Negative | ||||
| Missing positive | 65.3 | 71.6 | 1.34 (.5–3.6) | 0.56 |
| Missing missing | 79.5 | 81.0 | 0.96(31–2.9) | 0.94 |
| Cocaine Negative | ||||
| Missing positive | 53.7 | 57.9 | 1.19 (.42–3.36) | 0.75 |
| Missing missing | 65.4 | 65.5 | .88 (.27–2.86) | 0.83 |
| Collected Samples | 82.1 | 88.4 | 1.66(0.43–6.52) | 0.47 |
| Thrice Weekly Urinalysis | ||||
| Opiate Negative | ||||
| Missing positive | 51.8 | 66.2 | 1.82 (.81–4.1) | 0.15 |
| Missing missing | 76.0 | 86.5 | 1.76 (.67–4.64) | 0.25 |
| Cocaine Negative | ||||
| Missing positive | 45.3 | 54.6 | 1.45 (.6–3.53) | 0.41 |
| Missing missing | 66.5 | 71.3 | 1.36 (.44–4.23) | 0.60 |
| Collected Samples | 68.2 | 76.6 | 1.64(0.53–5.02) | 0.36 |
| Days in Attendance | 57.5 | 64.5 | 1.35 (0.65–2.78) | 0.42 |
Figure 2.
The percentage of participants retained in XR-NTX treatment (continued to take scheduled injections; top panel) and the therapeutic workplace (continued attending the workplace; bottom panel) across study weeks.
3.3 Opiate and cocaine use
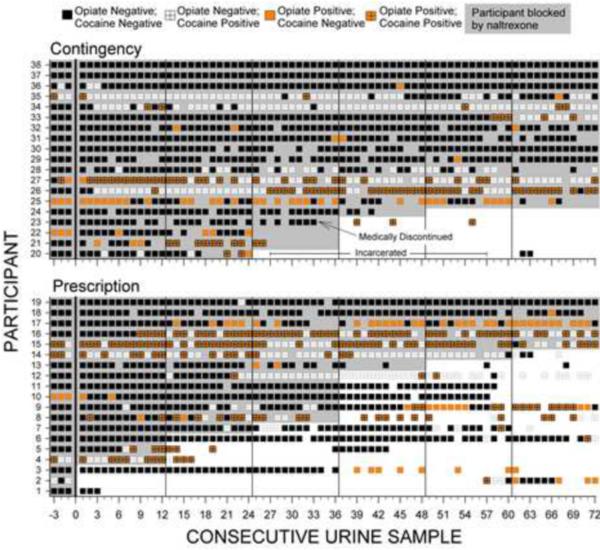
As shown in Table 2, urinalysis collection rates were high and similar across conditions. There were no between group differences in opiate or cocaine use as assessed by urinalysis. As in our prior study (Everly et al., 2011), review of individual naltrexone adherence and urinalysis results (see Figure 3) showed that most urine samples that were positive for opiates were also positive for cocaine, (258 of 366; 70%), and that this occurred whether the sample was provided by a participant currently under naltrexone blockade (222 of 309; 72%) or not (36 of 57; 63%).2 GEE analyses (excluding missing samples) were conducted to determine whether opiate urinalysis results were associated with condition (contingency/prescription), naltrexone blockade (samples collected within four weeks of the last injection; yes/no), or cocaine use (urine sample positive for cocaine; yes/no), or interactions of those variables. These analyses showed that opiate positive urine samples were associated with cocaine positive urine samples, both for monthly (χ2 = 12.98, p < .001) and thrice weekly (χ2 = 9.75, p = .002) urine samples, even when controlling for condition and naltrexone blockade.3 A Spearman's rank correlation test showed that opiate abstinence at monthly assessments was not correlated with naltrexone blockade (r = .11, p = .16).
Figure 3.
Naltrexone blockade and opiate and cocaine urinalysis results across consecutive thrice weekly urine samples collected when participants attended the therapeutic workplace. Within each panel, rows of data represent the results for individual participants. Urinalysis results are based on samples collected three times per week, typically on Monday, Wednesday and Friday of each week. Samples prior to the left of the vertical black line at 0 on the horizontal axis were collected prior to random assignment while participants were taking oral naltrexone. Black squares indicate urine samples negative for both opiates and cocaine; orange squares indicate opiate positive urine samples; white squares with crosses indicate cocaine positive urine samples; orange squares with crosses indicate samples positive for both opiates and cocaine. Empty sections indicate missing samples. Shaded portions show when participants were blocked by naltrexone (i.e., the sample was collected within 4 weeks of the last naltrexone injection). Vertical lines after urine samples 0, 12, 24, 36, 48, and 60 indicate the time of scheduled injections. Within each panel, participants are arranged from top to bottom from those with the most to least naltrexone blockade, most to least opiate negative samples, and then most to least cocaine negative samples.
