Abstract
Background
Major depressive disorder (MDD) frequently co-occurs in adolescents with substance use disorders (SUD) and attention deficit hyperactivity disorder (ADHD), but the impact of MDD on substance treatment and ADHD outcomes and implications for clinical practice are unclear.
Methods
Adolescents (n=303; ages 13-18) meeting DSM-IV criteria for ADHD and SUD were randomized to Osmotic Release Methylphenidate (OROS-MPH) or placebo and 16 weeks of cognitive behavioral therapy (CBT). Adolescents with (n=38) and without (n=265) MDD were compared on baseline demographic and clinical characteristics as well as non-nicotine substance use and ADHD treatment outcomes.
Results
Adolescents with MDD reported more non-nicotine substance use days at baseline and continued using more throughout treatment compared to those without MDD (p<0.0001 based on Timeline Followback; p<0.001 based on urine drug screens). There was no difference between adolescents with and without MDD in retention or CBT sessions attended. ADHD symptom severity (based on DSM-IV ADHD Rating Scale) followed a slightly different course of improvement although with no difference between groups in baseline or 16-week symptom severity or 16 week symptom reduction. There was no difference in days of substance use or ADHD symptom outcomes over time in adolescents with MDD or those without MDD treated with OROS-MPH or placebo. Depressed adolescents were more often female, older, and not court ordered.
Conclusions
These preliminary findings suggest that compared to non-depressed adolescents with ADHD and SUD, those with co-occurring MDD have more severe substance use at baseline and throughout treatment. Such youth may require interventions targeting depression.
Keywords: Major depressive disorder, substance use disorder, attention deficit hyperactivity disorder, adolescents, comorbid disorder, treatment outcomes
1.0 Introduction
Identifying efficacious treatments is a substantial concern in the treatment of adolescents with substance use disorders (SUD). The majority of adolescents with SUD have co-occurring psychiatric disorders (Rowe et al., 2004; Riggs et al., 2007). Psychiatric comorbid disorders have been associated with poorer treatment outcomes (Buckstein and Horner, 2010; Magura et al., 2008; Baker et al., 2007) although not consistently (Crowley et al., 1998; Rivers et al., 2001) and little is known about the impact of specific comorbid disorders on treatment outcomes. If SUD treatment outcomes differ based on the presence of specific co-occurring psychiatric disorders, comprehensive strategies can be developed to target SUD in the context of these disorders in order to maximize treatment outcomes.
Both externalizing disorders (e.g., attention deficit hyperactivity disorder [ADHD], conduct disorder) and internalizing disorders (e.g., major depressive disorder [MDD], anxiety) commonly co-exist in adolescents with SUD (Shane et al., 2003; Rowe et al., 2001). In pooled data from 4930 adolescents in multisite studies of SUD, 72-74% had conduct disorders, 61-64% had ADHD, 53% had depression, and 61% had both internalizing and externalizing disorders (Chan et al., 2008).
Depression has been associated with increased substance use severity. Adolescents with MDD or dysthymia and SUD had more substance dependence diagnoses (Riggs et al., 1995), and MDD severity was associated with more symptoms of substance dependence (Whitmore et al., 1997). In a community sample of high school students, increased lifetime occurrence of MDD or dysthymia was associated with increased lifetime alcohol use disorders (Rohde et al., 1996).
Findings have been mixed about the relationship between depressive symptoms and substance treatment outcomes in studies that do not include specific treatment for depression. Number of MDD symptoms at substance treatment initiation was not related to drug use at 2-year follow-up in delinquent adolescents with SUD (Crowley et al., 1998), although symptoms of depression at intake were associated with lack of improvement in drug use in adolescents in another study (Dobkin et al., 1998). Only two of the studies reporting associations between depression and substance use severity or treatment outcome, however, have used diagnostically driven measures of MDD such as the Diagnostic Interview Schedule for Children (Costello et al., 1982; Fisher et al., 1991) and reported outcomes specific to MDD (Whitmore et al., 1997; Crowley et al., 1998).
There are a few randomized controlled trials (RCTs) of MDD pharmacotherapy in adolescents with SUD. An RCT of fluoxetine versus placebo (n=126) in adolescents with MDD and SUD receiving concurrent CBT for SUD showed greater reduction in symptoms of depression on one of two measures with fluoxetine. Although days of past month substance use decreased in both groups there was no difference between groups (Riggs et al., 2007). Another RCT of fluoxetine versus placebo (n=50) in adolescents with MDD receiving concurrent Motivation Enhancement Therapy (MET)/CBT for alcohol use disorder (AUD) showed reductions in depression and alcohol use but no difference between groups on either outcome (Cornelius et al., 2009). Similarly, in an RCT of fluoxetine versus placebo in adolescents and young adults (n=70) with MDD and cannabis use disorder (CUD), receiving MET and CBT for depression and CUD, reductions in depression did not differ between groups and amount of cannabis use did not improve significantly in either group (Cornelius et al., 2010). However, in these trials, CBT even when targeted to SUD, may have also contributed antidepressant effects and minimized SUD outcome differences between medication and placebo groups (Riggs et al., 2007; Nunes and Levin, 2004). Furthermore, none of these trials were adequately powered to detect the effect of treating depression on substance use outcomes. It therefore remains unclear whether pharmacological or other treatments targeted to co-occurring MDD impact SUD outcomes. However, adolescents whose depression remitted did have a significant reduction in drug use compared with those whose depression did not remit, regardless of whether taking fluoxetine or placebo (Riggs et al., 2007), which is consistent with findings in adults (Brown et al., 1997).
