Abstract
Depression is a major public health concern, and the period from late childhood through early adolescence is a critical time in the development of depressive symptoms. In adults, depression and depressive symptoms are associated with a reduction in the feedback negativity (FN), an ERP component elicited by feedback indicating rewards versus losses. The current study sought to extend these findings to a sample of 64 children aged 8 to 13, and to examine developmental differences in the FN. Consistent with previous work in adults, higher depressive symptom scores were associated with a blunted FN across the sample. When responses to losses and gains were examined separately, only reduction in the response to monetary gain was associated with increased depressive symptoms. In the current study, the vast majority of children were pre-pubertal, and the FN was unrelated to both age and pubertal development. The FN may be an ideal biomarker for studying changes in reward sensitivity and depression that emerge as children transition through puberty.
Keywords: reward, depression, feedback negativity, feedback-related negativity, developmental, childhood
Introduction
Depression is a debilitating, costly (Berto, D'Ilario, Ruffo, Di Virgilio, & Rizzo, 2000; Luppa, Heinrich, Angermeyer, Konig, & Riedel-Heller, 2007), and widespread disorder that affects children as well as adults, with 2.2% of 9- to 16-year-old children meeting criteria for depression in a given three-month period and with an estimated cumulative prevalence of 9.5% by age 16 (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). Early symptoms of depression in youth are arguably the strongest predictor of adolescent and adult depression. Each additional depressive symptom at ages 8–10 is associated with a 50–80% increase in risk for developing a depressive disorder by age 11–13 (Keenan, Feng, Hipwell, & Klostermann, 2009), and subthreshold depression in adolescence confers an estimated risk of 67% for a diagnosis of depression by the time individuals are in their early 30s (Klein, Shankman, Lewinsohn, & Seeley, 2009). A core diagnostic criterion of depression is anhedonia (American Psychiatric Association, 2000), a lack of interest in pleasurable activities. Anhedonia has been conceptualized in terms of dysfunction of an approach-related behavioral system (cf. Davidson, 1998; Henriques & Davidson, 2000), and reduced responsiveness to reward (Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008; Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009).
In line with behavioral conceptualizations of reward responsiveness, aberrant neural processing of rewards has been consistently reported in association with mood disorders in adults. For instance, adult patients diagnosed with major depression show decreased mPFC and striatal activation in response to monetary reward and positively-valenced visual stimuli, and decreased amygdala activation in response to happy words using fMRI (for a review, see Diekhof, Falkai, & Gruber, 2008).
Children with depression show abnormal patterns of reward responsiveness, as well as more general negative biases in memory (Bishop, Dalgleish, & Yule, 2004) and in their interpretation of events (Krackow & Rudolph, 2008), similar to those seen in adults. Reward responsiveness is often evaluated using guessing tasks, in which the participant makes a selection that can result in a win or a loss, but is not directly tied to performance. In contrast to their non-depressed counterparts, who are more likely to choose the chance for a high-magnitude reward in a guessing task than a low-magnitude reward when the probability of receiving the reward is high, 11-year-old boys with depression do not distinguish between the high- and low-magnitude reward options (Forbes, Shaw, & Dahl, 2007). Forbes et al. (2006) found that 9- to 17-year-old boys and girls with depression show less activation in reward-related brain areas (i.e., anterior cingulate cortex, left caudate, and orbitofrontal cortex) than healthy age-matched controls in response to feedback about rewards and losses. These findings suggest that hyposensitivity to reward may be a relatively early-emerging and stable marker of depression.
In light of the drastic increase in depression from childhood to adolescence (Cohen et al., 1993; Costello, et al., 2003), it may be especially important to identify early neural markers of reward-related dysfunction that precede the emergence of a full-threshold mood disorder in adolescence and adulthood. Given that early symptoms of depression in youth are arguably the strongest predictor of adolescent and adult depression (Keenan, et al., 2009; Klein, et al., 2009; Pine, Cohen, Gurley, Brook, & Ma, 1998), it is important to identify markers in childhood that may help to signal the emergence of early depressive symptoms. Hence, the primary goal of this study was to examine the association between depressive symptoms and a putative neural marker of diminished responsiveness to reward in a sample of 8- to 13-year-old children. This is an important first step toward the eventual goal of linking a neural marker seen in childhood to the development of depression in adulthood.
The feedback negativity (FN) is a frontally maximal ERP component (Dunning & Hajcak, 2007; Gehring & Willoughby, 2002; Hajcak, Moser, Holroyd, & Simons, 2006; Miltner, Braun, & Coles, 1997) that is associated with reward processing in healthy adults. The FN peaks approximately 300 ms after the presentation of feedback indicating a favorable versus unfavorable outcome in paradigms in which the participant loses or wins money (Dunning & Hajcak, 2007; Hajcak, et al., 2006; Hajcak, Moser, Holroyd, & Simons, 2007). It is more negative for undesirable outcomes than for desirable ones, but is insensitive to the magnitude of the outcome; that is, the FN reflects a binary evaluation of outcomes as either good or bad (Hajcak, et al., 2006). This evaluation is not based on the absolute value of the outcome (Holroyd, Hajcak, & Larsen, 2006); rather, it is the value of an outcome relative to alternative outcomes that drives the FN. For instance, even a feedback stimulus indicating a loss of points or money may be evaluated as good if the alternative would have been an even greater loss. Although the FN has traditionally been interpreted as a negative deflection in response to unfavorable outcomes, recent reports suggest that the apparent negativity may actually be an epiphenomenon created by the absence of a reward-related positivity on non-rewarded trials (Baker & Holroyd, 2011; Foti & Hajcak, 2009; Foti, Weinberg, Dien, & Hajcak, in press; Holroyd, Pakzad-Vaezi, & Krigolson, 2008). That is, the FN may be better thought of as a positivity that is increased for favorable outcomes, and that variation in FN magnitude is driven by neural sensitivity to rewards.