3.4 Other Drug Use
Of the 197 monthly samples collected, 1 was positive for methadone, 1 was positive for amphetamines, 4 were positive for benzodiazepines, and 13 were positive for buprenorphine. No sample was positive for more than one of these four substances.
3.5 Voucher Earnings and Attendance
Prescription participants earned about the same amount in vouchers as contingency participants [M(SD) = $3,111($1562) and $3,427($1,630), respectively; t = −.61, p = .55]. Participants in the two conditions attended the workplace a similar number of days (Table 2), and for a similar number of total hours [M(SD) = 235(117) and 257(134), respectively; t = −.53, p = .6].
4. Discussion
The FDA recently approved a formulation of XR-NTX, a medication designed to improve adherence to naltrexone. The clinical availability of XR-NTX has the potential to enhance the treatment of opioid dependence. However, the results of this study suggest that under routine prescription procedures many patients discontinue XR-NTX soon after beginning treatment, or refuse it entirely, even when the medication is conveniently available at no cost. Only 26% of prescription participants completed the 24-week course of XR-NTX. This occurred under highly favorable conditions for adherence: participants had free access to the medication, the medication was available at a facility that was within a short walk of the therapeutic workplace that the large majority of participants attended regularly, and participants received routine reminders at the workplace when injections were scheduled. Despite these highly favorable circumstances, most prescription participants stopped taking XR-NTX injections within the 6-month study period. Importantly, and in contrast, this study showed that employment-based reinforcement for XR-NTX adherence can be highly effective in promoting and maintaining adherence to XR-NTX. Relative to prescription participants, contingency participants accepted a significantly greater percentage of XR-NTX; and nearly 74% of these participants completed the entire course of the medication.
The majority of the results of this study closely parallel the results of our prior study with the investigational Depotrex® formulation of XR-NTX (Everly et al., 2011). Both studies showed that many participants discontinue use of XR-NTX under usual care conditions, and that employment-based reinforcement was effective in promoting and maintaining adherence to XR-NTX, establishing the generality of these findings. Both studies also showed that many individuals use opiates while under XR-NTX blockade, particularly when they also use cocaine. These latter results suggest that additional interventions will be needed to reduce the opiate and cocaine use that persists under XR-NTX blockade.
Most participants who were simply prescribed the FDA-approved XR-NTX at no cost did not sustain long-term adherence to this medication in the absence of special procedures designed to improve adherence. In contrast, a contingency in which participants were required to adhere to XR-NTX in order to work and earn wages significantly improved adherence to XR-NTX in opioid-dependent adults. The excellent retention and adherence in the contingency condition suggests that employment-based reinforcement can be an effective means of promoting long-term adherence to XR-NTX treatment. The relatively poor retention in XR-NTX treatment in the control group suggests that reinforcement-based adherence interventions may be critical to the long-term success of XR-NTX pharmacotherapy for many heroin dependent adults.
This study highlights the importance of addressing cocaine use in polysubstance users who are prescribed XR-NTX. The substantial and significant difference in adherence to XR-NTX across the two study conditions was not associated with a significant difference in opiate abstinence. Secondary analyses that controlled for study condition and naltrexone blockade showed that urine samples were significantly more likely to be opiate positive when they were cocaine positive, suggesting that heroin use and cocaine use are related behaviors. This is a well established finding in the context of methadone treatment (Silverman et al., 1998; Avants et al., 1994). An especially noteworthy aspect of the relationship between these two behaviors is that abstinence contingencies that target only one type of drug use can have collateral effects on the other. For example, methadone patients who can earn monetary vouchers contingent upon opiate abstinence display collateral decreases in cocaine use (Robles et al., 2002). Similarly, methadone patients who can earn monetary vouchers contingent upon cocaine abstinence display collateral decreases in opiate use (Silverman et al., 1998; Silverman et al., 2004).