A recent RCT of psychostimulant medication (osmotic release methylphenidate, OROS-MPH) was conducted in adolescents (n=303) with ADHD concurrently receiving CBT for co-occurring SUD. There were reductions in adolescent-reported ADHD symptoms and substance use in both medication and placebo groups with no differences between groups, although participants treated with OROS-MPH had fewer parent-rated ADHD symptoms and more negative urine drug screens (Riggs et al., 2010). This trial provides a unique opportunity to compare substance treatment outcomes in adolescents who have both an internalizing disorder, MDD, and an externalizing disorder, ADHD. This report asks the following exploratory questions:
Is co-occurring MDD associated with any demographic or clinical characteristics at substance treatment entry?
Is co-occurring MDD associated with treatment response (non-nicotine substance use, ADHD symptoms)?
Is co-occurring MDD associated with adherence to substance use treatment visits or retention?
2.0 Methods
Data from the 16-week RCT of OROS-MPH or placebo in combination with weekly individual CBT targeting SUD were used to compare adolescents with MDD (n=38) and without MDD (n=265). The trial was conducted at 11 community treatment sites affiliated with the NIDA Clinical Trials Network.
2.1 Participants
Participants were 303 adolescents (ages 13-18) meeting DSM-IV criteria for ADHD and at least one non-nicotine SUD. Diagnoses of MDD, ADHD, and conduct disorder were determined by clinician-administered, semi-structured diagnostic interview (Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Epidemiological Version, 4th Edition (K-SADS-E) (Orvaschel and Puig-Antich, 1987; Geller et al., 2000). Scores of ≥ 22 on the DSM-IV ADHD Rating Scale (ADHD-RS; DuPaul et al., 1998) were required for inclusion. Substance abuse and dependence diagnoses were determined from the Composite International Diagnostic Interview (CIDI) (Cottler et al., 1989; Robins, 1988; Crowley et al., 2001). Adolescents with a history of tic disorder, pregnancy, current or lifetime psychotic or bipolar disorder, serious medical illness, significant suicidal risk, or taking psychotropic medications were excluded from participation as were adolescents with abuse, dependence or past month use of methamphetamine, or current or past year opioid dependence.
Participants were recruited from existing outpatient substance treatment referral sources and community-outreach (e.g., media advertising, schools, primary care providers). The study was approved and overseen by the institutional review boards of the community treatment sites and academic centers affiliated with each site. Participants provided assent and their parent or guardian provided written consent if a non-emancipated minor. Participants 18 years or older provided written informed consent.
2.2 Measures
Substance use measures
The primary outcome measure for substance use was number of days of non-nicotine substance use, including drugs and alcohol, collected for the past 28 days at baseline and weekly throughout the 16-week trial using standardized Timeline Followback (TLFB) procedures (Sobell and Sobell, 1992; Miller and Del Boca, 1994). Urine drug screens were also collected at screening/baseline and weekly throughout the trial using a rapid system screening for amphetamine, barbiturates, benzodiazepines, cocaine, methamphetamine, opiates and THC. Results were analyzed based on the number of urine drug screens negative for drugs of abuse. This approach avoids imputation of “positive” urine toxicologies if an expected sample is missing (Ling et al., 1997).
ADHD measure
The primary outcome measure for ADHD was the DSM-IV ADHD-RS symptom checklist (DuPaul et al., 1998) administered by medical clinicians with the adolescent at baseline for the prior 28 days and weekly throughout the study. The scale is medication sensitive and correlated with ADHD in children and adolescents (Bostic et al., 2000; Prince et al., 2000).
2.3 Procedures
Participants were randomized to either OROS-MPH or matching placebo. Participants were titrated to a 72 mg daily dose or the highest dose tolerated. All participants received 16 weeks of individual, evidence-based CBT (Kadden et al., 1995; Monti et al., 1989; Waldron et al., 2001) as a manual-standardized outpatient substance treatment.
2.4 Data Analysis
Baseline demographic and clinical characteristics were compared in adolescents with and without MDD using t-tests for continuous variables and chi-square tests (or Fisher's exact test) for categorical variables.