A prominent theory of the FN, the reinforcement-learning (RL) theory, suggests that the FN reflects phasic activity in the midbrain dopamine system (Holroyd & Coles, 2002). Consistent with this perspective, source localization techniques have implicated the ACC (Gehring & Willoughby, 2002; Potts, Martin, Burton, & Montague, 2006) and the striatum (Foti, Weinberg, et al., in press; Martin, Potts, Burton, & Montague, 2009) as likely neural generators of the FN. The generation of the FN within reward-sensitive brain networks is also supported by pharmacological (Santesso et al., 2009) and combined EEG/fMRI studies (Martin, et al., 2009). In one recent guessing-task study, FN amplitude (measured as a difference between principal components analysis scores for losses and gains) correlated directly with reward-related activation in the ventral striatum measured using fMRI, as well as with the mesocorticolimbic reward circuit more broadly, including regions in the mPFC/ACC and orbitofrontal cortex (Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011).
Given its association with reward processing, the FN is a logical choice to investigate with respect to individual differences in depressive symptoms. One study in a non-clinical sample of undergraduate students, in which participants chose one of two doors on a computer screen that concealed either a monetary reward or loss, found that participants with higher depression scores showed smaller differences in FN amplitude between losses and gains than did participants with lower depression scores; that is, depressive symptoms were associated with reduced neural differentiation between losses and gains (Foti & Hajcak, 2009). Similarly, self-reported sadness after a mood induction also predicted a blunted FN in undergraduate students in a similar door-guessing task (Foti & Hajcak, 2010), and this effect was recently replicated and larger among 15- to 17-year-old girls with a parental history of depression in a door-guessing task (Foti, Kotov, Klein, & Hajcak, 2011). In the study by Foti, Kotov, and colleagues (Foti, et al., 2011), participants at risk for depression showed a stronger association between sadness and blunted FN after a mood induction than did those not at risk.
To date, however, little research has been conducted to investigate the FN in children, especially in relation to individual differences in depressive symptoms. Some studies with healthy participants have reported a monotonic decrease in FN magnitude from childhood to young adulthood and into old age (Eppinger, Kray, Mock, & Mecklinger, 2008; Eppinger, Mock, & Kray, 2009; Hammerer, Li, Muller, & Lindenberger, 2011; Pietschmann, Simon, Endrass, & Kathmann, 2008). Hammerer and colleagues (2011) additionally found that reward sensitivity – as represented by the difference between the FN magnitude in response to losses and gains – takes the shape of an inverted U across the lifespan, peaking during adolescence and declining through adulthood. However, in contrast to the type of gambling task often used to study the FN, many of these studies used probabilistic paradigms in which participants learned to distinguish between stimuli that were more or less likely to result in a reward. In this type of paradigm, the FN might reflect both reward- and learning-related (i.e., performance-based) information.
The current study aimed to extend findings with teenage participants (e.g. Foti, et al., 2011) to a younger, non-clinical sample of children and to examine the associations between pubertal development and responsiveness to reward. We employed a relatively simple paradigm to elicit an unambiguous measure of reward sensitivity: on each trial, participants chose between one of two doors, and subsequently could win or lose a nominal amount of money. We administered this task to a non-clinical sample of 8- to 13-year-olds in order to investigate the relationship between depressive symptoms and neurophysiological response to rewards (i.e., the FN). Moreover, we examined the relationship between the FN and both age and pubertal development, as assessed by child and parent self-report. We predicted that, consistent with findings from behavioral and neuroimaging studies, the FN would differentiate monetary losses from monetary gains and that, as in adults, the difference between loss and gain trials would be attenuated among children with higher depressive symptom scores. We expected that this relationship might specifically be driven by the neural response to rewards (e.g., Foti & Hajcak, 2009). Furthermore, based on the findings from Hammerer and colleagues (2011) and the widely-supported finding that reward responsiveness increases in adolescence (Galvan, 2010), we predicted that reward sensitivity, as reflected by the FN difference, would increase as a function of age and pubertal development. There is little in the literature to provide information about gender effects on the FN; therefore, we did not have a specific hypothesis regarding associations between gender and FN, but we investigated possible relationships as an exploratory analysis.
Methods
Participants
Participants were recruited from Stony Brook and the surrounding community using a commercial mailing list targeting families with 8- to 13-year-old children. Letters were sent to 800 families from the mailing list, and the letters were followed-up with phone calls. Of those families who were contacted, 101 initially expressed interest in participating and were scheduled for appointments. Out of those 101 families, 30 did not participate due to one or more of the following reasons: did not have time, decided not to participate for personal reasons, or did not show up for scheduled appointments more than two times and were not contacted again. Seventy-one families showed up for their scheduled appointments, and two of these did not participate in any EEG-based paradigm (one child had Asperger’s syndrome and was unable to complete the tasks, and the other child was unable to sit still and eventually refused to participate in tasks). Thus, in total, 69 children participated in the overall study.