The robust association between opiate and cocaine positive urine samples and the relatively rare occurrence of samples that were positive for opiates alone during XR-NTX blockade suggests that persistent cocaine use should be addressed via psychosocial intervention in order to enhance the clinical effectiveness of XR-NTX treatment. Importantly, employment-based reinforcement contingencies used to promote naltrexone adherence in the present study could be arranged to simultaneously reinforce drug abstinence by simply requiring that patients take naltrexone and abstain from drug use in order to access the workplace and maintain the maximum rate of pay. The treatment model of combined XR-NTX adherence and drug abstinence reinforcement contingencies could prove to be a highly effective means of reducing or eliminating opiate use, and could be used widely in community workplaces. This is especially feasible in the U.S., where the existing infrastructure and guidelines for drug-free workplaces in safety-sensitive jobs could be harnessed for therapeutic purposes (for guidelines see US Department of Transportation, 2010). Overall, this study suggests that contingent access to workplaces could be used therapeutically to promote consistent long-term use of XR-NTX in opiate dependent adults.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
* Supplementary material showing: a complete CONSORT diagram; the results of the monthly assessments in Figure 3; a table of all results from the GEE analysis and a figure that summarizes this data can be found byaccessing the online version of this paper at http://dx.doi.org and by entering doi:…
A complete CONSORT diagram can be viewed as supplementary material by accessing the online version of this article at http://dx.doi.org and by entering doi:…
A figure similar to Figure 3 that shows the results of the monthly assessments can be viewed as supplementary material by accessing the online version of this article at http://dx.doi.org and by entering doi:…
A table of all results from these GEE analyses and a figure that summarizes the data included in those analyses can be viewed as supplementary material by accessing the online version of this article at http://dx.doi.org and by entering doi:…
References
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, Bayliss S, Roberts T, Burls A. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol. Assess. 2007;11:1–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. Task Force on DSM-IV. [Google Scholar]
- Avants SK, Margolin A, Kosten TR. Cocaine abuse in methadone maintenance programs: integrating pharmacotherapy with psychosocial interventions. J. Psychoactive Drugs. 1994;26:137–146. doi: 10.1080/02791072.1994.10472261. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl.) 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin. Investig. Drugs. 2007;16:1285–1294. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Mager DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug Alcohol Depend. 1996;41:179–187. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: a randomized controlled trial. Addiction. 2009;104:1530–1538. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. J. Appl. Behav. Anal. 2008;41:499–516. doi: 10.1901/jaba.2008.41-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everly JJ, DeFulio A, Koffarnus MN, Leoutsakos MS, Donlin WD, Aklin WM, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: a randomized controlled trial. Addiction. 2011;106:1309–1318. doi: 10.1111/j.1360-0443.2011.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD. ALIVE Study. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort, 1988–2000: description and determinants. Am. J. Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch. Gen. Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horowitz RI. Stratified randomization for clinical trials. J. Clin. Epidemiol. 1999;52:19–26. doi: 10.1016/s0895-4356(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicenre randomised trial. Lancet. 2011:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Effects in man. Arch. Gen. Psychiatry. 1973;28:784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J. Pharmacol. Exp. Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp. Clin. Psychopharmacol. 2002;10:213–227. doi: 10.1037//1064-1297.10.3.213. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Robles E, Stitzer ML, Strain EC, Bigelow GE, Silverman K. Voucher-based reinforcement of opiate abstinence during methadone detoxification. Drug Alcohol Depend. 2002;65:179–189. doi: 10.1016/s0376-8716(01)00160-0. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl.) 1999;145:162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- Silverman K, Bigelow GE, Stitzer ML. Treatment of cocaine abuse in methadone maintenance patients. In: Higgins ST, Katz JL, editors. Cocaine Abuse. Academic Press; San Diego: 1998. pp. 363–388. [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone maintained patients who inject drugs. J. Consult. Clin. Psychology. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp. Clin. Psychopharmacol. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Exp. Clin. Psychopharmacol. 2002;10:228–240. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, Fingerhood M, Nuzzo P, Kolodner K. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. J. Appl. Behav. Anal. 2007;40:387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J. Educ. Behav. Stat. 1998;24:323–355. [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, Anen SJ, Brooks AC, Jiang H, Akerele E, Nunes EV. Management of relapse in naltrexone maintenance for heroin dependence. Drug Alcohol Depend. 2007;91:289–292. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Transportation, Office of Drug and Alcohol Policy and Compliance, 49 CFR Part 40 (Drug and Alcohol Regulation) Washington DC: [Accessed May 2011]. Available: http://www.dot.gov/ost/dapc/NEW_DOCS/PART40.pdf. [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J. Pharmacol. Exp. Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test Administration Manual. Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.