Data plots were developed based on raw data, and longitudinal data analyses of the prospectively collected data were conducted using all data points to evaluate trajectories of change in non-nicotine substance use and ADHD symptoms. For the longitudinal analyses and data plots, past 28-day measures of non-nicotine substance use were converted to past 7-day use (by dividing by 4) to be consistent with the weekly time frame for administration of the ADHD-RS and collection of urine drug screens. All longitudinal analyses were adjusted for baseline differences between the groups in age, gender, and court-ordered status. Population-average linear mixed models with an AR(1) correlation structure, and MDD status, time (linear and potentially higher order terms), and MDD by time interaction(s) as predictors were fitted. If the group by time interaction was not significant, it was deleted for the longitudinal model.
To further assess whether there was any interaction between treatment group (OROS-MPH or placebo) and the presence or absence of MDD, analyses were conducted to compare trajectories of substance use and ADHD symptoms over time between those receiving OROS-MPH and those receiving placebo within the MDD group (n=19 receiving OROS-MPH; n=19 receiving placebo) and within the group without MDD (n=133 receiving OROS-MPH; n=132 receiving placebo) in addition to exploring an interaction model involving treatment group, presence or absence of MDD, and time.
Study retention, CBT visit adherence, abstinence, and negative urine drug screens were compared in adolescents with and without MDD using t-tests and chi-square tests.
3.0 Results
3.1 Baseline characteristics in adolescents with and without MDD
Baseline demographic and clinical characteristics of adolescents with and without MDD are presented in Table 1. Adolescents with MDD were older (17.0 vs. 16.4 years old; p=0.01), more often female (42% vs. 18%; p=0.0007), less likely to be court mandated to substance treatment (11% vs. 26%; p=0.04), and had more days of past month non-nicotine substance use (18.8 vs. 14.1 days; p=0.005). There were no differences between the groups in ADHD symptom severity (ADHD-RS scores), presence of current conduct disorder, total number of substance abuse or dependence diagnoses, or any specific substance abuse or dependence diagnosis at baseline. Groups were balanced with regard to random assignment of participants with and without MDD to OROS-MPH or placebo.
Table 1. Baseline Demographic and Clinical Characteristics Associated with Major Depressive Disorder.
| Major Depressive Disorder | |||
|---|---|---|---|
| Demographic |
Present N=38 |
Absent N=265 |
p |
| % | % | ||
| Randomized to OROS –MPH | 50.0 | 49.8 | 0.98 |
| Female Sex | 42.1 | 18.1 | 0.0007 |
| Race1 | 0.23 | ||
| Caucasian | 69.4 | 60.7 | |
| African-American | 5.0 | 22.9 | |
| Native American/Asian/Pacific Islander/Mixed Race/Other | 5.6 | 16.4 | |
| Hispanic Ethnicity | 7.9 | 16.2 | 0.18 |
| Abuse & Dependence Diagnoses | |||
| Alcohol | 55.3 | 56.2 | 0.91 |
| Cannabis | 92.1 | 92.1 | 0.995 |
| Cocaine | 13.2 | 9.4 | 0.565 |
| Amphetamine | 7.9 | 3.8 | 0.215 |
| Hallucinogen | 10.5 | 12.8 | 0.995 |
| PCP | 0.0 | 0.8 | 0.995 |
| Inhalant | 2.6 | 1.9 | 0.565 |
| Opiate | 21.1 | 11.3 | 0.115 |
| Sedative | 10.5 | 9.8 | 0.785 |
| Other | 0.0 | 1.1 | 0.995 |
| Conduct Disorder (current) | 36.8 | 31.7 | 0.53 |
| Court Mandated | 10.5 | 26.0 | 0.04 |
|
|
|||
| Mean(SD) | Mean(SD) | ||
| Age (years) | 17.0 (1.3) | 16.4 (1.3) | 0.01 |
| ADHD symptom severity2 | 40.2 (8.1) | 38.5 (9.0) | 0.29 |
| Number of days non-nicotine substance use-past 28 days3 | 18.8 (8.8) | 14.1 (9.4) | 0.005 |
| Number of days non-nicotine substance use-past 7 days3,4 | 4.7 (2.2) | 3.5 (2.4) | 0.005 |
| Number of days nicotine use-past 28 days3 | 19.4 (11.8) | 15.8 (12.5) | 0.10 |
| Number of days nicotine use-past 7 days3,4 | 4.8 (2.9) | 4.0 (3.1) | 0.10 |
| Number of non-nicotine abuse/dependence diagnoses | 2.1 (1.2) | 2.0 (1.3) | 0.52 |
Race was not available for 5 subjects (2 for MDD, 3 for no MDD).
Based on the Attention Deficit Hyperactivity Disorder Rating Scale.
Based on the Timeline Followback.
Past 7-day use calculated by dividing 28 day measure by 4. T-tests for continuous variables and chi-square tests for categorical variables were used, except
Fisher's exact test. P-values < 0.05 were bolded.
3.2 Substance use and ADHD symptoms in adolescents with and without MDD
Figures 1 and 2 show the longitudinal raw data plots for adolescents with and without MDD for days of non-nicotine substance use and ADHD symptom severity over the 16-week trial. Raw data plots are shown because the use of multiple covariates in the longitudinal models makes clear visual presentation of these models difficult.