Out of the 69 study participants, two did not complete the guessing task and therefore were not included in data analysis. Furthermore, participants with FN difference scores, CDI:SR scores, or CDI:P scores greater than three standard deviations from the mean were excluded from analysis; an additional three participants were excluded according to this criterion. Thus, a total of five out of the 69 participants in the overall study were excluded from analysis. The final sample comprised 64 participants (26 females, 38 males; age: mean = 10.66, SD = 1.60).
Children received $40.00 for study completion, plus a bonus of $5.00 for the guessing task. Assent was obtained from child participants, and informed consent was obtained from their parent before the experiment. This study was formally approved by the Stony Brook University Institutional Review Board.
Measures
After assent and consent were obtained, participants were given several computer-administered questionnaires. Child participants were given the short version of the Children’s Depression Inventory self-report measure (CDI:SR), a 10-item questionnaire (items on a 0–2 scale, possible total scores 0–20) used to gauge depressive symptomatology in children (Kovacs, 1992). Kovacs (2003) suggests a clinical cutoff for the CDI at the 85th percentile; for the CDI:SR short form, this corresponds to a raw score of 6 for children aged 7–12. Children completed the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997) to assess for anxiety symptoms. The SCARED consists of 41 items rated “not true or hardly ever true,” “somewhat true or sometimes true,” and “very true or often true;” item ratings are converted to scores of 0, 1, or 2, respectively, and then summed for a total score. Children were also given the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988), which assesses the degree to which several indicators of puberty (e.g. growth spurt, body hair) are present. The PDS consists of 5 items (each on a 1–4 scale representing no development to completed development) that are averaged together into a summary score. If children were unable to understand a given question on any measure, an experimenter of the same gender as the participant was available to explain it further. Parents filled out the parent versions of the CDI (CDI:P), SCARED, and the PDS in reference to their child. For the CDI:P, the 85th-percentile clinical cutoff score is 20 for boys aged 7–12 and 17 for girls aged 7–12 (Kovacs, 2003).
Guessing Task
The guessing task was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, California, USA) and was similar to the version used in previous studies (Dunning & Hajcak, 2007; Foti & Hajcak, 2009, 2010). The task consisted of 60 trials, presented in three blocks of 20 trials separated by a break that lasted as long as the participant chose. At the beginning of each trial, participants were presented with an image of two doors. They were instructed to choose one door by clicking on the left mouse button for the left door or the right mouse button for the right door. Participants were told that they could either win or lose real money on each trial, and that the goal of the task was to correctly guess which door hid the reward and to earn as much money as possible. Participants were not explicitly told that feedback was random or that they should use a strategy. The image of the doors remained on the screen until the participant made a choice. After stimulus offset, a fixation mark (+) appeared for 1000 ms, and feedback was presented on the screen for 2000 ms. Participants were told that they could either gain $0.50 or lose $0.25 on each trial; these values were chosen in order to equalize the subjective values of the gains and losses (Tversky & Kahneman, 1981, 1992). A gain of $0.50 was indicated on the feedback screen by a green “↑,” and a loss of $0.25 was indicated by a red “↓.” After offset of the feedback stimulus, a fixation mark appeared for 1500 ms and was followed by the message “Click for the next round”, which remained on the screen until the participant responded and the next trial began. The probability of winning over the course of the experiment was exactly 50%; 30 gain and 30 loss feedback trials were presented to each participant, in a fully random order.
Following the questionnaires, the computer tasks were briefly explained to children and electroencephalograph (EEG) electrodes were applied. All participants performed other tasks in addition to the guessing task, to be described elsewhere; tasks were counterbalanced across the session.
Psychophysiological recording, data reduction, and analysis
EEG recordings were collected using the ActiveTwo BioSemi System (BioSemi, Amsterdam, Netherlands) with a custom cap, which used 34 electrodes arranged according to the 10/20 system, including FCz and Iz. Additional recordings were taken from the right and left mastoids. Electrooculogram (EOG) activity generated from eye movements and blinks was recorded from four electrodes: one placed approximately 1 cm to the right of the right eye, one approximately 1 cm to the left of the left eye, and one each approximately 1 cm above and below the left eye. The signal was pre-amplified at the electrode with a gain of one; electroencephalogram data was digitized at 24-bit resolution with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. Each electrode was measured online with respect to a common mode sense electrode that formed a monopolar channel.
Off-line analyses were conducted using BrainVision Analyzer (Brain Products, Munich, Germany) with all channels re-referenced to the average of the two mastoid recordings and band-pass filtered with cutoffs of .1 and 30 Hz. EEG was segmented for each trial, beginning 200 ms before onset of the feedback stimulus and ending 600 ms after onset of the feedback stimulus. EEG was corrected for EOG artifact using the procedure developed by Gratton and colleagues (Gratton, Coles, & Donchin, 1983). Physiological artifacts were rejected using a semi-automated procedure with a maximal allowed voltage step of 50 µV/ms between sample points, a maximal voltage difference of 300.0 µV in a given trial, and a minimum allowed voltage of .50 µV within an interval of 100 ms.
Stimulus-locked ERPs were averaged separately for losses and gains, using the 200 ms before stimulus onset as baseline. ERP activity was quantified on gains and losses as the pooled activity at Fz, FCz, and Cz, as the average activity from 275 ms to 375 ms after stimulus onset. The FN was defined as the activity on losses minus the activity on gains. The difference score provides a measure of neural sensitivity to outcome valence regardless of source; this scoring method has been used in a number of previous studies (e.g. Christie & Tata, 2009; Dunning & Hajcak, 2007; Foti & Hajcak, 2009, 2010; Gu, Huang, & Luo, 2010; Hajcak, et al., 2007; Hammerer, et al., 2011; Moser & Simons, 2009; Nieuwenhuis, Slagter, von Geusau, Heslenfeld, & Holroyd, 2005). This scoring method is limited to some extent by the fact that it does not allow for investigating the individual contributions of responses to gains and losses. However, individual scoring of gain and loss responses can be misleading, in that it can indicate an ERP component where one does not exist (Luck, 2005). In a follow-up analysis, we also examined ERP responses to gains and losses separately.
Results
Psychological and Developmental Measures
Means, standard deviations, and ranges for the psychological and developmental measures are reported in Table 1. Parent-rated PDS score was positively correlated both with age (r = .65, p < .001) and children’s self-rated PDS score (r = .67, p < .001); children’s self-reported PDS score was also positively correlated with age (r = .46, p < .001). Parent-rated CDI and children’s self-rated CDI were only moderately correlated with one another (r = .35, p < .01). Using the summation of the z-scores for the parent- and self-rated CDI, we created an aggregate measure of total depressive symptoms (CDI:T), which we used as our primary depressive symptomatology variable. Aggregate CDI was not correlated with either parent-reported PDS or child-reported PDS (all p values > .05). Girls (M = 1.89, SD = .70) had significantly higher parent-rated PDS scores than boys (M = 1.52, SD = .68; t(62) = 2.12, p < .05; Cohen’s d = .54). However, boys and girls did not differ on self-reported PDS or aggregate CDI (p values > .05).
Table 1.
Means and standard deviations of psychological and developmental measures.
| Measure | Possible Range |
Observed Range |
Mean | Standard Deviation |
|---|---|---|---|---|
| Pubertal development | ||||
| PDS:P | 1–4 | 1–3.4 | 1.67 | 0.71 |
| PDS:SR | 1–4 | 1–3.8 | 1.93 | 0.68 |
| Depressive symptoms | ||||
| CDI:P | 0–51 | 0–20 | 7.95 | 5.21 |
| CDI:SR | 0–20 | 0–6 | 1.15 | 1.45 |
Using the five-level Puberty Category Score criteria from Carskadon and Acebo (Carskadon & Acebo, 1993), which calculates degree of development from certain items of the self-reported PDS, 11 participants were categorized as prepubertal, 24 as early pubertal, 22 as midpubertal, 5 as late pubertal, and 2 as postpubertal. When calculated from parents’ ratings, 34 children were categorized as prepubertal, 5 as early pubertal, 19 as midpubertal, 6 as late pubertal, and 0 as postpubertal. Thus, in the current sample, the vast majority of participants were in early or mid puberty—and only a couple of participants had completed puberty.
Feedback Negativity
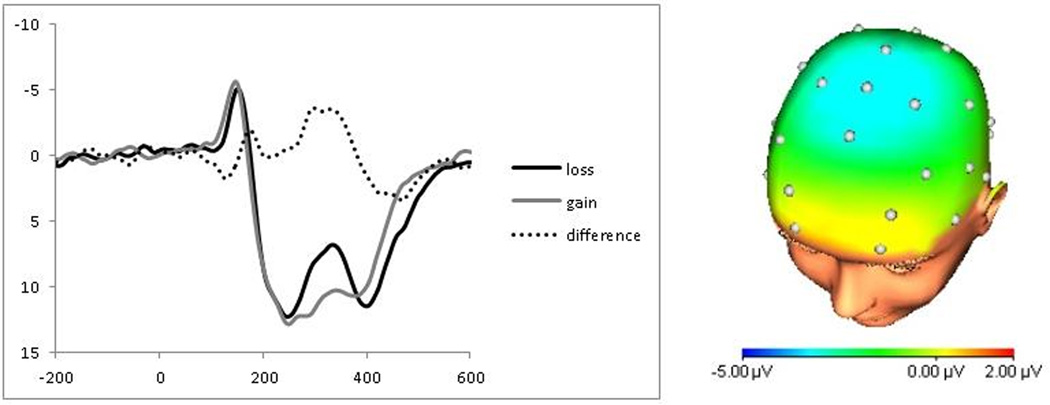
A repeated-measures ANOVA with a Greenhouse-Geisser correction found no significant difference between sites Fz, FCz, and Cz (F(1.49, 93.90) = .48, p = .57; ηp2 = .01); thus, the three electrode sites were pooled for analysis. The feedback-locked ERPs at Fz/FCz/Cz are presented in Figure 1 (left). Consistent with previous work in adults, feedback indicating monetary loss was associated with a relative negativity around 330 ms compared to feedback indicating monetary gain. The difference between losses and gains (i.e., the FN) was maximal at frontocentral sites (Figure 1, right). A paired-samples t-test confirmed that monetary losses (M = 8.05 µV, SD = 8.49 µV) were associated with a greater negativity than gains (M = 11.01 µV, SD = 9.11 µV; t(63) = −3.79, p < .001; Cohen’s d = .34).