Figure 1.
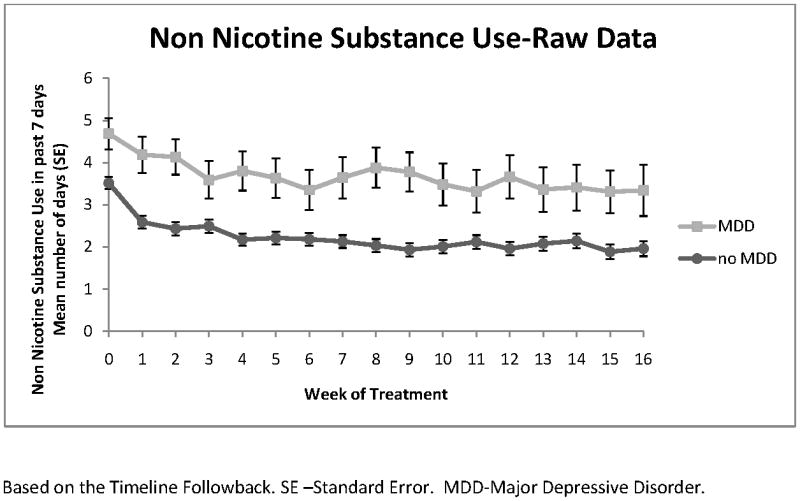
Non Nicotine Substance Use
Figure 2.
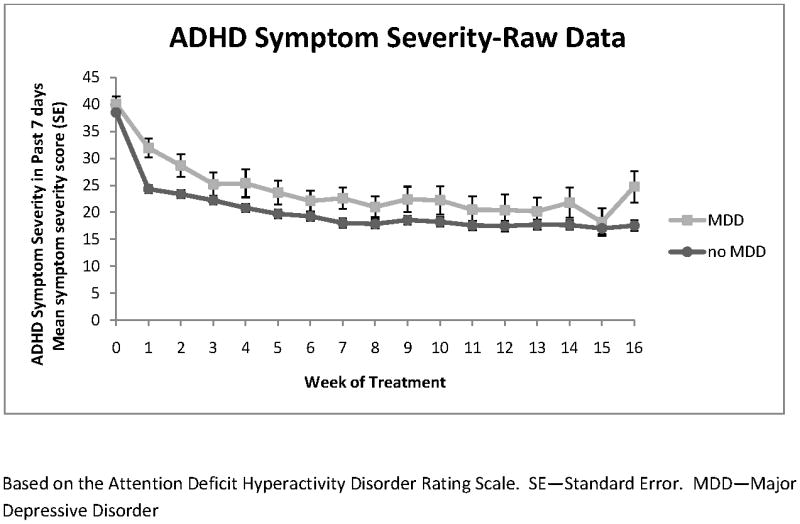
ADHD Symptom Severity
The trajectory for each of these outcomes followed a cubic model in time (weeks). In the longitudinal model, controlling for covariates, (estimated) mean days of non-nicotine substance use in the prior 7 days began 1.18 days higher at baseline in the adolescents with MDD (t298=4.64, p 〈0.0001) and this difference persisted throughout treatment with a mean reduction of 1.53 days of use per week by week 16 (95% CI=1.12 - 1.94). In the longitudinal model, controlling for covariates, there were no significant differences in ADHD symptom severity at baseline between the groups (mean 1.03 point higher for those with MDD; t300=0.53, p=0.59). ADHD symptom severity did follow a slightly but significantly different course of improvement in the two groups (MDD by week effect, p=0.007; MDD by week2 effect; p =0.02; MDD by week3 effect; p= 0.048). Adolescents with MDD had a 19.91 point reduction (95% CI=14.36 -25.46) and those without MDD had a 22.03 point reduction in symptom severity by week 16 (95% CI=20.00 - 24.05), which did not differ significantly between groups (t3212=0.70, p=0.48). There was no significant difference in ADHD symptom severity between groups at week 16 (mean 3.15 point higher for those with MDD; t3212=1.29, p=0.20).
In interaction models, including medication treatment, there was no main or interaction effect of OROS-MPH for either days of non-nicotine substance use or ADHD symptom severity outcomes. In a subgroup longitudinal model for non-nicotine substance use in adolescents without MDD the group receiving OROS-MPH had 0.36 fewer days of substance use at baseline (t261=-2.14, p=0.03) but there was no significant interaction of OROS-MPH with time. However, in adolescents with MDD a significant time interaction with OROS-MPH was found for ADHD symptom severity, and cubic curves for OROS-MPH and placebo were compared. There were no significant differences in ADHD symptom severity at baseline between treatment groups (mean 3.68 point lower for OROS-MPH, t36=-1.04, p=0.31). The OROS-MPH group had a 19.38 point reduction (95% CI=11.95 - 26.81) and the placebo group had a 20.29 point reduction in symptom severity by week 16 (95% CI=13.24 - 27.34), which did not differ significantly between treatment groups (t386=0.17, p=0.86). There were also no significant differences in ADHD symptom severity between treatment groups at week 16 (mean 2.77 point lower for OROS-MPH; t386 =-0.63, p=0.53).