Figure 1.
Stimulus-locked event-related potentials (left) to feedback indicating monetary loss (dark) and gain (light), as well as the difference waveform for loss minus gain trials (dashed). Scalp distribution of the difference between loss and gain trials from 275 to 375 ms following stimulus onset (right).
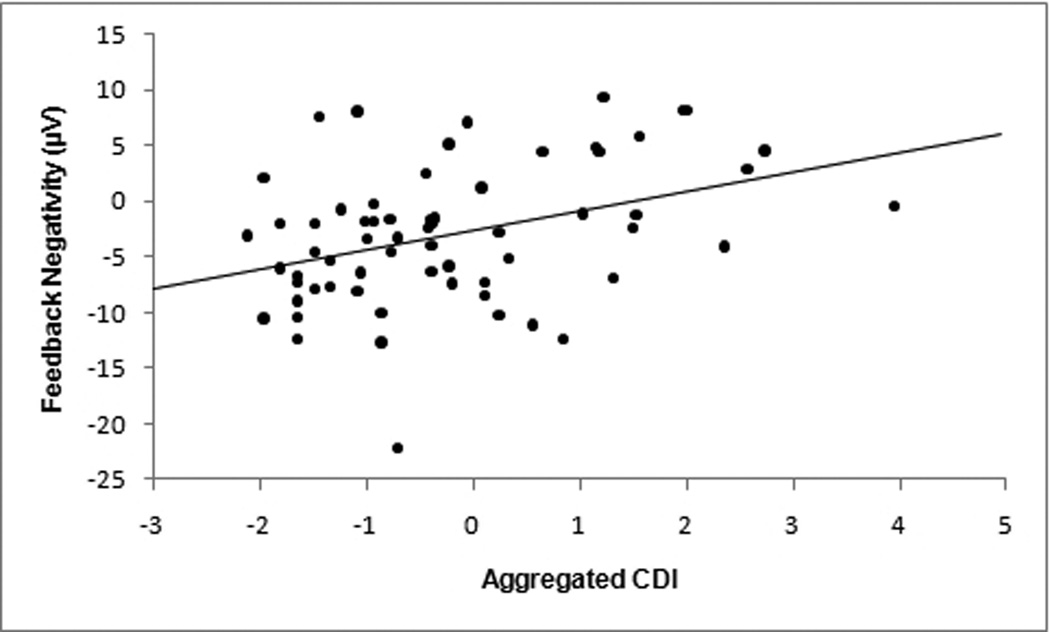
A series of bivariate correlations was performed in order to relate the FN (i.e., losses minus gains) to psychological and developmental measures. The FN did not differ between boys and girls (t(62) = 1.67, p = .10; Cohen’s d = .42), and was unrelated to age (r = −.04, p = .78), child-reported PDS (r = −.06, p = .65), and parent-reported PDS (r = .03, p = .80). However, the FN was significantly correlated with CDI:T (r = .38, p < .01; see Figures 2 and 3). When CDI:T was broken down into its component measures, FN was significantly correlated with CDI:SR (r = .33, p < .01), and with CDI:P (r = .30, p < .05) 1,2. Since the FN difference score is numerically negative, these positive correlations indicate that an increase in both self-rated and parent-rated depressive symptom scores was associated with a reduction in the FN (i.e. less neural differentiation between losses and gains). Additional correlations were performed to examine the relationships between depressive symptom rating scores and participants’ neural responses to losses and gains separately. CDI:T predicted the ERP response to gains (r = −.27, p < .05), but not losses (r = −.01, p = .94), and the association with gains was significantly stronger (Z = 2.94, p < .01). A similar pattern emerged for the self-report measure: CDI:SR predicted the ERP response to gains (r = −.27, p < .05), but not losses (r = −.05, p = .71), and the association with gains was significantly stronger (Z = 2.49, p = .01). The correlation between CDI:P and response to gains did not reach significance (r = −.17, p = .17), nor did the correlation between CDI:P and losses (r = 0.03, p = .81).
Figure 2.
Scatter plot of feedback negativity (FN; calculated as a difference score between loss and gain trials) versus aggregated ratings of depression. Aggregated ratings of depression were calculated as the sum of z-scores for self-rated and parent-rated CDI.
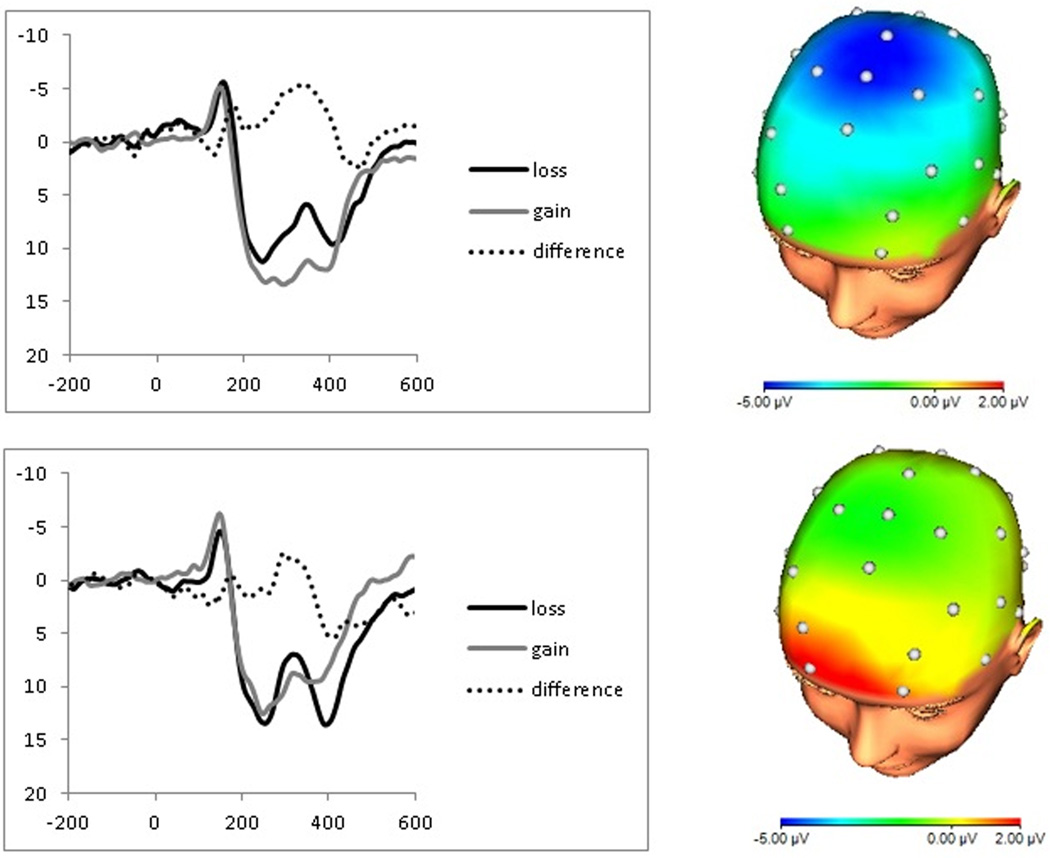
Figure 3.
Stimulus-locked event-related potentials (left) to feedback indicating monetary loss (dark) and gain (light), as well as the difference waveform for loss minus gain trials (dashed), and scalp distribution of the difference between loss and gain trials from 275 to 375 ms following stimulus onset (i.e., the FN; right) for individuals with low (top) and high (bottom) aggregate CDI scores.
FN was not correlated with either SCARED:SR (r = .13, p = .30) or SCARED:P (r = .20, p = .11). Similarly, a simultaneous regression (overall R2 = .15, F(3,60) = 3.40, p < .05) found no significant contribution of either SCARED:SR (t(60) = −.29, p = .78) or SCARED:P (t(60) = .21, p = .84) beyond the variance accounted for by CDI:T (β = .38, t(60) = 2.67, p = .01).
Discussion
As expected, FN amplitude in children was observed as a frontally maximal component that was more negative in response to losses than rewards. This is consistent with previous research in adults (e.g. Dunning & Hajcak, 2007; Foti & Hajcak, 2009) and children (Hammerer, et al., 2011). Also consistent with the adult literature, when FN was calculated as the difference between losses and gains, FN in children was reduced as a function of depressive symptoms. When the aggregate depressive symptom score was broken down into its component scales, FN was reduced as a function of children’s self-report of depressive symptoms as well as their parents’ reports of the children’s depressive symptoms. In both cases, as depressive symptom scores increased, electrocortical sensitivity to losses compared to rewards decreased. Based on the analysis of the CDI:P subscales, it also appears that association between FN and depressive symptoms are driven by emotional problems rather than functional problems. Interestingly, although both self-rated and parent-rated depressive symptom scores were associated with a decrease in sensitivity to outcome, the two factors were only moderately correlated with each other. This is not entirely surprising, given the generally low correlations between self-reports in children and reports by informants (Achenbach, McConaughy, & Howell, 1987), particularly for internalizing disorders such as depression. While such discrepancies are common, they may be even more so in children, who may be less developmentally prepared to make critical observations of themselves.
Although a large literature assumes that the FN is an increased negativity to losses, recent work suggests instead that the variation in FN amplitude is primarily driven by rewards (Holroyd, Krigolson, & Lee, 2011; Holroyd, et al., 2008). Similarly, Foti and colleagues (Foti, Weinberg, et al., in press) used temporospatial principal components analysis (PCA) to investigate the underlying structure of the ERP response to monetary feedback, and identified a positive component that was increased for rewards and reduced for losses. In other words, the apparent negativity to losses appears to reflect the absence of a reward-related positivity on loss trials, and thus the FN may be more aptly characterized as a reward-related positivity. It is notable that when responses to losses and rewards were examined separately in the current study, participants’ depressive symptom scores related to the neural response to rewards but not losses. Figure 3 likewise suggests that the neural response to losses was equivalent in the high- and low-symptom groups based on a median-split of the CDI:T; on the other hand, the groups differed in their responses to rewards, such that only participants low in depressive symptoms were characterized by a more positive ERP following gains. The current results contribute to the growing literature suggesting that individual differences in FN magnitude may be driven specifically by sensitivity to rewards.