3.3 Retention and adherence in adolescents with and without MDD
There was no difference between those with and without MDD in study completion (71.1% vs. 75.5%, respectively, p=0.56) or CBT visit adherence (67.3 vs. 70.3 percent of sessions attended, respectively; p=0.52). However, adolescents with MDD had significantly fewer days of abstinence (47.1 vs. 67.3 days; p=0.001), a lower percent of days abstinent (47.2 vs. 68.8 percent of days; p< 0.0001) as well as fewer negative urine drug screens (1.6 vs. 3.6; p= 0.0007) (Table 2).
Table 2. Substance Use, Visit Adherence, and Retention Outcomes Associated with Major Depressive Disorder.
| Major Depressive Disorder | |||
|---|---|---|---|
| Variable |
Present N=38 |
Absent N=265 |
p |
| % | % | ||
| Study completion-16 week1 | 71.1 | 75.5 | 0.56 |
| Mean(SD) | Mean(SD) | ||
| Number of days abstinent during trial2, 3 | 47.1 (37.5) |
67.3 (34.4) |
0.001 |
| Percent of days abstinent during trial2,3 | 47.2 (34.3) |
68.8 (28.6) |
<.0001 |
| Percent CBT visit attendance | 67.3 (24.7) |
70.3 (26.7) |
0.52 |
| Number of negative urine drug screens3 | 1.6 (2.8) |
3.6 (4.7) |
0.00074 |
Attendance at week-16 research visit.
Based on the Timeline Followback.
Timeline Followback and urine drug screens were not available for 6 subjects (1 for MDD, 5 for no MDD). T-tests for continuous variables and chi-square tests for categorical variables were used, except
t-test for unequal variances. P-values < 0.05 were bolded.
4.0 Discussion
In adolescents with ADHD and SUD, those with co-occurring MDD used drugs and/or alcohol on significantly more days prior to treatment entry, as well as throughout and at the end of treatment compared to those without MDD. Depressed adolescents also had fewer days of abstinence and fewer negative urine drug screens during treatment despite having similar rates of treatment completion and adherence and comparable reduction in ADHD symptom severity compared to non-depressed adolescents. There was no difference, however, in reduction in days of non-nicotine substance use in those with or without MDD during treatment for SUD.
These findings are consistent with most previous studies that reported depression was associated with more severe substance use problems in clinical and community samples (Rohde et al., 1996; Whitmore et al., 1997; Riggs et al., 1995). Our finding that adolescents who had co-occurring MDD but no reported therapy targeted to MDD had poorer SUD outcomes is consistent with findings in adolescents by Dobkin et al. (1998) but not Crowley et al. (1998). However, most prior studies have not reported the association between MDD and SUD treatment outcomes in well-characterized samples of adolescents with MDD such as this one.
The presence of MDD was not associated with ADHD treatment response in this sample. Adolescents with and without MDD began the trial with no difference in severity of ADHD symptoms. Although those without MDD had a larger decrease in symptom severity during the initial weeks, this difference between groups leveled out and does not appear to be clinically meaningful since there was no difference between the groups in symptom improvement or final symptom severity at the end of the trial. Similar to our finding that the presence of MDD was not meaningfully related to ADHD outcomes, the presence of symptoms of depression or anxiety did not appear to impede response to atomoxetine for ADHD in children and adolescents treated with atomoxetine alone or in combination with fluoxetine (Kratochvil at al., 2005).
Given the similarity in both ADHD symptom severity at treatment initiation and ADHD outcomes in both groups, it is also unlikely that ADHD symptoms or treatment response were responsible for the differences in non-nicotine substance use outcomes in those with and without MDD. ADHD was similarly unrelated to SUD outcomes in the controlled trial of fluoxetine for MDD in adolescents also receiving CBT for SUD where adolescents with or without ADHD had similar substance treatment as well as depression outcomes (Riggs et al., 2009).
Depressed adolescents in this sample tended to be older, female, and fewer were court-mandated to substance treatment compared to those without MDD. Depression and mood disorders have been reported as more likely in substance using adolescent females than males (Grella et al., 2001; Buckstein et al., 1992; Deykin et al., 1992).
Co-occurring MDD did not appear to impact CBT treatment adherence or study completion. Some prior studies similarly found no association between comorbid disorders and treatment attendance (Rivers et al., 2001; Rowe et al., 2004), although an association between internalizing symptoms and better treatment completion was found in an earlier study (Kaminer et al., 1992). Since attendance at CBT visits and retention rates in the study did not differ in adolescents with and without MDD, adherence to the psychosocial intervention or retention also cannot account for the difference between non-nicotine substance use in the adolescents with and without MDD.