Overall, the FN in the current study was quite similar to what has been reported in adults, both in terms of morphology and topographical distribution. Furthermore, both children and adults have in common the decreased differentiation between rewards and non-rewards as a function of depressive symptoms. These data suggest a developmentally consistent relationship between depressive symptoms and dysfunctional reward processing. Our results are consistent with recent fMRI findings (Diekhof, et al., 2008) that both clinically depressed adults (Pizzagalli et al., 2009) and clinically depressed adolescents (Forbes, et al., 2006) show blunted responsiveness to reward.
Importantly, the feedback used in the current study was independent of participants’ performance, allowing for a relatively unambiguous interpretation of the results. Studies using performance-based feedback have shown mixed results. Gotlib and colleagues (2010), for instance, used a task in which feedback was based on reaction time. They reported that 10- to 14-year-old daughters of mothers with a history of recurrent depression showed decreased neural reactivity to reward compared to girls of non-depressed mothers – namely, using fMRI, the high-risk girls had less activation in the left putamen and several areas in the cingulate gyrus in response to reward feedback, and more activation in the dorsal cingulate gyrus in response to loss feedback. Forbes et al. (2006) reported similar results: 9- to 17-year-olds with depression had decreased neural activation using fMRI in reward-related brain areas in a task where feedback was not as closely tied with performance. These findings are consistent with those of the current study and with other EEG studies using non-performance-based feedback, which likewise find decreased neural response associated with depressive symptomatology.
On the other hand, some EEG studies have used performance-based feedback (e.g. Tucker, Luu, Frishkoff, Quiring, & Poulsen, 2003), and found that depression is associated with a larger FN (although, interestingly, within the depression group, the more severely depressed participants had somewhat smaller FNs). However, such studies may conflate the effects of gaining or losing money with performing well or poorly, and their results could reflect a combination of the effects of response monitoring and reward processing. It is therefore unsurprising that some studies using performance-based feedback produce different results from those that use more pure measures of reward and non-reward that do not also convey performance-related information.
Contrary to our second hypothesis, there was no observed association between FN amplitude and either age or degree of pubertal development. The age group we targeted was expected to contain children at a variety of developmental stages; however, PDS scores indicated that participants were more developmentally homogeneous than expected: relatively few participants had reached even late puberty. Thus, there may not have been enough variability in pubertal development for an association with FN to emerge, and it is difficult to make any clear conclusions regarding the role of development in the topology of the FN. Given the existing work by Hammerer and colleagues (Hammerer, et al., 2011), there is reason to believe that FN may increase over the course of puberty, if the sample includes a sufficiently wide range of pubertal development. Therefore, although the current results do not provide support for this association, the FN retains the potential to be an excellent biomarker of depression. Future research that combines cross-sectional and longitudinal designs with children and young adolescents across a broader range of ages and developmental stages will be needed in order to more fully investigate potential changes in the FN over various stages of development throughout childhood and adolescence.
The difference-wave approach to ERPs employed in the current study carries some potential limitations; namely, using this approach, it is not possible to pinpoint effects specifically to gain or loss responses. However, evaluating the gain and loss waves individually leads to the possibility of erroneously identifying a component that may arise as an artifact of underlying components (Luck, 2005). The difference-wave approach circumvents this problem by removing the effects of common underlying components that are unlikely to change as a result of the win/loss manipulation. Thus, despite its limitations, the difference-wave approach offers sufficient advantages to justify its use in studies of the FN.
Another limitation of the current research is the non-clinical sample—and these findings may not generalize to a clinical population. Relatedly, it is possible that some participants may have been clinically depressed; because no diagnostic assessment was done, it was not possible to determine whether any participants met criteria for MDD or other comorbid disorders. Future work will be needed to establish that FN is reduced among clinically depressed children, extending the existing work on depression and rewards from neuroimaging (Forbes, et al., 2006; May et al., 2004; Smoski et al., 2009). Establishing the same association using electrocortical measures might be important, given that EEG may be a better tolerated and less expensive measure of reward in depressed samples, and that it is unaffected by some of the unique challenges inherent in this age group (e.g. orthodontic braces). EEG recording also has excellent temporal resolution, and as such can provide complementary information to behavioral and fMRI studies.
It also remains to be clarified whether comorbid disorders may impact the association between depressive symptoms and electrocortical sensitivity to reward. The current results suggest that anxiety symptoms do not affect this relationship, but since there was no diagnostic evaluation, it is possible that clinically significant anxiety may have an effect. Furthermore, future studies might investigate relationships between the FN and subjective reports of emotion in response to winning and losing, and with specific more specific facets of depression, such as anhedonia.
Given the correlational nature of the current study, we are unable to draw firm conclusions about the causal direction of our findings. It is possible that a reduced FN in children reflects a predisposition toward increased depressive symptomatology; however, it is also possible that the FN decreases as a result of depressed mood. Research with adults has shown that the FN has both state- and trait-like qualities. Greater self-reported sadness after a mood induction is associated with a blunted FN (Foti & Hajcak, 2010), suggesting that responsiveness to reward is state-dependent. In a recent study, however, this association between state sadness and FN amplitude was greater among older adolescents with a parental history of depression, suggesting that neural sensitivity to rewards is more strongly modulated by current mood state among high-risk populations (Foti, Kotov, Klein, & Hajcak, in press). In both studies, as in the current study, amplitude was greater in response to rewards than in response to non-rewards, resulting in a negative loss-win FN difference score. In future studies, it will be important to determine whether this effect is seen before the onset of a first diagnosable depressive episode, or whether it is a concomitant or consequence of depressive symptoms.