The current treatment literature does not provide a clear answer to whether providing targeted treatments for both MDD and SUD will improve SUD outcomes in adolescents (Riggs et al, 2007; Cornelius et al., 2009; Cornelius et al., 2010; Deas et al., 2000). However, given the finding from this study that MDD is associated with greater severity of SUD and continued higher use throughout and at the end of SUD treatment and preliminary evidence that depression remission is associated with improved SUD outcomes (Riggs et al., 2007), targeting both for treatment may be helpful. Studies in adults with MDD and SUD suggest that treating both may improve outcomes (Rao and Chen, 2008). Receiving psychiatric services in addition to SUD treatment was associated with improved outcome (Ray et al., 2005), and manualized behavioral therapies including CBT have been shown to decrease substance or alcohol use and/or depressive symptoms in adults (Carroll, 2004; Maude-Griffin et al., 1998; Brown et al., 1997).
There may be alternate factors impacting SUD outcomes for adolescents with MDD, SUD, and ADHD, such as the severity of the disorders, additional burden of other disorders, psychosocial functioning, or quality of life. For example, number of days of heavy alcohol use was associated with lack of remission of depression in adolescents (Cornelius et al., 2009). However, the association between SUD or MDD severity and outcomes was not evaluated in this sample.
Finally, we also evaluated whether the use of OROS-MPH or placebo might be associated with different outcomes in the adolescents with MDD or the adolescents without MDD. There was no difference in either days of non-nicotine substance use or ADHD symptom severity outcomes over time in either group when OROS-MPH and placebo were compared, consistent with the primary outcomes for the entire sample in the main study (Riggs et al., 2010). While there is some evidence for the efficacy of psychostimulants in reducing symptoms of depression (Candy et al., 2008), the finding that substance use outcomes do not differ with its use in the adolescents with MDD is consistent with the prior studies that found psychopharmacology for depression was not associated with improvement in substance use, at least in the presence of CBT (Riggs et al., 2007, Cornelius et al., 2009, Cornelius et al., 2010). Other possible benefits of treating ADHD with OROS-MPH, given the poor treatment completion and adherence and worse outcomes reported in adolescents with ADHD and SUD (Grella et al., 2001, Rowe et al., 2004, Wise et al., 2001) were also not apparent.
Since pharmacological and psychosocial treatments specifically targeted to MDD were not allowed in this study, we could not evaluate the impact of combining treatments for MDD and SUD, although the effects of such combined treatment may have been masked by possible antidepressant effects of the SUD-focused CBT here as well. Since there was also no measure of depression symptom change during the trial, it is also not clear whether a difference in depression response or remission, even absent treatment targeted to depression, would have helped close the gap in drug use outcomes between those with and without MDD, as findings from a prior study suggest (Riggs et al., 2007). The age of onset and timing of onset of MDD in relation to the SUD and measures of other concurrent psychiatric disorders were not available. Finally, with 38 patients with MDD, options for questions and analyses were limited. For example, a subgroup analysis for adolescents with MDD or estimating the group by time interaction would have been more reliable with a larger sample. Further validation is needed to provide support for the generalizability of these findings.
Outcomes with SUD treatments in adolescents remain modest, and co-occurring conditions are the rule rather than the exception. Preliminary results from this study, which includes thorough diagnostic assessment of MDD with the K-SADS-E, extend previous research by suggesting that the presence of co-occurring MDD in the context of ADHD and SUD is associated with greater severity of SUD and continued higher use in the absence of treatment specifically targeted to MDD. It is also associated with specific clinical characteristics (older age, female gender, fewer court referrals). Adequately powered trials evaluating treatments focused on MDD in the context of SUD and other frequently present, co-occurring psychiatric disorders are needed. In the interim, mechanisms for assessing and treating these disorders are essential in SUD treatment settings since their presence appears to interfere with maximizing SUD outcomes.
Acknowledgments
Role of funding source: Funding for the main study was provided by NIDA (U10DA013732, U10DA013716, U10DA020024). Data analysis for this secondary analysis was also supported by NIDA. NIDA's Publications Committee approved the manuscript for submission for publication.
Paula D. Riggs, M.D. has received funding from the National Institute on Drug Abuse in the past five years and has received honoraria and/or travel support for giving scientific presentations at the American Academy of Child and Adolescent Psychiatry, Sheppard Pratt Hospital; McGill University; University of Washington; California Society of Addiction Medicine; Science and Management of Addiction Foundation; and the Colorado Department of Behavioral Health
Footnotes
Contributors: Drs. Warden, Riggs, Winhusen, Min, Mikulich-Gilbertson, and Tamm developed the plan for the secondary analysis presented. Drs. Min and Mikulich-Gilbertson conducted the statistical analyses. All authors have contributed to and have approved the final manuscript. Dr. Riggs and Dr. Winhusen designed the larger multisite trial and Dr. Riggs was the Lead Investigator for that trial.