Research highlights.
Feedback negativity (i.e., greater negativity for losses than gains) evident in 8 to 13 year-olds.
No observed relation between FN and developmental measures or gender.
Increased depressive symptoms predicted a decreased FN.
Depressive symptoms were specifically related to reduced neural response to rewards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
When the scatterplot in Figure 2 was examined visually, it appeared that the participants with the highest CDI:T score and the most negative FN may have been driving this result. When these participants were removed from the analysis, CDI:T remained significantly correlated with FN(r = .41, p = .001), as did CDI:SR (r = .33, p = .01) and CDI:P (r = .33, p < .01).
The CDI:P has two subscales: Emotional Problems, which includes items such as “looks sad” and “is cranky or irritable,” and Functional Problems, which includes items such as “enjoys being with people” and “is showing worse school performance than before” (Kovacs, 2003). FN was significantly correlated with Emotional Problems (r = .42, p = .001), but not with Functional Problems (r = .09, p = .49).
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull. 1987;101(2):213–232. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Berto P, D'Ilario D, Ruffo P, Di Virgilio R, Rizzo F. Depression: cost-of-illness studies in the international literature, a review. J Ment Health Policy Econ. 2000;3(1):3–10. doi: 10.1002/1099-176x(200003)3:1<3::aid-mhp68>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Dalgleish T, Yule W. Memory for emotional stories in high and low depressed children. Memory. 2004;12(2):214–230. doi: 10.1080/09658210244000667. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Christie GJ, Tata MS. Right frontal cortex generates reward-related theta-band oscillatory activity. Neuroimage. 2009;48(2):415–422. doi: 10.1016/j.neuroimage.2009.06.076. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Streuning EL. An epidemiological study of disorders in late childhood and adolescence--I. Age-and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34(6):851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective Style and Affective Disorders: Perspectives from Affective Neuroscience. Cognition and Emotion. 1998;12(3):307–330. [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59(1):164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Hajcak G. Error-related negativities elicited by monetary loss and cues that predict loss. Neuroreport. 2007;18(17):1875–1878. doi: 10.1097/WNR.0b013e3282f0d50b. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mock B, Mecklinger A. Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia. 2008;46(2):521–539. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Mock B, Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46(5):1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. State sadness reduces neural sensitivity to nonrewards versus rewards. Neuroreport. 2010;21(2):143–147. doi: 10.1097/WNR.0b013e3283356448. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein DN, Hajcak G. Abnormal Neural Sensitivity to Monetary Gains Versus Losses Among Adolescents at Risk for Depression. J Abnorm Child Psychol. 2011 doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to non-rewards versus rewards among adolescents at risk for depression. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-011-9503-9. in press. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. doi: 10.1002/hbm.21182. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gu R, Huang YX, Luo YJ. Anxiety and feedback negativity. Psychophysiology. 2010;47(5):961–967. doi: 10.1111/j.1469-8986.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44(6):905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hammerer D, Li SC, Muller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J Cogn Neurosci. 2011;23(3):579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14(5):711–724. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006;1105(1):93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE, Lee S. Reward positivity elicited by predictive cues. Neuroreport. 2011;22(5):249–252. doi: 10.1097/WNR.0b013e328345441d. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Keenan K, Feng X, Hipwell A, Klostermann S. Depression begets depression: comparing the predictive utility of depression and anxiety symptoms to later depression. J Child Psychol Psychiatry. 2009;50(9):1167–1175. doi: 10.1111/j.1469-7610.2009.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome depressive disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(7):703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory Manual. New York: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- Kovacs M. Children's Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY: Multi-Health Systems; 2003. [Google Scholar]
- Krackow E, Rudolph KD. Life stress and the accuracy of cognitive appraisals in depressed youth. J Clin Child Adolesc Psychol. 2008;37(2):376–385. doi: 10.1080/15374410801955797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, Massachusetts: The MIT Press; 2005. [Google Scholar]
- Luppa M, Heinrich S, Angermeyer MC, Konig HH, Riedel-Heller SG. Cost-of-illness studies of depression: a systematic review. J Affect Disord. 2007;98(1–2):29–43. doi: 10.1016/j.jad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF, Burton PC, Montague PR. Electrophysiological and hemodynamic responses to reward prediction violation. Neuroreport. 2009;20(13):1140–1143. doi: 10.1097/WNR.0b013e32832f0dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Miltner W, Braun CH, Coles MG. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a "generic" neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6) doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Moser JS, Simons RF. The neural consequences of flip-flopping: the feedback-related negativity and salience of reward prediction. Psychophysiology. 2009;46(2):313–320. doi: 10.1111/j.1469-8986.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci. 2005;21(11):3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A Self-Report Measure of Pubertal Status - Reliability, Validity, and Initial Norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pietschmann M, Simon K, Endrass T, Kathmann N. Changes of performance monitoring with learning in older and younger adults. Psychophysiology. 2008;45(4):559–568. doi: 10.1111/j.1469-8986.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. J Cogn Neurosci. 2006;18(7):1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30(7):1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol. 2003;112(4):667–678. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in Prospect-Theory - Cumulative Representation of Uncertainty. Journal of Risk and Uncertainty. 1992;5(4):297–323. [Google Scholar]