Conflict of Interest: Diane Warden, Ph.D., M.B.A. has owned stock in Pfizer, Inc. and Bristol Meyers Squibb within the last five years and has received funding from the National Alliance for Research in Schizophrenia and Depression.
Theresa Winhusen, Ph.D. has no disclosures to report.
Susan K. Mikulich-Gilbertson, Ph.D. has no disclosures to report.
Sung-Joon Min, Ph.D. has no disclosures to report.
Leanne Tamm, Ph.D. has no disclosures to report.
Kathlene Trello-Rishel, M.D. was on the speakers' bureau for Shire Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker KD, Lubman DI, Cosgrave EM, Killackey EJ, Yuen HP, Hides L, Baksheev GN, Buckby JA, Yung AR. Impact of co-occurring substance use on 6 month outcomes for young people seeking mental health treatment. Aust N Z J Psychiatry. 2007;41:896–902. doi: 10.1080/00048670701634986. [DOI] [PubMed] [Google Scholar]
- Bostic JQ, Biederman J, Spencer TJ, Wilens TE, Prince JB, Monuteaux MC, Sienna M, Polisner DA, Hatch M. Pemoline treatment of adolescents with attention deficit hyperactivity disorder: a short controlled trial. J Child Adolesc Psychopharmacol. 2000;10:205–216. doi: 10.1089/10445460050167313. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans M, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckstein O, Glancy LJ, Kaminer Y. Patterns of affective comorbidity in a clinical population of dually diagnosed adolescent substance abusers. J Am Acad Child Adolesc. 1992;31:1041–1045. doi: 10.1097/00004583-199211000-00007. [DOI] [PubMed] [Google Scholar]
- Buckstein OG, Horner MS. Management of the adolescent with substance use disorders and comorbid psychopathology. Child Adolesc Psychiatr Clin N Am. 2010;19:609–23. doi: 10.1016/j.chc.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Candy B, Jones L, Williams R, Tookman A, King M. Psychostimulants for depression (intervention review) Cochrane Database Syst Rev. 2008;2:CD006722. doi: 10.1002/14651858.CD006722.pub2. [DOI] [PubMed] [Google Scholar]
- Carroll K. Behavioral therapies for co-occurring substance use and mood disorders. Biol Psychiatry. 2004;56:778–784. doi: 10.1016/j.biopsych.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, Brown SJ. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112:39–45. doi: 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy A, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34:905–909. doi: 10.1016/j.addbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AJ, Edelbrock CS, Kalas R, Kessler MK, Klaric SA. NIMH Diagnostic Interview Schedule for Children. National Institute of Mental Health; Bethesda, MD: 1982. [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Crowley T, Mikulich S, Ehlers K, Whitmore E, Macdonald M. Validity of structured clinical evaluations in adolescents with conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2001;40:265–273. doi: 10.1097/00004583-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, MacDonald M, Young SE, Zerbe GO. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug Alcohol Depend. 1998;49:225–237. doi: 10.1016/s0376-8716(98)00016-7. [DOI] [PubMed] [Google Scholar]
- Deykin EY, Buka SL, Zeena TH. Depressive illness among chemically dependent adolescents. J Am Psychiatry. 1992;149:1341–1347. doi: 10.1176/ajp.149.10.1341. [DOI] [PubMed] [Google Scholar]
- Dobkin PL, Chabot L, Maliantovitch K, Craig W. Predictors of outcome in drug treatment of adolescent inpatients. Psychol Rep. 1998;83:175–186. doi: 10.2466/pr0.1998.83.1.175. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretations. The Guilford Press; New York, NY: 1998. [Google Scholar]
- Fisher P, Shaffer D, Piacentini J, Lapkin J, Wicks J, Rojas M. Completion of Revisions of the NIMH Diagnostic Interview Schedule for Children (DISC-2) Epidemiology and Psychopathology Research. National Institute of Mental Health; Washington, D.C.: 1991. [Google Scholar]
- Geller D, Biederman J, Faraone SV, Frazier J, Coffey BJ, Kim G, Bellordre CA. Clinical correlates of obsessive compulsive disorder in children and adolescents referred to specialized and non-specialized clinical settings. Depress Anxiety. 2000;11:163–168. doi: 10.1002/1520-6394(2000)11:4<163::AID-DA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser Y, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. J Nerv Ment Dis. 2001;189:384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive-behavioral coping skills therapy manual. Vol. 3. National Institute of Alcohol Abuse and Alcoholism; Rockville, MD: 1995. [Google Scholar]
- Kaminer Y, Tarter RE, Bukstein OG, Kabene M. Comparison between treatment completers and noncompleters among dually diagnosed substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 1992;31:1046–1049. doi: 10.1097/00004583-199211000-00008. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Newcorn JH, Arnold LE, Duesenberg D, Emslie GJ, Quintana H, Sarkis EH, Wagner KD, Gao H, Michelson D, Biederman J. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry. 2005;44:915–924. doi: 10.1097/01.chi.0000169012.81536.38. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- Magura S. Effectiveness of dual focus mutual aid for co-occurring substance use and mental health disorders: a review and synthesis of the “double trouble” in recovery evaluation. Subst Use Misuse. 2008;43:1904–1926. doi: 10.1080/10826080802297005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude-Griffin OM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66:832–837. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Monti PM, Abrams DB, Kadden RM, Cooney NL. Treating Alcohol Dependence: A Coping Skills Training Guide. Guilford Press; New York, NY: 1989. [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic, 4th Version. Nova University, Center for Psychological Study; Ft. Lauderdale, FL: 1987. [Google Scholar]
- Prince JB, Wilens TE, Biederman J, Spencer TJ, Millstein R, Polisner DA, Bostic JQ. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2000;10:193–204. doi: 10.1089/10445460050167304. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA. Neurobiological and psychosocial processes associated with depressive and substance-related disorders in adolescents. Curr Drug Abuse Rev. 2008;1:68–80. doi: 10.2174/1874473710801010068. [DOI] [PubMed] [Google Scholar]
- Ray GT, Weisner CM, Mertens JR. Relationship between use of psychiatric services and five-year alcohol and drug treatment outcomes. Psychiatr Serv. 2005;56:164–71. doi: 10.1176/appi.ps.56.2.164. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Baker S, Mikulich SK, Young SE, Crowley TJ. Depression in substance-dependent delinquents. J Am Acad Child Adolesc Psychiatry. 1995;34:764–771. doi: 10.1097/00004583-199506000-00017. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Mikulich-Gilbertson SK, Davies RD, Lohman M, Klein C, Stover SK. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161:1026–1034. doi: 10.1001/archpedi.161.11.1026. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Winhusen T, Davies R, Leimberger J, Mikulich-Gilbertson SK. A randomized controlled trial of OROS-MPH + CBT in adolescents with ADHD and substance use disorders Symposium Presentation and Symposium Chair. NIDA-sponsored symposium at AAAP annual meeting; Honolulu, HI. 2009. [Google Scholar]
- Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, Macdonald M, Lohman M, Bailey GL, Haynes L, Jaffee WB, Hodgkins C, Whitmore E, Trello-Rishel K, Tamm L, Acosta MC, Royer-Malvestuto C, Subramaniam G, Holmes BW, Kaye ME, Vargo MA, Woody GE, Nunes EV, Liu D. Randomized controlled trial of osmotic-release methylphenidate with CBT in adolescents with ADHD and substance use disorders. J Am Acad Child Adolesc Psychiatry. doi: 10.1016/j.jaac.2011.06.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN. An overview of the Diagnostic Interview Schedule and the Composite International Diagnostic Interview. In: Mezzich JE, von Cranach M, editors. International Classification in Psychiatry: Unity and Diversity. Cambridge University Press; New York: 1988. pp. 205–220. [Google Scholar]
- Rivers SM, Greenbaum RL, Goldberg E. Hospital-based adolescent substance abuse treatment: comorbidity, outcomes and gender. J Nerv Ment Dis. 2001;189:229–237. doi: 10.1097/00005053-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. J Am Acad Child Adolesc Psychiatry. 1996;35:101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Rowe CL, Liddle HA, Dakof GA. Classifying clinically referred adolescent substance abusers by level of externalizing and internalizing symptoms. J Child Adolesc Subst Abuse. 2001;11:41–65. [Google Scholar]
- Rowe CL, Liddle HA, Greenbaum PE, Henderson CE. Impact of psychiatric comorbidity on treatment of adolescent drug abusers. J Subst Abuse Treat. 2004;26:129–140. doi: 10.1016/S0740-5472(03)00166-1. [DOI] [PubMed] [Google Scholar]
- Shane PA, Jaiukaitis P, Green RS. Treatment outcomes among adolescents with substance abuse problems: the relationship between comorbidities and post-treatment substance involvement. Eval Program Plann. 2003;26:393–402. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc.; Rockville, MD: 1992. pp. 207–224. [Google Scholar]
- Waldron HB, Slesnick N, Brody JL, Turner CW, Peterson TR. Treatment outcomes for adolescent substance abuse at 4- and 7-month assessments. J Consult Clin Psychol. 2001;69:802–813. [PubMed] [Google Scholar]
- Whitmore EA, Mikulich SK, Thompson LL, Riggs PD, Aarons GA, Crowley TJ. Influences on adolescent substance dependence: conduct disorder, depression, attention deficit hyperactivity disorder, and gender. Drug Alcohol Depend. 1997;47:87–97. doi: 10.1016/s0376-8716(97)00074-4. [DOI] [PubMed] [Google Scholar]
- Wise BK, Cuffe SP, Fischer T. Dual diagnosis and successful participation of adolescents in substance abuse treatment. J Subst Abuse Treat. 2001;21:161–165. doi: 10.1016/s0740-5472(01)00193-3. [DOI] [PubMed] [Google Scholar]


